Abstract
Background: This study aimed to investigate GABA effects on blood pressure and blood dynamics of anesthetic rats by observing spontaneously hypertensive rats under both anesthesia and waking state. Materials and methods: 72 male waking Wistar-Kyokos (WKY) rats and 72 male anesthetized spontaneously hypertensive (SHR) rats were randomly divided into control group and experimental group (N = 36 each). Rats were further divided into three subgroups (N = 12 each), which received 15 μmol GABA, 35 nmol muscimol, or 4 nmol dicentrine into unilateral paraventricular nucleus, respectively. Rats in the control group (WKY1) and experimental group (SHR1) were compared for the GABA effect on blood pressure (MAP), heart rate (HR), and arterial baroreceptor reflex function (BRS) changes under waking state. Anesthetic WKY rats (WKY2) and spontaneously hypertensive rats (SHR2) were compared for the GABA effect on those abovementioned indexes. Abdominal aorta mean arterial pressure, heart rate, and arterial baroreceptor reflex function changes were compared in all rats. Results: MAP, HR, and BRS were slightly lower in the rats under anesthetic state than in waking state before treatment (P < 0.05); they did not show significant changes between anesthetic and waking state, however, after treatment (P > 0.05). Unilateral paraventricular nucleus injection of GABA or muscimol elevated MAP, HR, and BRS in both normal and spontaneously hypertensive rats under waking or anesthetic state (P < 0.05). In addition, the amplitudes of changes of three indicators in spontaneously hypertensive group were markedly higher than those of control group (P < 0.05). Dicentrine could induce MAP and HR to increase, while BRS decreased significantly (P < 0.05). The amplitudes of changes in spontaneously hypertensive group were larger than those of normal group (P < 0.05). Conclusion: Centrally GABA injection can enhance the BRS function in spontaneously hypertensive rats and adjust heart rate to reduce the blood pressure fluctuation. It may play a role in reducing blood pressure and protecting cardiovascular function.
Keywords: γ-aminobutiric acid, blood pressure, arterial baroreceptor reflex function, blood dynamics, cardiovascular
Introduction
γ-aminobutiric acid (GABA), as a kind of important neurotransmitter in plants and animals, participates in regulating a variety of neural signaling pathways and physiological functions. It is involved in various biological activities including antihypertension, analgesia, antianxiety, reducing blood ammonia, and improving brain activity, and has been confirmed to participate in the regulation of cardiovascular activity [1,2]. Studies have reported [3,4] that GABA can reduce the irritating hypertension in experimental animals, and GABA intake has good function to the human body. As hypertension patients are often complicated with decreased renal function, GABA also can potentiate renal diuresis. It exerts the function of the prevention and treatment of hypertension by secreting excess salt to reduce the blood pressure [5,6]. γ-aminobutiric acid can act on GABA receptor to regulate the chloride ion concentration distribution between the nerve cell membrane. Activated γ-aminobutiric acid receptors on the cell membrane may open the chlorine ion channels for influx of chloride ions into the nerve cells, resulting the cell membrane hyperpolarization and excitability reduction, thereby reducing animal activity and lowering blood pressure [7]. In addition, γ-aminobutiric acid can also regulate the antidiuretic hormone vasopressin levels for vasodilation and decreasing blood pressure [8].
Paraventricular nucleus (PVN) of the hypothalamus has neuroendocrine function to regulate cardiovascular autonomic nervous system. Studies have demonstrated that the GABA concentration in PVN is higher with GABA-noid immune positive cells [9]. There were some studies focusing on the effect of GABA ergic neurons and its receptors in cardiovascular activities. The GABA-related effect on spontaneous hypertension and hemodynamics under anesthesia and waking state, however, still lacks investigation. This study aimed to explore GABA effect on cardiovascular activities and antihypertension under anesthetic state by comparing the MAP, HR, and BRS in spontaneously hypertensive rats under both anesthesia and waking state.
