Abstract
Early brain injury (EBI), following subarachnoid hemorrhage (SAH), includes blood-brain barrier (BBB) disruption and consequent edema formation. This study aims to evaluate the effect of lycopene on early brain injury and inflammation in SAH. Neurological deficits, brain water content and Evans blue dye extravasation were evaluated after the treatment with lycopene. Besides neuronal apoptosis,some inflammatory cytokines were also detected. The results suggested that administration of lycopene following SAH significantly ameliorated EBI, including brain edema, blood-brain barrier (BBB) impairment, cortical apoptosis, and neurological deficits. In addition, it also ameliorated inflammation triggered by SAH. In conclusion, post-SAH lycopene administration may attenuate EBI in SAH, possibly through ameliorating neuronal apoptosis, maintaining BBB integrity and attenuating inflammation.
Keywords: Lycopene, subarachnoid hemorrhage, early brain injury, inflammation
Introduction
Subarachnoid hemorrhage (SAH) can be very devastating and of poor prognosis. In acute situations, 30% of patients will die, and those who survive may have poor prognosis in the future [1,2]. Despite the recent progress in microsurgical techniques, the outcome of patients who suffer from SAH remains unsatisfactory. SAH is a complex neurosurgical disease, which is frequently associated with many complications such as acute/delayed cerebral vasospasm, brain edema, obstructive/communicating hydrocephalus, diffuse/focal cerebral ischemia, etc [3].
Lycopene, which is a carotenoid compound, has been found naturally in tomato and other fruits like papaya, pink guava and watermelon.It has long been known for its health-promoting benefits [4]. Among natural carotenoids, lycopene has the strongest ability to scavenge free radicals: being 10-fold, 47-fold and 100-fold more effective at quenching singlet oxygen than α-tocopherol, β-carotene and vitamin E respectively [5,6]. Additionally, more studies have suggested that lycopene have other properties such as anti-apoptosis [7], anti-inflammation [8], anti-ischemia [9], and anti-tumor [10]. Therefore, we would like to investigate whether lycopene may be effective in SAH. And here we reported that lycopene might be protective in SAH.
Materials and methods
Animals
Sixty adult male Sprague-Dawley rats (250-300 g) were purchased from theCenter of Experimental Animal in Zhejiang University, China. All experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee of Zhejiang University.
SAH model
The endovascular perforation model of SAH was performed as previously described [11]. Briefly, rats were anesthetized, intubated, and kept on artificial ventilation during surgery with 3% isoflurane in 70%/30% medical-air/oxygen. Body temperature was monitored by a rectal probe, and normothermia was maintained with a heating lamp. A sharpened 4-0 nylon suture was inserted into the left internal carotid artery and advanced until a resistance was felt (approximately 18 mm from the common carotid bifurcation). The suture was then further advanced to perforate the bifurcation of the anterior and middle cerebral arteries and withdrawn immediately after artery perforation. During sham operations, the suture was inserted into the left carotid artery; however, no perforation was performed. After removal of the suture, the skin incision was sutured, and rats were individually housed in heated cages until recovery.
Treatments
Animals were randomly assigned into three groups: sham group, SAH group and SAH + lycopene group. Animals received the following agents as intraperitoneal (i.p.) injections 2 h after surgery: lycopeneat a dose of 40 mg/kg (dissolved in tetrahydrofuran (THF)). Sham group and SAH group rats were injected solely with an equal concentration of THF.
Neurological score
At 24 h after surgery, immediately before euthanasia, neurological scores were evaluated in a blinded fashion, using a modification of the Garcia scoring system as previously described [11,12]. This composite sensorimotor assessment evaluates the rodent’s spontaneous activity (0-3 points), its reaction to side stroking (1-3 points) and vibrissae touch (1-3 points), as well as limb symmetry (0-3 points), forelimb outstretching (0-3 points), as well as its climbing (0-3 points) and beam walking ability (0-4 points). The latter evaluated the walking distances on a wooden beam for 1 min. The sum of all subtests was calculated to determine neurological function; best test performances were scored with 22, and worst performances were scored with 2 points.
