Abstract
Anterior temporal lobe resection (ATLR) is often complicated by superior quadrant visual field deficits (VFDs) because of damage to the anterior portion of the optic radiation (Meyer’s loop). This study reports the evaluation of optic radiation mapping in protecting against VFDs in the ATLR. We retrospectively analyzed 52 patients with medically refractory temporal lobe epilepsy undergoing ATLR between January 2012 and December 2013. The surgical operations in Group I (n=32) were performed with the modified ATLR, and the operations in Group II (n=20) were aided by combining optic radiation mapping by diffusion tensor imaging, microscopic-based neuronavigation and the intraoperative magnetic resonance imaging (iMRI) technique. A t-test was used to compare the size of ATLR, and a chi square test was applied for the postoperative VFD and seizure outcomes. The optic radiation was reconstructed in all patients in Group II. The size of ATLR was 5.11±1.34 cm (3.3-8 cm), and 3.24±0.75 cm (2.2-4.8 cm) in Groups I and II, respectively; the size of ATLR was significantly smaller in Group II (F=9.803; P=0.00). The visual fields assessment by the Humphrey Field Analyser 30-2 test showed 27 patients (84.4%) in Group I suffered VFDs at 3 months post-operation, whereas only eight patients (40.0%) in Group II showed VFDs (Pearson chi square =11.01; P=0.001). The 6-month follow-up survey showed that 90.6% of patients in Group I achieved a good outcome (Engel class I-II), outperforming 85.0% in Group II, however, there was no statistically significant difference (chi square =0.382, P=0.581). This techniques of combining optic radiation mapping, microscopic-based neuronavigation and iMRI aided in precise mapping and hence reduction of the risk of visual field deficits in ATLR. The size of ATLR guided by optic radiation mapping was significantly smaller but the seizure outcome was not significantly affected.
Keywords: Intraoperative magnetic resonance imaging (iMRI), optic radiation mapping; diffusion tensor imaging (DTI), visual field deficits (VFD), anterior temporal lobe resection (ATLR)
Introduction
Up to 40% of patients with temporal lobe epilepsy (TLE) are refractory to medication [1,2] and anterior temporal lobe resection (ATLR) is a well-established and effective means of treatment [3]. ATLR is often complicated by superior quadrant visual field deficits (VFDs) because of damage to the Meyer’s loop of the optic radiation. Meyer’s loop, the most anterior portion of the optic radiation, passes through the temporal lobe, and is therefore at risk during surgery. The ability to drive is a key goal of patients who are undergoing surgery [4], but postoperative VFDs are significant enough to preclude driving in 4-50% of patients, even if a patient is free of seizures [5-7].
Anatomic dissection and experience from temporal lobe surgery since the 1940s have provided copious information on the anatomy of the optic radiation and the consequences of surgery. However, the optic radiation cannot be distinguished using clinical magnetic resonance imaging (MRI) sequences. More recently, diffusion tensor imaging (DTI) tractography has enabled depiction of the optic radiation in vivo, with these data now routinely used to guide tumor neurosurgery. The DTI technique has also been applied to evaluate the relationship between white matter fibers and the epileptic foci in the temporal lobe, in sensorimotor cortex epilepsy or in occipital lobe epilepsy [8-13] for better decision making on resection sizes and for better estimation of the potential functional deficits [14].
Using DTI, the location of lesions can be visualized relative to the adjacent eloquent cortex and fiber tracts, which allows a surgeon to preoperatively design optimal resection trajectories for maximally removing the lesions as well as preserving function. Beyond preoperative mapping and planning, the intraoperative MRI (iMRI) technique further provides immediate evaluation of the surgical outcome [15]. To make full use of the neuroimaging data, microscope-based functional neuronavigation allows on-line evaluation of the extent of resection by continuously updated intraoperative images with brain shift addressed, which makes intraoperative adaptation of the surgical procedure feasible.
Here we describe the size of ATLR, the postoperative VFD and the seizure outcomes of operations on 52 patients with temporal lobe epilepsy. Operations for 32 patients were performed with the modified anterior temporal lobe resection between January 2012 and December 2012, and operations for 20 patients were guided by combining optic radiation mapping, microscopic-based neuronavigation and the iMRI technique between January 2013 and December 2013. In this paper, we focus on comparisons of the size of ATLR, the postoperative VFD and the seizure outcomes between the two patient groups who underwent different surgical procedures.
Methods
Subjects
We studied 52 patients with medically refractory temporal lobe epilepsy undergoing ATLR at the General Hospital of PLA, Beijing, China, between January 2012 and December 2013. Of the 52 patients, 30 had left- and 22 right-sided resections. The subjects were 31 males and 21 females. The types of seizure experienced by the subjects included complex partial seizures, generalized tonic-clonic seizures, and status epilepticus. Common pre-surgical evaluation procedure was applied to all patients, including continuous video electroencephalography (EEG) monitoring and MRI. Additional examinations including magnetoencephalography (MEG), positron emission tomography (PET), and intracranial grids were performed for a few patients. The pre-surgical examination of visual fields was assessed by the Humphrey Field Analyser 30-2 test in all patients; only one patient had a partial quadrant visual field deficit. All patients were divided into two groups chronologically; each group underwent different surgical procedures. Group I (n=32) were surgically treated with the modified ATLR [16,17] (3-8 cm of lateral temporal cortex, the uncus, the amygdala, and 2-4 cm of anterior hippocampus) between January 2012 and December 2012. Group II (n=20) was surgically treated with the guidance of optic radiation mapping, microscopic-based neuronavigation, and the iMRI technique between January 2013 and December 2013. The clinical characteristics of Groups I and II are summarized in Table 1.
Table 1.