Materials and methods
Main reagents and instruments
GABA, muscimol, phenylephrine, and dicentrine were bought from Sigma (USA) and diluted by artificial cerebrospinal fluid. Ketamine was provided by Shanghai No.1 biochemical and pharmaceutical co. LTD. (Shanghai, China). Blood gas analyzer was got from Millipore (MAB341, USA). MPA-2000M multichannel biological signal analysis system was provided by Alcott biotech co., LTD. (Shanghai, China). Blood pressure monitoring device and type I rats stereotaxic instrument were from The Anqiu People’s Hospital (Anqiu, China).
Grouping
72 waking male WKY rats and 72 anesthesia spontaneous hypertensive male rats weighted 200-230 g were provided by in Shandong university animal experiment center/ABSL-III lab. Experimental animal qualified number was SCXK 2009-0007. Each type of the rats was randomly divided into two groups as WKY1, WKY2, SHR1 and SHR2 group. Each group of rats was further divided into three groups (n = 12): GABA, muscimol, and dicentrine group. And they were marked as 1a, 1b, and 1c group.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of our hospital.
Animal blood pressure monitor
Animals were fasted for 12 h before experiments. The rats received 5 ml/kg 0.7% chloral hydrate peritoneal injection and endotracheal intubation. The ventilator parameters were set as follows: FiO2 = 0.3, breathing frequency at 80/min, tidal volume as 10 ml/kg, and inspiration and expiration ratio as 1:2. Hydroxyethyl starch solution was given to maintain stable hemodynamics. One side femoral artery received intubation to connect with MPU-0.5A pressure converter and physiological recorder. MAP, electrocardiogram, and HR were recorded synchronously. Appropriate electromagnetic flow meter was selected to record the arterial flow at the root of rat aorta. Left common carotid artery was intubated to record the systolic pressure, diastolic pressure, and MAP to finally calculate the rat systemic vascular resistance and cardiac index. The left femoral vein was intubated. RM-6000 physiological recorder was applied to collect the abovementioned indicators. 1 g/L tubocurarine was intraperitoneal injected at 2 mg/kg and artificial ventilator (DH-140-B) was used to maintain ventilation. The experiment started after the blood pressure smooth, and the rectal temperature was maintained at 36~38°C.
PVN microinjection
The rat head was leaned on the stereotaxic instrument and located according to the Watson map after exposing skull [10]. 0.7 mm stainless steel casing was vertically inserted to PVN through the skull hole. Microinjection syringe with 0.3 mm diameter was applied to inject drugs to the PVN evenly through the casing. 0.5 μL fluid was injected for 1 min each time. 50 g/L methylene blue was injected after the experiment. After killing the rat, the brain was fixed in formaldehyde solution and sliced for injection point identification. Inaccurate positioning results were excluded. 15 μmol GABA, 35 nmol muscimol, or 4 nmol dicentrine at pH 6.4 was injected to unilateral paraventricular nucleus, respectively.
BRS detection
Modified Smyth method [11,12] was applied. Epinephrine was intravenous injected to stimulate arterial baroreceptor, and the systolic blood pressure and cardiac cycle were recorded at the same time. Linear regression was drawn to calculate the BRS in rats.
Statistical analysis
All statistical analyses were performed using SPSS20.0 software (Chicago, IL). Measurement data in normal distribution was presented as mean ± standard deviation (x ± s). Differences between groups were analyzed by 2-samle student t test, with P < 0.05 considered to indicate a statistically significant result.
Results
GABA effects on rat hemodynamics
MAP and HR were slightly lower in the rats under anesthetic state than in waking state before treatment (P < 0.05, t-test); they did not show significant changes between anesthetic and waking state after treatment (P > 0.05, t-test). Unilateral paraventricular nucleus injection of GABA or muscimol significantly decreased MAP and HR in both SHR rats and WKY rats under waking or anesthetic state (P < 0.05, t-test). In addition, the changes of MAP and HR in SHR group were significantly higher than that of WKY group (P < 0.05, t-test). Dicentrine significantly increased MAP and HR (P < 0.05, t-test), and their amplitudes of changes in SHR group were larger than those of WKY group (P < 0.05, t-test) (Tables 1 and 2).
Table 1.