Brain water content (cerebral edema)
Cerebral edema was determined by measuring the brain water content according to the wet-dry method [13]. These rats were killed and brains were immediately removed and placed on a frozen plate. Tissue samples were dissected out from infarct areas in ischemic rats and from corresponding areas in sham-operated and non-operated animals. Samples were immediately weighed to obtain wet weight. Then, samples were dried in a desiccating oven at 110°C for 24 h and weighed again to obtain the dry weight. Brain water content was calculated as follows: brain water content (%) = (wet weight-dry weight) × 100/wet weight.
Blood-brain barrier disruption
At 24 h after surgery, Evans blue dye (2%; 5 ml/kg) was injected into the right femoral vein, over a period of 2 min, allowing the dye to circulate for a total of 60 min [14]. Under deep isoflurane anesthesia, rats were subjected to transcardial perfusion with phosphate-buffered saline (PBS), and brains were removed. Brain specimens were weighed, homogenized in 1 ml of PBS, and centrifuged at 15,000 g for 30 min. Then 0.6 ml of the resultant supernatant was added to an equal volume of trichloroacetic acid. After overnight incubation at 4°C and centrifugation at 15,000 g at 4°C for 30 min, the supernatant was used for spectrophotometric quantification of extravasated Evans blue dye at 615 nm.
TUNEL staining
The brain tissue was fixed with the 10% neutral buffered formalin and embedded in paraffin. Briefly, paraffin-embedded sections were mounted on positively charged slides, deparaffinized, rehydrated, and washed thoroughly with distilled water. The tissues were digested with 20 g/ml proteinase K (Boehringer Mannheim, Mannheim, Germany) at room temperature for 15 min to retrieve antigen. Endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide/methanol in phosphate-buffered saline at 37°C for 30 min. The sections were then incubated with terminal deoxynucleotidyl transferase at 37°C for 60 min. Anti-digoxigenin antibody peroxidase was applied to the sections to detect the labeled nucleotides. The sections were stained with DAB and counter-stained slightly with hematoxylin. The positive cells were analyzed under a light microscope by another investigator in a blinded way.
RNA extraction and RT-PCR
The levels of TNF-α, IL-1β, and ICAM-1 mRNA expression were determined by RT-PCR. Total RNA was extracted with TriPure Reagent (Roche Diagnostics Corp. Indianapolis, IN, USA) according to the manufacture’s instruments. The cDNA synthesis from the isolated RNA was performed using a reverse transcriptional system. Briefly, 4 mg of total RNA was subjected to the first strand cDNA synthesis for 1 h at 42°C in a 20 ml reaction mixture containing 20 U RNase inhibitor, 0.04 μmol of each dNTP, 0.5 μg oligo (dT)15 and 15 U AMV reverse transcriptase (Promega). The reaction was terminated by incubation at 95°C for 5 min. PCR amplification was performed in a total volume of 25 μL containing 4 μl cDNA, 0.05 μmol MgCl2, 2.5 U Taq polymerase, 0.02 μmol of each dNTP, specific oligonucleotide primers (Table 1) for TNF-α, IL-1β, ICAM-1 or GAPDH genes, and 2.5 μl 10 × Taq polymerase reaction buffer. Each PCR cycle included a denaturation step at 94°C for 30 s, a primer annealing step for 30 s, an extension step at 72°C for 50 s, and a final extension step at 72°C for 7 min. Then, the amplified fragments were detected by agarose gel electrophoresis and visualized by ethidium bromide staining. The gel was captured as a digital image and analyzed using Scion Image software (Scion Corp, Maryland, USA). Values for each sample were normalized by the GAPDH as the control.
Table 1.