Clinical characteristics of patients in Groups I and II
| Characteristic | Group I | Group II | p-value |
|---|---|---|---|
| No. of patients | 32 (61.5) | 20 (38.5) | |
| Mean age (years) | (26.8±8.5) | (27.5±8.9) | .797a |
| Mean duration of epilepsy (years) | (9.6±8.8) | (6.4±5.0) | .094a |
| Identifiable lesions on magnetic resonance imaging | 17 (53.1) | 9 (45.0) | .569b |
| Intracranial grids | 11 (33.3) | 6 (30.0) | .801b |
| Left temporal lobe epilepsy | 17 (53.1) | 14 (70.0) | .228b |
Values are presented as mean ± SD or number (%).
t-test;
Chi square test.
The research protocol was approved by the Ethics Committee of The General Hospital of People’s Liberation Army and all patients gave informed consent for the study.
Image acquisition
MRI was performed for all patients using 1.5 T scanners (Siemens Espree, Erlangen, Germany). For Group I, only conventional MRI was performed to show the identifiable lesions or hippocampal sclerosis. For Group II, Both conventional MRI and DTI were performed. A T1-weighted 3D magnetization-prepared rapid-acquisition gradient echo sequence was measured with an echo time of 3.02 milliseconds, a repetition time of 1650 milliseconds, a matrix size of 256×256, a field of view of 250×250 mm, a slice thickness of 1 mm, and a slab of 16 cm. In addition, T2-weighted images (echo time, 93 milliseconds; repetition time, 5500 milliseconds; matrix size, 512×512; field of view, 230×230 mm; slice thickness, 3 mm), T2 fluid-attenuated inversion recovery images (echo time, 84 milliseconds; repetition time, 9000 milliseconds; matrix size, 256×256; field of view, 230×230 mm; slice thickness, 3 mm), and postcontrast 3D T1-weighted images were scanned.
For DTI, we applied a single-shot spin-echo diffusion-weighted echoplanar imaging sequence (echo time, 147 milliseconds; repetition time, 9400 milliseconds; matrix size, 128×128; field of view, 251×251 mm; slice thickness, 3 mm; bandwidth, 1502 Hz per pixel; diffusion encoding gradients in 12 directions using b values of 0 and 1000 s/mm2; and voxel size, 1.9×1.9×3 mm). We used 40 slices, no intersection gap, 40 continuous free interval collection slices, and 5 time repetitions. The total scan time was 10 minutes 22 seconds).
Intraoperative scans were performed immediately when the anterior temporal lobe was completely removed or when intraoperative scanning was necessary for brain shift correction in Group II.
Image processing (tractography)
For fiber tracking, we implemented a tracking algorithm based on a tensor deflection algorithm. Generally, the course of a fiber is defined by following the direction of maximum diffusion. We used the fiber-tracking module of the neuronavigation planning software iPlan 2.6 (BrainLab, Feldkirchen, Germany) to reconstruct the optic radiation. Details of the method have previously been published. Before tracking was initiated, the fractional anisotropy threshold was adjusted to 0.15, the angle threshold to 20, and the minimum fiber length to 50 mm (stop criteria). Tract seeding was performed by defining a rectangular volume of interest (VOI) in the coregistered standard T1 anatomic data sets. We used a multi-VOI algorithm for fiber tracking of the optic radiation. For the ventral bundle of the optic radiation (the Meyer loop), the first VOI was placed on the lateral geniculate body. The second VOI was placed to cover the lower lip of the visual occipital cortex (calcarine cortex). We identified the lateral geniculate body by selecting the axial slice at the level of the transition from the posterior limb of the internal capsule to the cerebral peduncle. At this level, the lateral geniculate body is visible posterolateral to the peduncle. To reconstruct the dorsal bundle of the optic radiation (central and posterior bundle), the first VOI was also placed on the lateral geniculate body, and the second VOI was placed to cover the middle and upper lip of the visual occipital cortex. The fiber tract that passed through both VOIs was the final tract of interest. After selection of the appropriate fiber bundle, a 3D object was “created” automatically by wrapping neighboring fibers with a hull. The closing lines around all fibers from all slices together resulted in the 3D object (optic radiation). The anterior limit of the optic radiation was the Meyer loop. The time required to process the DTI data and to acquire the 3D object was approximately 10 minutes.
For segmentation and 3D reconstruction of the lesion and hippocampus, we used the “object creation” module. Segmentation of the lesion was performed on the T2 fluid-attenuated inversion recovery (FLAIR) anatomical data set, on a slice-by-slice basis. After all slices containing the lesion were outlined, a 3D image of the lesion was reconstructed. If the MRI showed hippocampal sclerosis only or no identifiable lesions, we only reconstructed the optic radiation (Figure 1).
Figure 1.