The effect of PVN injection of GABA, muscimol, or dicentrine on rat’s hemodynamics under waking state (x̅ ± s)
| Group | Cases | MAP (mmHg) | HR (Beat/min) | ||
|---|---|---|---|---|---|
|
| |||||
| Before treatment | Changes | Before treatment | Changes | ||
| 15 μmol GABA | |||||
| WKY | 12 | 96.12 ± 10.23 | -16.43 ± 3.42* | 350.2 ± 16.1 | -10.1 ± 1.4* |
| SHR | 10 | 142.45 ± 11.34 | -30.21 ± 7.91*,# | 370.4 ± 14.2 | -26.6 ± 1.1*,# |
| 35 nmol muscimol | |||||
| WKY | 11 | 97.26 ± 10.78 | -14.33 ± 3.21* | 351.4 ± 13.3 | -5.3 ± 1.6* |
| SHR | 11 | 140.93 ± 10.67 | -29.21 ± 4.87*,# | 369.6 ± 12.3 | -27.6 ± 1.7*,# |
| 4 nmol dicentrine | |||||
| WKY | 12 | 98.37 ± 9.45 | 18.34 ± 3.78* | 352.1 ± 14.2 | 15.2 ± 9.8* |
| SHR | 10 | 139.89 ± 10.67 | 27.56 ± 6.93*,# | 372.3 ± 20.1 | 32.2 ± 10.3*,# |
Compared with before treatment, P < 0.05 by t-test;
Compared with WKY group, P < 0.05 by t-test.
Table 2.
The effect of PVN injection of GABA, muscimol, or dicentrine on rat’s hemodynamics under anesthetic state (x̅ ± s)
| Group | Cases | MAP (mmHg) | HR (Beat/min) | ||
|---|---|---|---|---|---|
|
| |||||
| Before treatment | Changes | Before treatment | Changes | ||
| 15 μmol GABA | |||||
| WKY | 11 | 76.72 ± 9.26 | -15.2 ± 2.52* | 315.2 ± 10.3 | -9.1 ± 2.7* |
| SHR | 12 | 105.69 ± 10.24 | -24.21 ± 7.34*,# | 336.4 ± 11.6 | -28.6 ± 3.5*,# |
| 35 nmol muscimol | |||||
| WKY | 12 | 69.27 ± 8.76 | -13.64 ± 3.51* | 301.74 ± 12.1 | -7.2 ± 1.4* |
| SHR | 11 | 103.89 ± 7.38 | -32.75 ± 3.87*,# | 328.93 ± 10.2 | -25.6 ± 1.5*,# |
| 4 nmol dicentrine | |||||
| WKY | 12 | 78.34 ± 7.64 | 19.35 ± 2.32* | 325.83 ± 10.2 | 16.3 ± 5.3* |
| SHR | 11 | 105.68 ± 8.36 | 29.67 ± 4.38*,# | 343.62 ± 8.4 | 29.8 ± 6.1*,# |
Compared with before treatment, P < 0.05 by t-test;
Compared with WKY group, P < 0.05 by t-test.
GABA effect on BRS
BRS was significantly lower in the rats under anesthetic state than in waking state before treatment (P < 0.05, t-test); it did not, however, present significant changes between anesthetic and waking state after treatment (P > 0.05, t-test). Unilateral paraventricular nucleus injection of GABA or muscimol increased SBR significantly in both SHR rats and WKY rats under waking or anesthetic state (P < 0.05, t-test). The amplitude of change of SBR in SHR group was markedly higher than that of WKY group (P < 0.05, t-test). Dicentrine could significantly decrease MAP and HR (P < 0.05, t-test), with larger amplitude in SHR group than that of WKY group (P < 0.05, t-test) (Table 3).
Table 3.