Specific oligonucleotide primers
| Target gene | Sense primer (5’ to 3’) | Antisense primer (5’ to 3’) | Annealing temperature (°C) | Number of cycles | Size (bp) |
|---|---|---|---|---|---|
| TNF-α | GCTGCACTTCAGGCTGATC | CTTGTTCGGGTAGGAGACG | 57 | 34 | 352 |
| IL-1β | ATCTCCTGCCAACCCTACA | CTTTCAGCTCATACGTGCC | 54 | 34 | 274 |
| ICAM-1 | GCGGCTCAGTGTCTCATTCC | CACGCAGTCCTCGGCTTCT | 58 | 34 | 184 |
| GAPDH | GGAGCCAAAAGGGTCATC | CCAGTGAGTTTCCCGTTC | 57 | 30 | 347 |
Statistical analysis
SAH grades and neurological scores were expressed as median and 25th to 75th percentiles, analyzed by Mann-Whitney U test or Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks followed by Dunn’s post hoc analysis. All other results were expressed as mean ± standard deviation and were analyzed by one-way ANOVA followed by Tukey post hoc analysis. P < 0.05 was considered statistically significant.
Results
Effect of lycopene on neurological score at 24 h after surgery
At 24 h after surgery, the neurological score of rats in SAH group was lower than that in sham group (P < 0.05). However, lycopene administration ameliorated neurological score compared with the SAH group (P < 0.05), which suggested that lycopene attenuated neurological injury of SAH (Figure 1).
Figure 1.
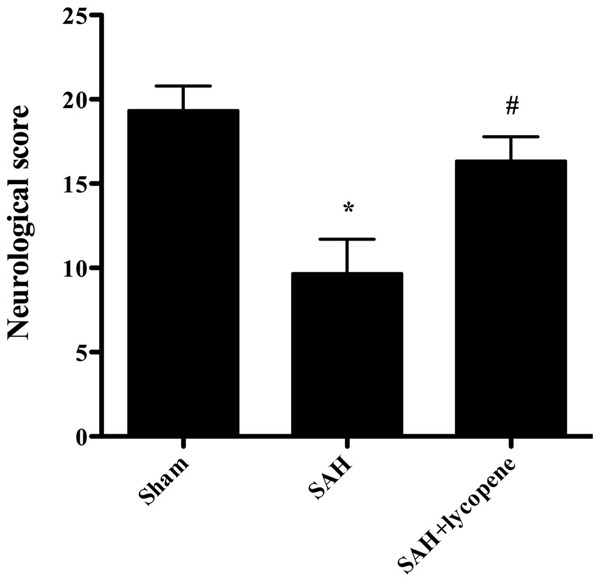
Effect of lycopene on neurological deficits in SAH. Lycopene significantly improved neurological deficits compared with the SAH group. Data were expressed as mean ± SD (n = 6 in each group). r*P < 0.05 versus thesham group, #P < 0.05 versus theSAH group.
Effect of lycopene on brain water content at 24 h after surgery
At 24 h after surgery, brain water content of rats in SAH group was higher than that in sham group (P < 0.05). However, lycopene administration attenuated brain water content compared with the SAH group (P < 0.05) (Figure 2).
Figure 2.
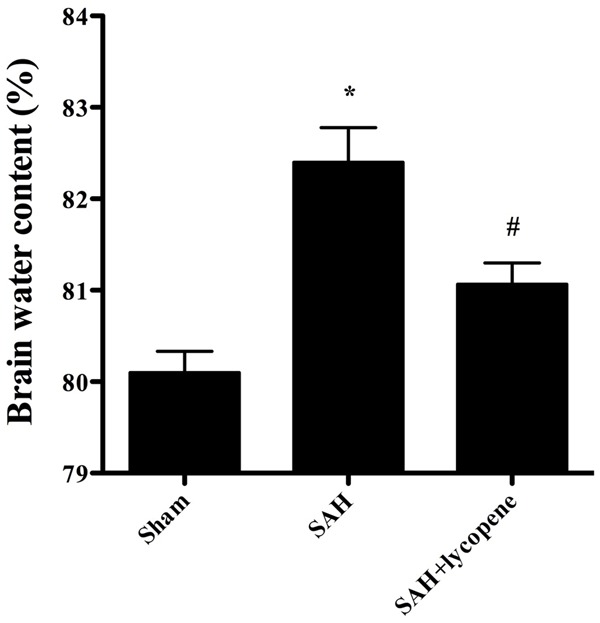
Effect of lycopene on brain water content at 24 h after SAH in rats. Data were expressed as mean ± SD (n = 6 in each group). *P < 0.05 versus thesham group, #P < 0.05 versus the SAH group.