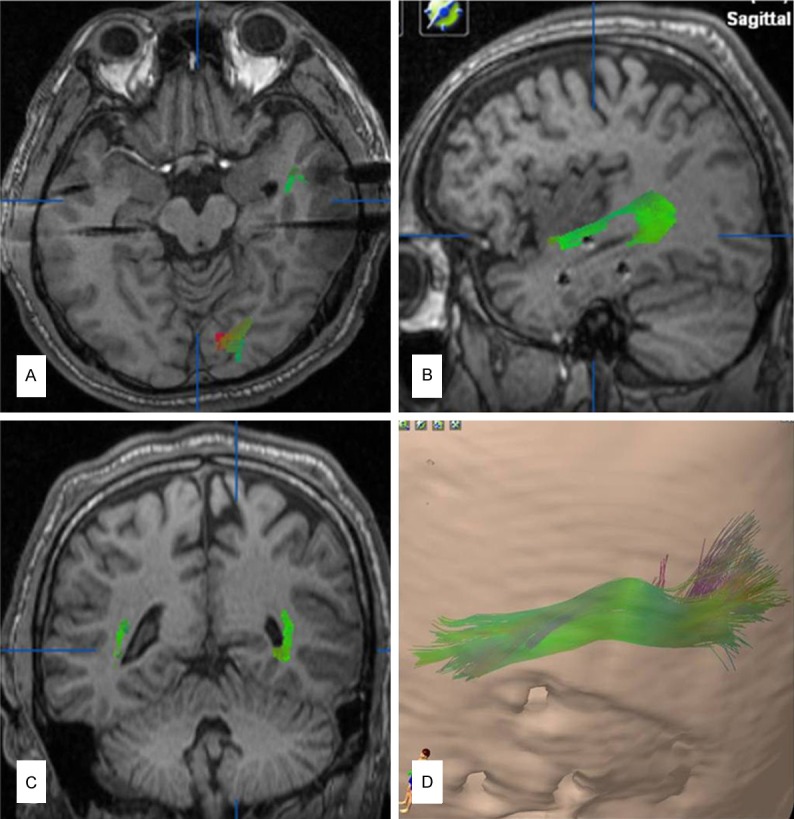
Preoperative surgical plan. A-C: The left optic radiation (green) was partially shown in the axial (lateral region of temporal horn), sagittal (outside of temporal stem) and coronal (outside of lateral ventricular trigone), T1 magnetic resonance imaging views. D: The left optic radiation was completely shown in 3D views.
Microscope-based neuronavigation and intraoperative MRI
In Group II, the DTI was registered with anatomical images. The technical details have been published elsewhere [18-20]. The contours of lesions, hippocampal sclerosis and optic radiation were displayed in the viewing field of the neuronavigation microscope (Pentero, Carl Zeiss, Oberkochen, Germany) (Figure 2). We resected the anterior temporal lobe anterior to the anterior limit of the optic radiation for each patient in Group II. iMRI was performed to examine whether the optic radiation was damaged, to make the decision if we should continue the resection of the anterior temporal lobe (Figure 3). In the cases when further resection was needed, the neuronavigation was updated based on the intraoperative MRI images and corrected for shifting of the brain. Mapping of the optic radiation was also updated with the fiber tracking module.
Figure 2.
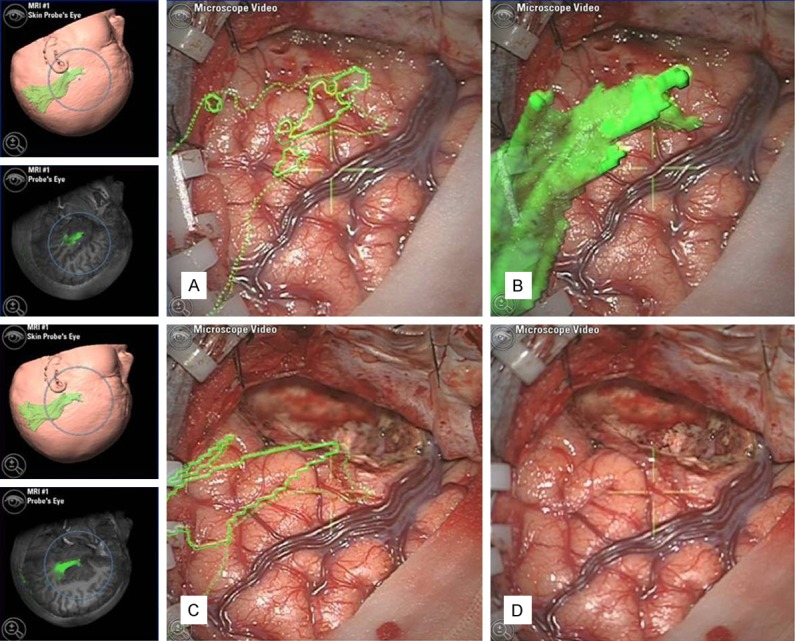
The optic radiation (green) was projected onto the cortex of the anterior temporal lobe in the viewing field of the neuronavigation microscope. The operation was performed anterior to the anterior limit of the optic radiation. A: A preoperative two-dimensional projection of the optic radiation, B: A preoperative three-dimensional projection of the optic radiation, C: A postoperative two-dimensional projection of optic radiation, D: A view of the temporal lobe resection.
Figure 3.
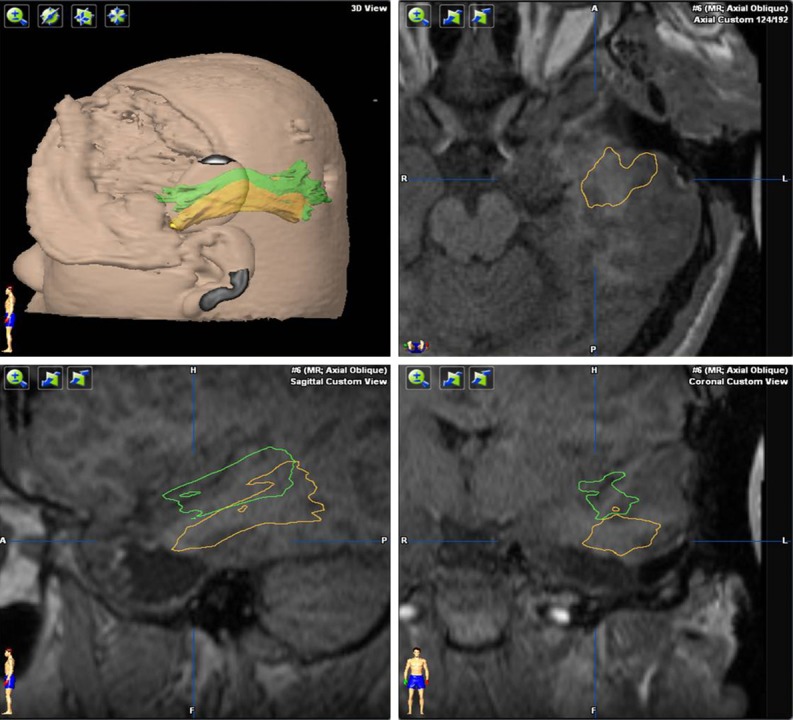
Fusion of preoperative and intraoperative magnetic resonance imaging (MRI) scans. After removing the anterior temporal lobe according to the projection of the optic radiation onto the cortex, an intraoperative MRI scan was performed and the optic radiation was reconstructed (green). We then judged whether the residual temporal lobe tissue needed to be removed. The intraoperative MRI scan was then fused to the preoperative MRI. Comparing the intraoperative optic radiation (green) with the preoperative optic radiation (yellow), we found that shifting of the brain because of cerebrospinal fluid movement during surgery was very common. The temporal lobe usually shifted toward the center line and the top of the head, but there was very little shift from front to back if the patient’s head was positioned on the side.