The effect of PVN injection of GABA, muscimol, or dicentrine on rat’s BRS under different state (x̅ ± s)
| Group | Cases | Waking status (ms/mmHg) | Anesthetic status (ms/mmHg) | ||
|---|---|---|---|---|---|
|
| |||||
| Before treatment | Changes | Before treatment | Changes | ||
| 15 μmol GABA | |||||
| WKY | 12 | 2.12 ± 0.03 | 0.31 ± 0.04* | 1.93 ± 0.16o | 0.37 ± 0.05* |
| SHR | 12 | 1.25 ± 0.14 | 0.72 ± 0.11*,# | 0.95 ± 0.04o | 0.79 ± 0.09*,# |
| 35 nmol muscimol | |||||
| WKY | 11 | 2.26 ± 0.08 | 0.30 ± 0.06* | 1.95 ± 0.07o | 0.35 ± 0.04* |
| SHR | 10 | 1.31 ± 0.07 | 0.86 ± 0.07*,# | 1.07 ± 0.02o | 0.92 ± 0.02*,# |
| 4 nmol dicentrine | |||||
| WKY | 12 | 2.17 ± 0.05 | -0.22 ± 0.08* | 2.01 ± 0.06o | -0.26 ± 0.06* |
| SHR | 11 | 1.29 ± 0.07 | -0.52 ± 0.03*,# | 0.96 ± 0.11o | -0.64 ± 0.04*,# |
Compared with before treatment, P < 0.05 by t-test;
Compared with WKY group, P < 0.05;
Compared with waking statues, P < 0.05 by t-test.
Indexes comparison under waking and anesthetic state
Under the waking state, rats in both groups exhibited lowering MAP, HR reduction, and BRS enhancement after PVN injection of 15 μmol GABA or 35 nmol muscimol; while PVN injection of 4 nmol dicentrine induced the opposite effect. Rats under the anesthetic state showed similar results between the two groups with no significant difference (P > 0.05, t-test) (Figures 1, 2 and 3).
Figure 1.
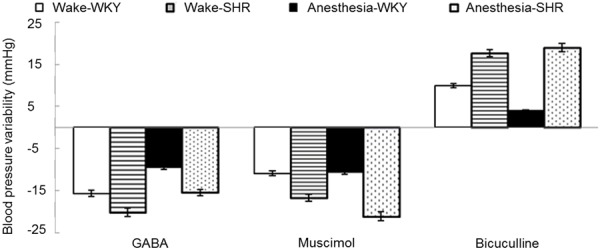
Blood pressure changes after the rats received PVN injection of GABA, muscimol, or dicentrine under waking or anesthetic state.
Figure 2.
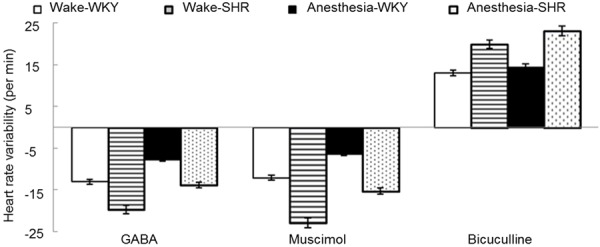
HR changes after the rats received PVN injection of GABA, muscimol, or dicentrine under waking or anesthetic state.
Figure 3.
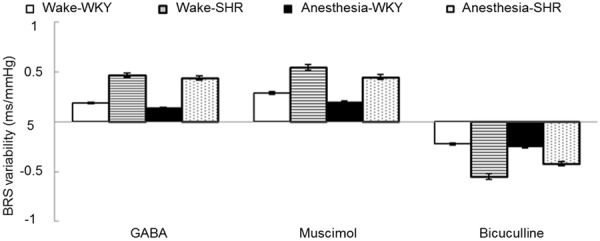
BRS changes after the rats received PVN injection of GABA, muscimol, or dicentrine under waking or anesthetic state.
Discussion
Hypertension is a common chronic disease causing severe complications such as stroke and myocardial infarction. Blood pressure elevation and fluctuation have important influence to cardiovascular, cerebrovascular and other viscera functions. Therefore, prevention and management of blood pressure, reduction of blood pressure fluctuation, and repairment of arterial baroreceptor reflex function play a significant role for the treatment and prevention of hypertension. Monitoring the changes of blood pressure, heart rate, and the arterial baroreceptor reflex function in hypertensive and normal rats can effectively reflect the GABA effect on rat blood pressure and hemodynamics.