Effect of lycopene on blood-brain barrier permeability at 24 h after surgery
At 24 h after surgery, rats in the SAH group showed a significant increase in BBB permeability to Evans blue (P < 0.05). However, lycopene administration attenuated Evans blue extravasation compared with the SAH group (P < 0.05) (Figure 3).
Figure 3.
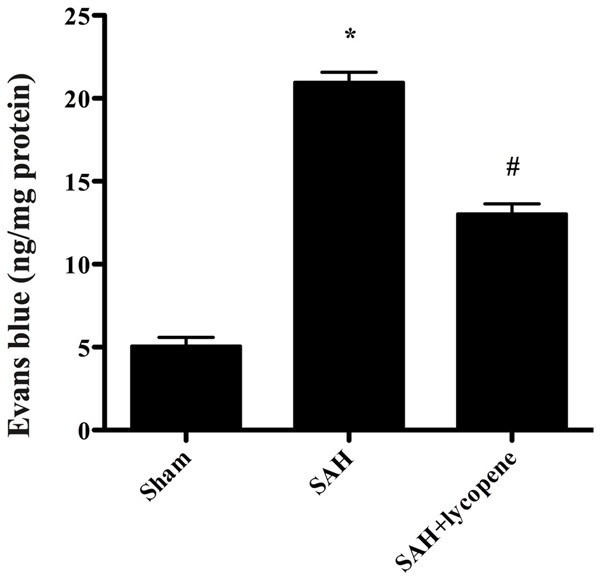
Alterations in Evans blue (EB) extravasation in each group. SAH induced a significant increase in blood-brain barrier extravasation in the rat brain compared with the sham group. After lycopene administration, the EB extravasation was dramatically reduced compared with the SAH group. Data were expressed as mean ± SD (n = 6 in each group). *P < 0.05 versus thesham group, #P < 0.05 versus the SAH group.
Effect of lycopene on neuronal apoptosis in the brain
Neuronal apoptosis was assessed by using TUNEL staining (Figure 4). The number of TUNEL-positive cells increased dramatically in SAH group compared with that in the sham group (P < 0.05). After lycopene was given, the number of TUNEL-positive cells decreased significantly compared with that in SAH group (P < 0.05). Besides, the expression of cleaved caspase-3 increased dramatically compared with that in the sham group (P < 0.05), which was down-regulated by lycopene (P < 0.05) (Figure 5). The results suggested that lycopene protect the brain from SAH via inhibiting neuronal apoptosis.
Figure 4.
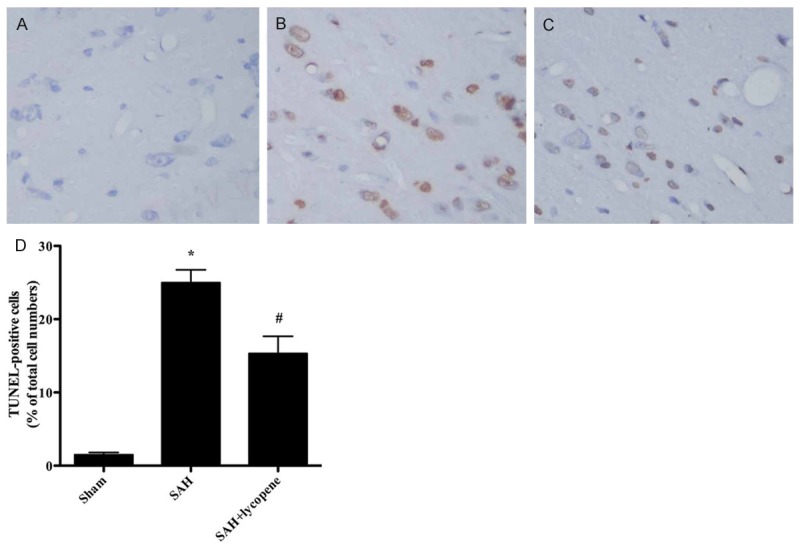
The neuronal apoptosis in the rat brain detected by TUNEL staining. A-C. It was suggested that the cells are light blue-stained in the sham group. In the SAH group, the brown-stained TUNEL-positive cells are observed in the cortex. After lycopene administration, the number of TUNEL-positive cells is less than that in the SAH group. D. Quantification of the TUNEL staining showed that the number of TUNEL-positive cells is significantly increased in the SAH group compared with that in the sham group. In the SAH + lycopene group, the number of TUNEL-positive cells isdramatically decreased compared with that in the SAH group. Data were expressed as mean ± SD (n = 6 in each group). *P < 0.05 versus thesham group, #P < 0.05 versus the SAH group.