Evaluation of sizes of ATLR, postoperative VFD and seizure outcomes
We obtained the size of the ATLR according to the medical records in Group I, and the results measured during operation in Group II. A t-test was used to compare the size of the ATLR between Group I and Group II. All patients were followed for at least 6 months and the seizure outcome was compared at 6-month follow-up. The seizure outcome was evaluated according to Engel’s classification [21]. Class I patients were those who showed absence of seizures or presence of auras only or presence of seizures only during drug withdrawal. Class II patients had rare seizures or nocturnal seizures only. Class III patients showed worthwhile improvement, and Class IV patients showed no improvement. Automated static perimetry was performed, and the preoperative and postoperative visual fields were assessed by the Humphrey Field Analyser 30-2 test. The postoperative VFD was evaluated at 3 months post-operation. The chi square test was used to compare the seizure outcome and postoperative VFD across patient groups.
Results
In Group II, the presurgical diffusion tensor images reconstructed the optic radiation in all cases. We measured the distance from the tip of the optic radiation to the temporal pole in all subjects and removed a length of the anterior temporal lobe no greater than this distance. iMRI was performed at least once and the optic radiation was reconstructed in all patients in Group II. The size of the ATLR was 5.11±1.34 cm (3.3-8 cm), and 3.24±0.75 cm (2.2-4.8 cm) individually in Groups I and II, respectively. The size of the ATLR was significantly smaller in Group II (F=9.803; P=0.00).
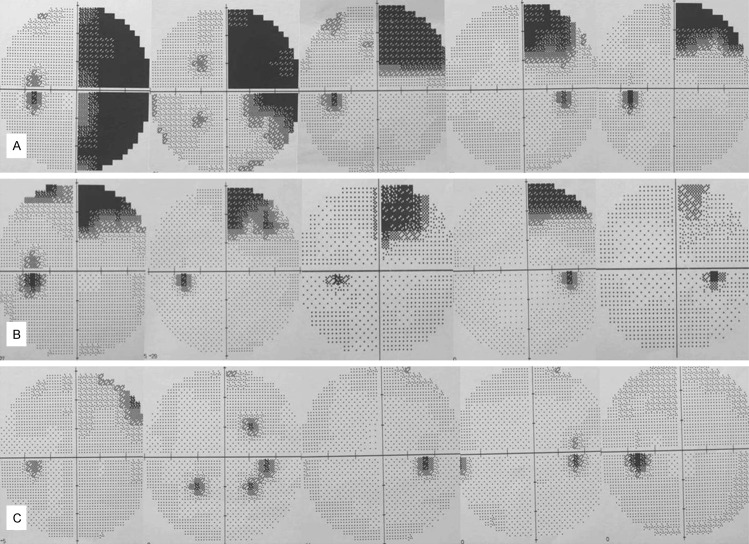
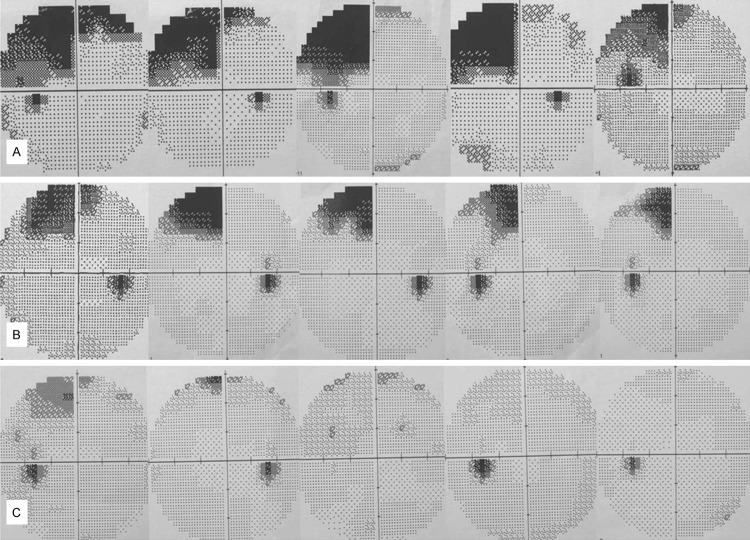
All patients had preoperative and postoperative visual fields assessed by the Humphrey Field Analyser 30-2 test; only one patient in Group I had a partial quadrant visual field deficit before the operation. Twenty-seven patients (84.4%) in Group I suffered VFDs at 3 months post-operation, whereas only eight patients (40.0%) in Group II suffered VFDs at 3 months post-operation (Pearson chi square =11.01; P=0.001). Only one of the eight patient with VFDs in Group II suffered contralateral upper quadrant VFDs; the other patients showed less than quadrant VFDs. Of the 27 patients with VFDs in Group I, one showed hemianopia, 16 patients had quadrant VFDs, and the remaining patients had partial quadrant VFDs (Figures 4, 5).