GABA is the most important inhibitory neurotransmitter in the mammalian central nervous system. The influence of GABA signaling pathway activation on the central nervous system and the pathophysiological mechanisms has always been a research hot spot [13]. GABA receptor has 3 subtypes, named GABAA, GABAB, and GABAC receptors. There are certain GABA neurons and its corresponding receptors in mammals cerebrovascular tissues. GABAA receptor is a kind of ligand gated ion channel. Postsynaptic GABAA receptor has a role to dilate blood vessels. GABAB receptor is a type of G protein coupled receptor that can effectively suppress the sympathetic nerve ending excitement. Aminobutyric acid can stimulate GABAA and GABAB receptors in neurocytes to promote angiectasis and lower blood pressure [14]. GABA is one of the main neurotransmitters in the central nervous system. Though GABA produced inhibitory effect in the process of the neural transmission, GABA itself is a stimulating instead of inhibitory neurotransmitter. GABAA receptor activation can selectively let Cl- pass and cause neurons hyperpolarization [15]. The hyperpolarization may cause neural signaling inhibition. GABAA produced inhibitory postsynaptic potential reversion under normal conditions may lower the action potential. The active sites of GABAA receptor can combine with GABA and many drugs such as muscimol, dicentrine, and gaboxadol, etc. It also contains multiple heterogeneous adjustments that may adjust the receptor activity indirectly. Drugs that can regulate heterogeneous locus include neurosteroids and barbital. Studies have suggested that [16] GABA neurons and its receptor, GABA transporter and glutamic acid enzyme mRNA existed in parabrachial nuclei by immunohistochemistry and in situ hybridization. GABA participates in regulating cardiovascular activity in many parts such as hypothalamus, ventrolateral medulla, and amygdaloid nucleus [17].
Our results showed that MAP, HR, and BRS were slightly lower in the rats under anesthetic state than in waking state before treatment; they did not show significant changes between anesthetic and waking state after treatment. This indicated that animals under anesthesia presented as having less activity and weak response to external stimuli. Anesthetic drug has an inhibitory effect on hemodynamics and arterial baroreceptor reflex function, which implied that GABA can not only reduce hypertension, but also can smooth blood pressure fluctuation, regulate heart rate, and improve BRS function under both waking and anesthetic state to protect the rat cardiovascular tissues. In addition, unilateral paraventricular nucleus injection of GABA or muscimol can cause MAP and HR reduction, and BRS elevation in both SHR group and SKY group under two states. It revealed that exogenous GABA can effectively reduce hypertension and heart rate, improve hemodynamics, increase arterial baroreceptor reflex function, and improve the rat’s ability to adjust blood pressure change itself. Unilateral paraventricular nucleus injection of dicentrine could induce MAP and HR increase, BRS decrease significantly in both the SHR group and SKY group. It implied that compared to normal group, GABA can obviously reduce the blood pressure, slow heart rate, and improve BRS in SHR group, which showed the same effect as those injected by GABAA receptor agonist muscimol and the opposite effect as those injected by GABAA receptor blocker dicentrine. Furthermore, most GABA receptors are distributed in hypothalamus, indicating that the blood pressure regulation effect of central γ-aminobutiric acid in spontaneously hypertensive rats was related to GABAA receptor activation.
GABA showed the same impact on hemodynamics and arterial baroreceptor reflex function in anesthetic rats with muscimol, which can decrease peripheral vascular resistance, increase cardiac output, lower blood pressure, and reduce blood pressure fluctuation. In addition, GABA can significantly reduce the diastolic blood pressure, but without significant effect on improving left ventricular pressure and left ventricular pressure maximum change rate. It can be speculated that it suppresses blood pressure effect mainly through arterial vessel expansion and cardiac afterload reduction. Anesthetic drug can reduce the myocardial contractility and contraction frequency in vitro, and inhibit the normal heart activity. Blood pressure reduction may excite sympathetic nerves and weaken the drugs’ direct heart inhibition, resulting in increased heart rate. At whole animal level, GABA’s direct role on heart rate is stronger than its indirect effect via sympathetic nerves, which may slow heart rate by inhibiting the sinoatrial node. Another possibility is that GABA may inhibit nervous excitation, with the exact reason remaining unclear.