Figure 5.
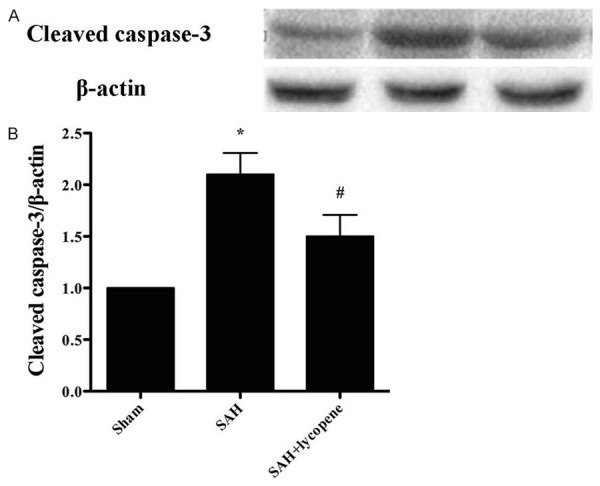
Effect of lycopene on the expression of cleaved caspase-3 detected by Western blot. SAH markedly increases the expression of cleaved caspase-3, while lycopene significantly lowers the level of cleaved caspase-3. Data were expressed as mean ± SD (n = 6 in each group). *P < 0.05 versus thesham group, #P < 0.05 versus the SAH group.
Effect of lycopene on inflammatory cytokines in the brain
The amount of inflammatory cytokines was detected by RT-PCR. The levels of inflammatory cytokines, including TNF-α, IL-1β, and ICAM-1, were all markedly increased in the SAH group (P < 0.05). Lycopene administration dramatically attenuated the levels of inflammatory cytokines (P < 0.05) (Figure 6). The results suggested the anti-inflammatory effect of lycopene in SAH.
Figure 6.
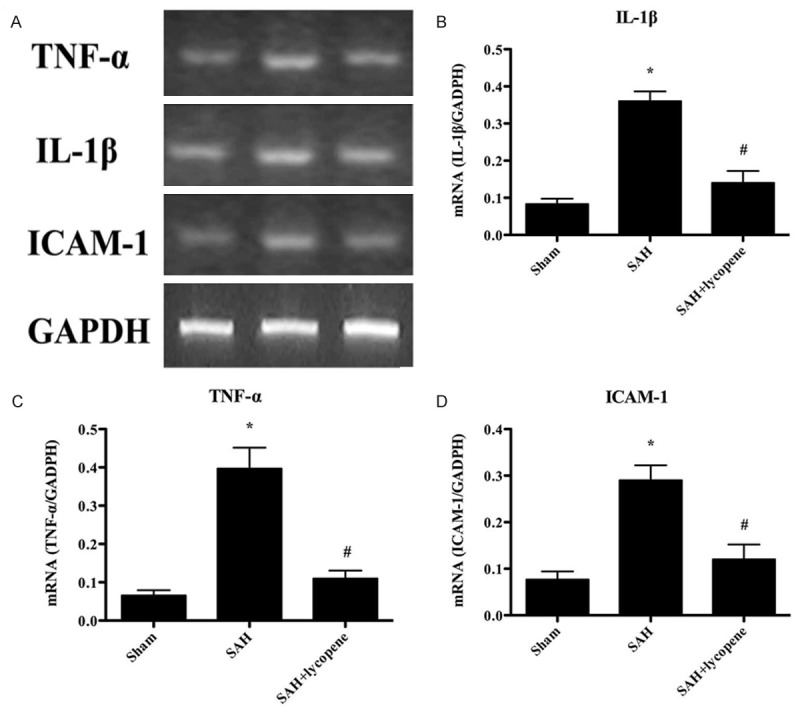
The gene expressions of TNF-α, IL-1β, and ICAM-1 in the brain detected by RT-PCR. A. The representative autoradiograms of TNF-α, IL-1β, and ICAM-1. B-D. Relative amount of TNF-α, IL-1β, and ICAM-1 mRNA. The levels of TNF-α, IL-1β, and ICAM-1 increased dramatically in the SAH group. After lycopene administration, the levels of TNF-α, IL-1β, and ICAM-1 were decreased. Data were expressed as mean ± SD (n = 6 in each group). *P < 0.05 versus thesham group, #P < 0.05 versus the SAH group.