Figure 4.
Left visual field deficits demonstrated by the Humphrey Field Analyser 30-2 test on right ATLR. One patient showed hemianopia and most others showed upper quadrant VFDs in Group I (A). In Group II, most of the patients had partial quadrant VFDs or no VFDs (B, C).
Figure 5.
Right VFDs demonstrated by the Humphrey Field Analyser 30-2 test on left ATLR. Most of the patients in Group I showed upper quadrant VFDs (A), and most of the patients in Group II had partial quadrant VFDs or no VFDs (B, C).
The 6-month follow-up survey showed a good outcome in 90.6% patients in Group I (Engel class I-II), outperforming 85.0% in Group II; however, there was no statistically significant difference (chi square =0.382, P=0.581). The seizure outcomes, postoperative VFD and ATLR size in Groups I and II are summarized in Table 2.
Table 2.
Seizure outcomes, postoperative VFD and the size of anterior temporal lobe resection (ATLR) between Groups I and II
| Total number (n=52) | Group I (n=32) | Group II (n=20) | p-value |
|---|---|---|---|
| Size of ATLR | 5.11±1.34 | 3.24±0.75 | 0.00a,* |
| Postoperative VFD | .001b,* | ||
| No VFD | 5 (15.6) | 12 (60.0) | |
| VFD | 27 (84.4) | 8 (40.0) | |
| Engel outcome | .537b | ||
| I-II | 29 (90.6) | 17 (85.0) | |
| III-IV | 3 (9.4) | 3 (15.0) |
Values are presented as number (%).
t-test;
Chi square test.
P<0.05.
Anterior temporal lobe resection (ATLR); Visual field deficits (VFD).
Discussion
ATLR for epilepsy was pioneered by Wilder Penfield from the Montreal Neurological Institute and Murray Falconer from the Guy’s-Maudsley Neurosurgical Unit, London from the late 1940s onward. Penfield found that when the line of removal was less than 6 cm posterior to the tip, it generally resulted in no postoperative visual field defect. If the removal included more than 6 cm posterior to the tip, it is apt to produce contralateral upper quadrant homonymous hemianopia. When the line was pushed back to 8 cm, there was likely to be a complete homonymous hemianopic defect [22]. Studies showed that the rates of quadrantanopia complicating lobectomy range from 50-70% [6,23-25] to 90-100% [26-29]. The original 6-cm safety margin proposed by Penfield [22] is clearly an overestimate, with subsequent studies suggesting margins of 30-40 mm [26] and 45 mm [27] from the temporal pole to avoid a VFD. Over time, estimates have gradually reduced further and a key finding is substantial variability among subjects, reflecting anatomic variability in the location of Meyer’s loop. Despite the anatomic variability, a linear relationship between the severity of a VFD and the degree of resection has been shown [30]. Studies have concluded that field loss is related to resection length, and that Meyer’s loop extends more anteriorly than estimated in traditional surgical studies.
The optic radiation cannot be distinguished using clinical MRI sequences. However, DTI tractography is an advanced MRI technique that enables the parcellation of white matter. Recently the DTI technique has also been applied to evaluate the relationship between white matter fibers and epileptic foci for better decision making on resection sizes and for better estimation of the potential functional deficits. Several authors have found that the visual field can be protected in ATLR by reconstructing the optic radiation [30-37].
In Group II of this study, the optic radiation was successfully reconstructed in all patients using the DTI technique. The size of ATLR in Group II was 3.24±0.75 cm (2.2-4.8 cm), and compared with Group I there was a smaller resection size. Regarding the size of ATLR and the anterior limit of the optic radiation, Jason J. S. Barton’s study [30] showed in 24 patients that the distance was 24 to 28 mm from the anterior limit of Meyer’s loop to the anterior temporal pole. Taoka [32] examined 14 patients who underwent temporal resection for temporal lobe epilepsy and found that the mean T-M distance (between the temporal tip and the anterior limit of the Meyer loop) was 36.6 mm. The interindividual variation of the distance ranged from 30.0 to 43.2 mm. Yogarajah’s study [33] showed that the distance from the tip of Meyer’s loop to the temporal pole was 24-43 mm (mean 34 mm), and 24-47 mm (mean 35 mm) in their two patient groups. Winston [35] showed that Meyer’s loop was 4.4 to 18.7 mm anterior to the resection margin in his patients. In Borius’s study [37], MRI studies performed in 18 patients and 13 controls with DTI with fiber tracking found a marked individual difference in the TP-ML distances (mean: 25.4 mm; range 18.2-38.3 mm; standard deviation: 4.7) but with no significant difference between patients and controls. All these studies showed a smaller size between the temporal pole and anterior limit of the optic radiation; however, those were preoperative or postoperative results. In our group, we obtained the size of ATLR from the presurgical plan and also updated intraoperative data, avoiding deviation because of the brain shift due to loss of cerebrospinal fluid, especially from the open temporal horn. Our result was 2.2-4.8 cm (mean: 3.24 cm); the considerable variation is consistent with others. We also found that compared with Group I, the reconstruction of optic radiation with DTI technique reduced the size of ATLR in Group II.