Central GABA can decrease blood pressure and slow heart rate by activating GABA receptor. GABA may increase the heart rate and tension booster effect by inhibiting the central norepinephrine neurotransmitter system to maintain normal blood pressure. Our results showed that GABA can significantly reduce blood pressure in spontaneously hypertensive rats, further confirming the antihypertension effect of central GABA as previously reported [18,19]. Central GABA exhibited stronger effects such as anesthetic animal blood pressure reduction, BRS elevation, heart rate slow down, and blood pressure regulation on SHR rats than those on WKY rats, including lower GABA level in hypertension animal’s central nervous system, which may be associated with the pathogenesis of hypertension [20,21]. PVN injection of GABA may rescue the GABA shortage in hypothalamus and lower blood pressure in spontaneous hypertensive rats. To sum up, GABA can not only lower blood pressure and improve BRS, but also regulate heart rate, suggesting its great therapeutic value for the prevention and treatment of cardiovascular disease caused by hypertension, especially in hypertensive patients with cardiac insufficiency. Therefore, GABA is expected to become an efficient cardiac protective agent, while its mechanism remains to be further studied.
Disclosure of conflict of interest
None.
References
- 1.Yalcin M, Cavun S, Yilmaz MS, Savci V. The involvement of central cholinergic system in the pressor effect of intracerebroventricularly injected U-46619, a thromboxane A2 analog, in conscious normotensive rats. Naunyn Schmiedebergs Arch Pharmacol. 2005;372:31–40. doi: 10.1007/s00210-005-1087-x. [DOI] [PubMed] [Google Scholar]
- 2.Root AR, Sanford JD, Kavanaugh SI, Sower SA. In vitro and in vivo effects of GABA, muscimol, and bicuculline on lamprey GnRH concentration in the brain of the sea lamprey (Petromyzon marinus) Comp Biochem Physiol A Mol Integr Physiol. 2004;138:493–501. doi: 10.1016/j.cbpb.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Shen FM, Wang MW, Su DF. Effects of nine antihypertensive drugs on blood pressure variability in sinoaortic-denervated rats. Acta Pharmacol Sin. 2006;27:1013–1017. doi: 10.1111/j.1745-7254.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 4.Brosnan RJ. GABA(A) receptor antagonism increases NMDA receptor inhibition by isoflurane at a minimum alveolar concentration. Vet Anaesth Analg. 2011;38:231–239. doi: 10.1111/j.1467-2995.2011.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu XW, Xie HH, Wang J, Shen FM, Su DF. Arterial baroreflex is not involved in salt preference in rats. Clin Exp Pharmacol Physiol. 2006;33:607–611. doi: 10.1111/j.1440-1681.2006.04414.x. [DOI] [PubMed] [Google Scholar]
- 6.Goncalves TC, Londe AK, Albano RI, de Araujo Junior AT, de Aguiar Azeredo M, Biagioni AF, Vasconcellos TH, Dos Reis Ferreira CM, Teixeira DG, de Souza Crippa JA, Vieira D, Coimbra NC. Cannabidiol and endogenous opioid peptide-mediated mechanisms modulate antinociception induced by transcutaneous electrostimulation of the peripheral nervous system. J Neurol Sci. 2014;347:82–89. doi: 10.1016/j.jns.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Vaz GC, Bahia AP, de Figueiredo Muller-Ribeiro FC, Xavier CH, Patel KP, Santos RA, Moreira FA, Frezard F, Fontes MA. Cardiovascular and behavioral effects produced by administration of liposome-entrapped GABA into the rat central nervous system. Neuroscience. 2014;285C:60–69. doi: 10.1016/j.neuroscience.2014.10.067. [DOI] [PubMed] [Google Scholar]