Discussion
The main findings of the present study are: 1) lycopene protect brain from SAH through attenuating early brain injury (EBI); 2) lycopene protect brain from SAH through inhibiting the expression of inflammatory cytokines.
EBI includes brain edema, neurological deficit, BBB disruption, and neuronal apoptosis. EBI is the major cause of mortality and morbidity in patientssuffering from aneurysmal SAH[15].Besides, study has suggested that EBI rather than vasospasm is the main cause of poor prognosis in patients with SAH [16]. So, EBI is an important target in the treatment of SAH. However, the treatment of EBI in the clinic is not so well. In the study, we have found that lycopene administration ameliorates neurological deficits, attenuated brain edema and BBB disruption, inhibited neuronal apoptosis, which indicates that lycopene exerts its protective effect through attenuating EBI.
Inflammation is also another important factor in SAH. The inflammatory cytokines involved in SAH includes TNF-α, IL-1β, and ICAM-1, NF-κB, etc. You et al have shown that activated NF-κB in neurons after SAH plays an important role in regulating the expressions of inflammatory genes in the brain, and ultimately contributes to delayed brain injury [17]. In addition, studies have shown that NF-κB pathway is also involved in SAH [18,19], which can regulate the expression of matrix metalloproteinase-9 (MMP-9). ICAM-1 can also regulate the expression of MMP-9 [20]. MMP-9 is one of the members in the MMP family. MMP-9 degrades the extracellular matrix of cerebral microvascular basal lamina, triggering BBB disruption after SAH [21]. And it is closely associated with the poor prognosis in the SAH patients. In this study, our results suggested that lycopene down-regulates the expression of TNF-α, IL-1β, and ICAM-1, which indicates its neuroprotective effect in SAH.
In summary, we demonstrate that lycopene alleviates EBI and inflammation in SAH. Lycopene ameliorates neurological deficits, brain water content, BBB disruption and neuronal apoptosis. Moreover, lycopene inhibits inflammatory reaction by attenuating the expression of TNF-α, IL-1β, and ICAM-1. Certainly, there may be some other molecules associated with the neuroprotective effect of lycopene, which we need to investigate. And lycopene may be of great value in the treatment of SAH in the future.
References
- 1.Hanel RA, Xavier AR, Mohammad Y, Kirmani JF, Yahia AM, Qureshi AI. Outcome following intracerebral hemorrhage and subarachnoid hemorrhage. Neurol Res. 2002;24(Suppl 1):S58–62. doi: 10.1179/016164102101200041. [DOI] [PubMed] [Google Scholar]
- 2.Quigley MR, Salary M. Defining survivorship after high-grade aneurysmal subarachnoid hemorrhage. Surg Neurol. 2008;69:261–265. doi: 10.1016/j.surneu.2007.02.013. discussion 265. [DOI] [PubMed] [Google Scholar]
- 3.de Rooij NK, Rinkel GJ, Dankbaar JW, Frijns CJ. Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke. 2013;44:43–54. doi: 10.1161/STROKEAHA.112.674291. [DOI] [PubMed] [Google Scholar]
- 4.Klipstein-Grobusch K, Launer LJ, Geleijnse JM, Boeing H, Hofman A, Witteman JC. Serum carotenoids and atherosclerosis. The Rotterdam Study. Atherosclerosis. 2000;148:49–56. doi: 10.1016/s0021-9150(99)00221-x. [DOI] [PubMed] [Google Scholar]
- 5.Conn PF, Schalch W, Truscott TG. The singlet oxygen and carotenoid interaction. J Photochem Photobiol B. 1991;11:41–47. doi: 10.1016/1011-1344(91)80266-k. [DOI] [PubMed] [Google Scholar]