Many studies have shown different levels of VFDs after ATLR. In some cases this can be severe enough to prohibit driving, even if a patient is free of seizures. Winston [35] studied 20 patients undergoing ATLR, with the help of structural MRI scans and DTI. Twelve of the twenty patients (60%) suffered a VFD (10-92% of upper quadrant; median, 39%) and eight patients had no VFD; the size of the resection was significantly greater in those developing a VFD than those without a VFD. In another study in 21 postoperative patients, 6 of the 11 patients undergoing a left ATLR suffered postoperative VFDs, compared with 3 of the 10 patients undergoing a right ATLR [33]. Taoka [32] using diffusion tensor tractography of Meyer’s loop in temporal lobe resection for temporal lobe epilepsy, found a statistically significant correlation between the degree of the visual field defect and the M-R distance (the anterior limit of the Meyer loop to the posterior limit of the temporal lobe resection); however, detailed studies of visual outcomes are lacking. In our cohort, 27 patients (84.4%) suffered from VFDs in Group I, whereas only 8 patients (40.0%) showed VFDs in Group II. Compared with other studies, our rate of VFDs is lower. All studies together show that the anterior limit of the optic radiation is in the anterior portion of the temporal lobe, and passes through it. The anterior limit of the optic radiation in most of the patients in Group I was damaged, so the patients suffered complete or partial superior quadrant VFDs. If the dorsal and ventral bundles of the optic radiation were damaged during surgery, the patients suffered hemianopia. For example, one patient received an 8-cm anterior temporal lobe resection in Group II, which resulted in hemianopia. Only one of the eight patients with VFDs in Group II suffered contralateral upper quadrant VFDs, the other seven patients had significantly lesser quadrant VFDs, showing that there was only partial damage to the anterior limit of the optic radiation in these seven patients. Analyzing the relationship between the size of ATLR and VFDs, we found that in Group I, the size of ATLR was larger, corresponding to a higher rate of postoperative VFDs, and in Group II, the size of ATLR was smaller, corresponding to a lower rate of the postoperative VFDs.
The 6-month follow-up survey showed that 90.6% of patients in Group I achieved a good outcome (Engel class I-II), outperforming 85.0% in Group II, however, this was not statistically significant (chi square =0.382, P=0.581). That is to say, in our study, the size of ATLR did not affect the efficacy of postoperative seizure control. In our groups, medial temporal lobe epilepsy was confirmed in all 52 patients by intracranial electrodes, explicit hippocampal sclerosis or lesion in the hippocampus-amygdala region by MRI. However, previous studies did not describe seizure outcomes [30-32].
Cushing [38] and Bjork [26] suggest that the optic radiation clothes the temporal horn and lies anterior to it. However, Van Buren [39] and Marino [23] depict the optic radiation ending just posterior to the temporal horn. Recent studies suggest that the optic radiation is anterior to the temporal horn, at least in the majority of cases. In one study, the average distance from temporal pole to temporal horn was 32 mm, with the optic radiation predicted to be 24 mm on average from the temporal pole [30], which is supported by dissection studies. In Nilsson’s study [31], seven healthy volunteers and two patients with previous temporal lobe resection were recruited. In the healthy subjects, the mean distance between the most anterior part of Meyer’s loop and the temporal pole was 44 mm (range 34-51 mm). Meyer’s loop did not reach the tip of the temporal horn in any subject. We thought that the distance of the temporal horn to the temporal pole may be constant; however, the anterior limit of optic radiation has a considerable variability in its anterior extent. So, in our study, we did not calculate the distance of the anterior edge of optic radiation to the temporal horn.
Shifting of the optic radiation and anterior hippocampus because of cerebrospinal fluid loss during surgery is very common. Intraoperative MRI has been proven to be a sound technique that allows a neurosurgeon to update neuronavigation data in real-time, to evaluate the extent of tumor resection, to correct for brain shift, to modify surgical strategy if necessary, to guide instruments to the lesion, and to evaluate presence of intraoperative complications at the end of surgery [40]. In this paper, we applied the iMRI technique, combined with microscopic-based neuronavigation to 20 patients in Group II. The iMRI technique confirmed that there was no hematoma; however, the shift of brain tissue occurred in every patient, and neuronavigation data was updated to help with accurate resection. This may be the reason that our rate of VFDs is lower than others. If the reconstruction of the optic radiation by iMRI scan showed remnant brain tissue anterior to the anterior limit of the optic radiation, the resection was continued. Then the intraoperative MRI scan was fused to the preoperative MRI, and, comparing the intraoperative optic radiation with the preoperative optic radiation, we found that shifting of the brain is very common. The temporal lobe most often shifted toward the center line and the top of the head, but there was very slight shift from front to back if the patient’s head was on the side.
Eight patients (40.0%) in Group II still suffered some VFDs. We suggest reasons that we could not completely protect the visual field as follows. 1) The size of anterior temporal lobectomy is too small; it is difficult to resect the hippocampus without excessively pulling the stump of the temporal lobe, causing secondary damage of optic radiation. 2) Brain shift may occur because of cerebrospinal fluid movement during the surgery; although this was partially corrected for by iMRI. 3) Currently, there is no standardized way to perform tractography. Choice of tractography method affected the visualized location of Meyer’s loop significantly in a heterogeneous, clinically relevant study group. Lilja et al. showed that to determine the anterior extent of Meyer’s loop, probabilistic tractography is superior to deterministic tractography, and the probability level of probabilistic tractography matters [41].
Conclusion
The techniques of combining optic radiation mapping, microscopic-based neuronavigation and iMRI aided in precise mapping and hence reduction of the risk of visual field deficits in ATLR. The size of ATLR with the guide of optic radiation mapping was significantly smaller but this had no significant effect on seizure outcome.
Acknowledgements
This work was supported by the fund of Beijing Key Laboratory of Epilepsy (No.2013DXBL01).
Disclosure of conflict of interest
None.