- 8.Tjen ALSC, Guo ZL, Longhurst JC. GABA in nucleus tractus solitarius participates in electroacupuncture modulation of cardiopulmonary bradycardia reflex. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1313–1323. doi: 10.1152/ajpregu.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawabe T, Ueyama T, Hano T, Sapru HN. Cardiovascular responses to microinjections of endomorphin-2 into the nucleus of the solitary tract are attenuated in the spontaneously hypertensive rat. Clin Exp Hypertens. 2015;37:197–206. doi: 10.3109/10641963.2014.933969. [DOI] [PubMed] [Google Scholar]
- 10.Tan S, Xu C, Zhu W, Willis J, Seubert CN, Gravenstein N, Sumners C, Martynyuk AE. Endocrine and neurobehavioral abnormalities induced by propofol administered to neonatal rats. Anesthesiology. 2014;121:1010–1017. doi: 10.1097/ALN.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter F, Lutz W, Eitner A, Leuchtweis J, Lehmenkuhler A, Schaible HG. Tumor necrosis factor reduces the amplitude of rat cortical spreading depression in vivo. Ann Neurol. 2014;76:43–53. doi: 10.1002/ana.24176. [DOI] [PubMed] [Google Scholar]
- 12.Wu JJ, Chang WP, Shih HC, Yen CT, Shyu BC. Cingulate seizure-like activity reveals neuronal avalanche regulated by network excitability and thalamic inputs. BMC Neurosci. 2014;15:3. doi: 10.1186/1471-2202-15-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viney TJ, Lasztoczi B, Katona L, Crump MG, Tukker JJ, Klausberger T, Somogyi P. Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat Neurosci. 2013;16:1802–1811. doi: 10.1038/nn.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer AP, Osorio I, Lunte CE. Microperfusion of 3-MPA into the brain augments GABA. Epilepsy Behav. 2013;29:478–484. doi: 10.1016/j.yebeh.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawabe T, Kawabe K, Sapru HN. Tonic gamma-aminobutyric acid-ergic activity in the hypothalamic arcuate nucleus is attenuated in the spontaneously hypertensive rat. Hypertension. 2013;62:281–287. doi: 10.1161/HYPERTENSIONAHA.113.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takechi K, Carstens MI, Klein AH, Carstens E. The antinociceptive and antihyperalgesic effects of topical propofol on dorsal horn neurons in the rat. Anesth Analg. 2013;116:932–938. doi: 10.1213/ANE.0b013e31827f560d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsukawa R, Hirooka Y, Ito K, Sunagawa K. Inhibition of neuregulin-1/ErbB signaling in the rostral ventrolateral medulla leads to hypertension through reduced nitric oxide synthesis. Am J Hypertens. 2013;26:51–57. doi: 10.1093/ajh/hps005. [DOI] [PubMed] [Google Scholar]
- 18.Patel KP, Salgado HC, Liu X, Zheng H. Exercise training normalizes the blunted central component of the baroreflex in rats with heart failure: role of the PVN. Am J Physiol Heart Circ Physiol. 2013;305:H173–181. doi: 10.1152/ajpheart.00009.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins SC, Zhao FY, Bowen CA, Fang X, Wei H, Heffernan ML, Spear KL, Spanswick DC, Varney MA, Large TH. Pharmacodynamic effects of a D-amino acid oxidase inhibitor indicate a spinal site of action in rat models of neuropathic pain. J Pharmacol Exp Ther. 2013;345:502–511. doi: 10.1124/jpet.113.204016. [DOI] [PubMed] [Google Scholar]
- 20.Garcia Mdel C, Godoy YC, Celuch SM. Impaired hypotensive responses induced by intrathecally injected drugs in fructose-fed rats. Eur J Pharmacol. 2013;706:17–24. doi: 10.1016/j.ejphar.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 21.Hatam M, Ganjkhani M. Effect of GABA(A) Receptors in the Rostral Ventrolateral Medulla on Cardiovascular Response to the Activation of the Bed Nucleus of the Stria Terminalis in Female Ovariectomized Rats. Iran J Med Sci. 2012;37:242–252. [PMC free article] [PubMed] [Google Scholar]