- 6.Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996;384:240–242. doi: 10.1016/0014-5793(96)00323-7. [DOI] [PubMed] [Google Scholar]
- 7.Fujita K, Yoshimoto N, Kato T, Imada H, Matsumoto G, Inakuma T, Nagata Y, Miyachi E. Lycopene inhibits ischemia/reperfusion-induced neuronal apoptosis in gerbil hippocampal tissue. Neurochem Res. 2013;38:461–469. doi: 10.1007/s11064-012-0952-5. [DOI] [PubMed] [Google Scholar]
- 8.Ip BC, Hu KQ, Liu C, Smith DE, Obin MS, Ausman LM, Wang XD. Lycopene metabolite, apo-10’-lycopenoic acid, inhibits diethylnitrosamine-initiated, high fat diet-promoted hepatic inflammation and tumorigenesis in mice. Cancer Prev Res (Phila) 2013;6:1304–1316. doi: 10.1158/1940-6207.CAPR-13-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiao G, Fong TH, Tzu NH, Lin KH, Chou DS, Sheu JR. A potent antioxidant, lycopene, affords neuroprotection against microglia activation and focal cerebral ischemia in rats. In Vivo. 2004;18:351–356. [PubMed] [Google Scholar]
- 10.Gunasekera RS, Sewgobind K, Desai S, Dunn L, Black HS, McKeehan WL, Patil B. Lycopene and lutein inhibit proliferation in rat prostate carcinoma cells. Nutr Cancer. 2007;58:171–177. doi: 10.1080/01635580701328339. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa Y, Suzuki H, Altay O, Zhang JH. Preservation of tropomyosin-related kinase B (TrkB) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke. 2011;42:477–483. doi: 10.1161/STROKEAHA.110.597344. [DOI] [PubMed] [Google Scholar]
- 12.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- 13.Hatashita S, Hoff JT, Salamat SM. Ischemic brain edema and the osmotic gradient between blood and brain. J Cereb Blood Flow Metab. 1988;8:552–559. doi: 10.1038/jcbfm.1988.96. [DOI] [PubMed] [Google Scholar]
- 14.Fujii M, Duris K, Altay O, Soejima Y, Sherchan P, Zhang JH. Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochem Int. 2012;60:327–333. doi: 10.1016/j.neuint.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 16.Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97:14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You WC, Wang CX, Pan YX, Zhang X, Zhou XM, Zhang XS, Shi JX, Zhou ML. Activation of nuclear factor-kappaB in the brain after experimental subarachnoid hemorrhage and its potential role in delayed brain injury. PLoS One. 2013;8:e60290. doi: 10.1371/journal.pone.0060290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang T, Su J, Guo B, Zhu T, Wang K, Li X. Ursolic acid alleviates early brain injury after experimental subarachnoid hemorrhage by suppressing TLR4-mediated inflammatory pathway. Int Immunopharmacol. 2014;23:585–591. doi: 10.1016/j.intimp.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Wang CX, Xie GB, Zhou CH, Zhang XS, Li T, Xu JG, Li N, Ding K, Hang CH, Shi JX, Zhou ML. Baincalein alleviates early brain injury after experimental subarachnoid hemorrhage in rats: Possible involvement of TLR4/NF-kappaB-mediated inflammatory pathway. Brain Res. 2015;1594:245–255. doi: 10.1016/j.brainres.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Xie X, Wu X, Cui J, Li H, Yan X. Increase ICAM-1 and LFA-1 expression by cerebrospinal fluid of subarachnoid hemorrhage patients: involvement of TNF-alpha. Brain Res. 2013;1512:89–96. doi: 10.1016/j.brainres.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 21.Sehba FA, Mostafa G, Knopman J, Friedrich V Jr, Bederson JB. Acute alterations in microvascular basal lamina after subarachnoid hemorrhage. J Neurosurg. 2004;101:633–640. doi: 10.3171/jns.2004.101.4.0633. [DOI] [PubMed] [Google Scholar]