References
- 1.Semah F, Ryvlin P. Can we predict refractory epilepsy at the time of diagnosis? Epileptic Disord. 2005;7(Suppl 1):S10–3. [PubMed] [Google Scholar]
- 2.Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- 3.Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporallobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 4.Taylor DC, McMacKin D, Staunton H, Delanty N, Phillips J. Patients’ aims for epilepsy surgery: desires beyond seizure freedom. Epilepsia. 2001;42:629–633. doi: 10.1046/j.1528-1157.2001.34400.x. [DOI] [PubMed] [Google Scholar]
- 5.Manji H, Plant GT. Epilepsy surgery, visual fields, and driving: a study of the visual field criteria for driving in patients after temporal lobe epilepsy surgery with a comparison of Goldmann and Esterman perimetry. J Neurol Neurosurg Psychiatry. 2000;68:80–82. doi: 10.1136/jnnp.68.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pathak-Ray V, Ray A, Walters R, Hatfield R. Detection of visual field defects in patients after anterior temporal lobectomy for mesial temporal sclerosis-establishing eligibility to drive. Eye (Lond) 2002;16:744–748. doi: 10.1038/sj.eye.6700152. [DOI] [PubMed] [Google Scholar]
- 7.Jeelani NU, Jindahra P, Tamber MS, Poon TL, Kabasele P, James-Galton M, Stevens J, Duncan J, McEvoy AW, Harkness W, Plant GT. ‘Hemispherical asymmetry in the Meyer’s Loop’: a prospective study of visual-field deficits in 105 cases undergoing anterior temporal lobe resection for epilepsy. J Neurol Neurosurg Psychiatry. 2010;81:985–991. doi: 10.1136/jnnp.2009.182378. [DOI] [PubMed] [Google Scholar]
- 8.Otte WM, van Eijsden P, Sander JW, Duncan JS, Dijkhuizen RM, Braun KP. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia. 2012;53:659–667. doi: 10.1111/j.1528-1167.2012.03426.x. [DOI] [PubMed] [Google Scholar]
- 9.Gross DW. Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia. 2011;52(Suppl 4):32–34. doi: 10.1111/j.1528-1167.2011.03149.x. [DOI] [PubMed] [Google Scholar]
- 10.Winston GP, Yogarajah M, Symms MR, McEvoy AW, Micallef C, Duncan JS. Diffusion tensor imaging tractography to visualize the relationship of the optic radiation to epileptogenic lesions prior to neurosurgery. Epilepsia. 2011;52:1430–1438. doi: 10.1111/j.1528-1167.2011.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radhakrishnan A, James JS, Kesavadas C, Thomas B, Bahuleyan B, Abraham M, Radhakrishnan K. Utility of diffusion tensor imaging tractography in decision making for extratemporal resective epilepsy surgery. Epilepsy Res. 2011;97:52–63. doi: 10.1016/j.eplepsyres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Salmenpera TM, Simister RJ, Bartlett P, Symms MR, Boulby PA, Free SL, Barker GJ, Duncan JS. High-resolution diffusion tensor imaging of the hippocampus in temporal lobe epilepsy. Epilepsy Res. 2006;71:102–106. doi: 10.1016/j.eplepsyres.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Cui ZQ, Ling ZP, Song HF, Hu S, Sun GC, Chen XL, Pan LS, Li C, Xu BN. Combining pyramidal tract mapping, microscopic-based neuronavigation, and intraoperative magnetic resonance imaging improves outcome of epilepsy foci resection in the sensorimotor cortex. Turk Neurosurg. 2014;24:538–545. doi: 10.5137/1019-5149.JTN.9517-13.0. [DOI] [PubMed] [Google Scholar]
- 14.Yang WD, Chen ZJ, Yu Q, Wang ZG, Hao ZD, Li H, Zhang CZ. Applications of blood oxygenation level dependent-functional magnetic resonance imaging, diffusion tensor imaging and intraoperative neurophysiology monitoring in secondary epileptic surgery in M1 area. Zhonghua Yi Xue Za Zhi. 2010;90:2755–2758. [PubMed] [Google Scholar]
- 15.Black PM, Moriarty T, Alexander E 3rd, Stieg P, Woodard EJ, Gleason PL, Martin CH, Kikinis R, Schwartz RB, Jolesz FA. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41:831–842. doi: 10.1097/00006123-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Falconer MA, Meyer A, Hill D, Mitchell W, Pond DA. Treatment of temporal-lobe epilepsy by temporal lobectomy; a survey of findings and results. Lancet. 1955;268:827–835. doi: 10.1016/s0140-6736(55)90421-9. [DOI] [PubMed] [Google Scholar]
- 17.MORRIS AA. Temporal lobectomy with removal of uncus, hippocampus, and amygdala; results for psychomotor epilepsy three to nine years after operation. AMA Arch Neurol Psychiatry. 1956;76:479–496. doi: 10.1001/archneurpsyc.1956.02330290023003. [DOI] [PubMed] [Google Scholar]
- 18.Gasser T, Ganslandt O, Sandalcioglu E, Stolke D, Fahlbusch R, Nimsky C. Intraoperative functional MRI: implementation and preliminary experience. Neuroimage. 2005;26:685–693. doi: 10.1016/j.neuroimage.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Nimsky C, Ganslandt O, Buchfelder M, Fahlbusch R. Intraoperative visualization for resection of gliomas: the role of functional neuronavigation and intraoperative 1.5 T MRI. Neurol Res. 2006;28:482–487. doi: 10.1179/016164106X115125. [DOI] [PubMed] [Google Scholar]
- 20.Nimsky C, Ganslandt O, von Keller B, Fahlbusch R. Preliminary experience in glioma surgery with intraoperative high-field MRI. Acta Neurochir Suppl. 2003;88:21–29. doi: 10.1007/978-3-7091-6090-9_5. [DOI] [PubMed] [Google Scholar]
- 21.Engel J, Van Ness P, Rasmussen T. Outcome with respect to epileptic seizures. In: Engel J, editor. Surgical Treatment of the Epilepsies. 2nd edition. New York: Raven Press; 1993. pp. 609–621. [Google Scholar]
- 22.Penfield W. Temporal lobe epilepsy. Br J Surg. 1954;41:337–343. doi: 10.1002/bjs.18004116802. [DOI] [PubMed] [Google Scholar]
- 23.Marino R Jr, Rasmussen T. Visual field changes after temporal lobectomy in man. Neurology. 1968;18:825–835. doi: 10.1212/wnl.18.9.825. [DOI] [PubMed] [Google Scholar]
- 24.Katz A, Awad IA, Kong AK, Chelune GJ, Naugle RI, Wyllie E, Beauchamp G, Lüders H. Extent of resection in temporal lobectomy for epilepsy. II. Memory changes and neurologic complications. Epilepsia. 1989;30:763–771. doi: 10.1111/j.1528-1157.1989.tb05336.x. [DOI] [PubMed] [Google Scholar]
- 25.Tecoma ES, Laxer KD, Barbaro NM, Plant GT. Frequency and characteristics of visual field deficits after surgery for mesial temporal sclerosis. Neurology. 1993;43:1235–1238. doi: 10.1212/wnl.43.6.1235. [DOI] [PubMed] [Google Scholar]
- 26.Bjork A, Kugelberg E. Visual field defects after temporal lobectomy. Acta Ophthalmol (Copenh) 1957;35:210–216. doi: 10.1111/j.1755-3768.1957.tb05407.x. [DOI] [PubMed] [Google Scholar]
- 27.Falconer MA, Wilson JL. Visual field changes following anterior temporal lobectomy: their significance in relation to Meyer’s loop of the optic radiation. Brain. 1958;81:1–14. doi: 10.1093/brain/81.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Hughes TS, Abou-Khalil B, Lavin PJ, Fakhoury T, Blumenkopf B, Donahue SP. Visual field defects after temporal lobe resection: a prospective quantitative analysis. Neurology. 1999;53:167–172. doi: 10.1212/wnl.53.1.167. [DOI] [PubMed] [Google Scholar]
- 29.Mengesha T, Abu-Ata M, Haas KF, Lavin PJ, Sun DA, Konrad PE, Pearson M, Wang L, Song Y, Abou-Khalil BW. Visual field defects after selective amygdalohippocampectomy and standard temporal lobectomy. J Neuroophthalmol. 2009;29:208–213. doi: 10.1097/WNO.0b013e3181b41262. [DOI] [PubMed] [Google Scholar]
- 30.Barton JJ, Hefter R, Chang B, Schomer D, Drislane F. The field defects of anterior temporal lobectomy: a quantitative reassessment of Meyer’s loop. Brain. 2005;128:2123–2133. doi: 10.1093/brain/awh544. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson D, Starck G, Ljungberg M, Ribbelin S, Jönsson L, Malmgren K, Rydenhag B. Intersubject variability in the anterior extent of the optic radiation assessed by tractography. Epilepsy Res. 2007;77:11–16. doi: 10.1016/j.eplepsyres.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Taoka T, Sakamoto M, Nakagawa H, Nakase H, Iwasaki S, Takayama K, Taoka K, Hoshida T, Sakaki T, Kichikawa K. Diffusion tensor tractography of the Meyer loop in cases of temporal lobe resection for temporal lobe epilepsy: correlation between postsurgical visual field defect and anterior limit of Meyer loop on tractography. AJNR Am J Neuroradiol. 2008;29:1329–1334. doi: 10.3174/ajnr.A1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yogarajah M, Focke NK, Bonelli S, Cercignani M, Acheson J, Parker GJ, Alexander DC, McEvoy AW, Symms MR, Koepp MJ, Duncan JS. Defining Meyer’s loop-temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain. 2009;132:1656–68. doi: 10.1093/brain/awp114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun GC, Chen XL, Zhao Y, Wang F, Hou BK, Wang YB, Song ZJ, Wang D, Xu BN. Intraoperative high-field magnetic resonance imaging combined with fiber tract neuronavigation-guided resection of cerebral lesions involving optic radiation. Neurosurgery. 2011;69:1070–1084. doi: 10.1227/NEU.0b013e3182274841. [DOI] [PubMed] [Google Scholar]
- 35.Winston GP, Daga P, Stretton J, Modat M, Symms MR, McEvoy AW, Ourselin S, Duncan JS. Optic radiation tractography and vision in anterior temporal lobe resection. Ann Neurol. 2012;71:334–341. doi: 10.1002/ana.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piper RJ, Yoong MM, Kandasamy J, Chin RF. Application of diffusion tensor imaging and tractography of the optic radiation in anterior temporal lobe resection for epilepsy: a systematic review. Clin Neurol Neurosurg. 2014;124:59–65. doi: 10.1016/j.clineuro.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Borius PY, Roux FE, Valton L, Sol JC, Lotterie JA, Berry I. Can DTI fiber tracking of the optic radiations predict visual deficit after surgery? Clin Neurol Neurosurg. 2014;122:87–91. doi: 10.1016/j.clineuro.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 38.Cushing H. Distortions of the visual fields in the case of brain tumours: the field defects produced by temporal lobe lesions. Trans Am Neurol Assoc. 1921;47:374–420. [Google Scholar]
- 39.Van Buren JM, Baldwin M. The architecture of the optic radiation in the temporal lobe of man. Brain. 1958;81:15–40. doi: 10.1093/brain/81.1.15. [DOI] [PubMed] [Google Scholar]
- 40.Keles GE. Intracranial neuronavigation with intraoperative magnetic resonance imaging. Curr Opin Neurol. 2004;17:497–500. doi: 10.1097/01.wco.0000137543.14610.fd. [DOI] [PubMed] [Google Scholar]
- 41.Lilja Y, Ljungberg M, Starck G, Malmgren K, Rydenhag B, Nilsson DT. Visualizing Meyer’s loop: A comparison of deterministic and probabilistic tractography. Epilepsy Res. 2014;108:481–490. doi: 10.1016/j.eplepsyres.2014.01.017. [DOI] [PubMed] [Google Scholar]