Abstract
Objectives: Epidemiological studies evaluating the association of tea consumption and the risk of thyroid cancer risk have produced inconsistent results. Thus, we conducted a meta-analysis to assess the relationship between tea consumption and thyroid cancer risk. Methods: Pertinent studies were identified by a search in PubMed and Web of Knowledge. The random effect model was used based to combine the results. Publication bias was estimated using Egger’s regression asymmetry test. Results: Finally, 11 articles with 14 studies (2 cohort studies and 12 case-control studies) involving 2,955 thyroid cancer cases and 106,447 participants were included in this meta-analysis. The relative risk (95% confidence interval) of thyroid cancer for the highest versus the lowest category of tea consumption was 0.774 (95% CI = 0.619-0.967), and the associations were also significant in Europe and America, but not in the Asia. No publication bias was found. Conclusions: Our analysis indicated that higher tea consumption may have a protective effect on thyroid cancer, especially in Europe and America.
Keywords: Tea consumption, thyroid cancer, meta-analysis
Introduction
The worldwide age-standardized incidence of thyroid cancer is 1.5 per 100,000 person-years for men and 4.7 for women, accounting for approximately 1% of all new cancer cases [1]. Although the incidence is relatively low, thyroid cancer is a major form of malignant neoplasm in women under 45 years of age [2]. Consumption of such dietary items as fish, vegetables, and beverages (such as tea) is suspected to be associated with thyroid cancer risk, but no conclusive evidence is available [3-5].
Tea, which is derived from the leaves of the plant Camellia sinensis, is one of the most popular beverages consumed worldwide, and generally the most consumed are black tea and green tea. Evidence from laboratory studies strongly showed the inhibition of tumorigenesis by tea and its constituents [6,7]. Up to date, a number of epidemiologic studies have been published to explore the relationship between tea consumption and thyroid cancer risk. However, the results are not consistent. Therefore, we conducted a meta-analysis to (1) first assess the thyroid cancer risk for the highest vs. lowest categories of tea consumption; (2) assess the heterogeneity among studies and publication bias.
Methods
Search strategy
We performed a literature search up to April 2015 using the databases of PubMed and Web of Knowledge. The following search terms were used: ‘tea’, or ‘diet’, or ‘lifestyle’ for exposure factors, and ‘thyroid cancer’, or ‘thyroid oncology’ for outcome factors. Moreover, we reviewed the reference lists from retrieved articles to search for further relevant studies. Two investigators searched articles and reviewed of all retrieved studies independently. Disagreements between the two investigators were resolved by consensus with a third reviewer.
Inclusion criteria
All relevant studies reporting the association of tea and thyroid cancer risk were considered for inclusion. The inclusion criteria were as follows: (1) case-control or cohort study; (2) the exposure of interest was variety of tea consumption; (3) the outcome of interest was thyroid cancer; (4) results including relative risk (RR) or odds ratio (OR) and its 95% confidence intervals (CI), or providing us with sufficient information to calculate them. Accordingly, the following exclusion criteria were also used: (1) reviews and (2) repeated or overlapped publications.
Data extraction
The following data were collected from all studies independently by two investigators: the design type (case-control study or cohort study), the first author’s last name, publication year, location where the study was performed, sample size and number of cases, RR estimates (we presented all results with RR for simplicity) with corresponding 95% CI for the highest versus lowest categories of tea and thyroid cancer, respectively. For studies that reported results from various covariate analyses, we abstracted the estimates based on the model that included the most potential confounders. If there was disagreement between the two investigators about eligibility of the data, it was resolved by consensus with a third reviewer.
Quality assessment
The quality of studies was examined and controlled in accordance with checklists of Preferred Reporting Items for Systematic reviews and Meta-Analyses for randomized trials (PRISMA) [8]. To determine the quality score of included studies, two reviewers independently performed the quality assessment by using the Newcastle-Ottawa Scale, which is a validated scale for non-randomized studies in meta-analyses [9]. The Newcastle-Ottawa Scale is a nine-point scale that allocates points based on the selection process of cohorts (0-4 points), the comparability of cohorts (0-2 points), and the identification of the exposure and the outcomes of study participants (0-3 points). We assigned scores of 0 -3, 4 -6, and 7 -9 for low, moderate, and high quality of studies, respectively.
Statistical analysis
The pooled measure was calculated as the inverse variance-weighted mean of the logarithm of RR with 95% CI, to assess the association between tea consumption and thyroid cancer risk. Random-effects model was used to combine study-specific RR (95% CI), which considers both within-study and between-study variation [10]. The I2 was used to assess heterogeneity, and I2 values of 0, 25, 50 and 75% represent no, low, moderate and high heterogeneity [11], respectively. Meta-regression with restricted maximum likelihood estimation was performed to assess the potentially important covariates that might exert substantial impact on between-study heterogeneity [12]. Publication bias was evaluated using Egger regression asymmetry test [13]. A study of influence analysis [14] was conducted to describe how robust the pooled estimator was to removal of individual studies. An individual study was suspected of excessive influence if the point estimate of its omitted analysis lay outside the 95% CI of the combined analysis. All statistical analyses were conducted with STATA version 12.0 (StataCorp LP, College Station, Texas, USA). Two-tailed p-value ≤ 0.05 was accepted as statistically significant.
Results
Search results and study characteristics
The search strategy identified 246 articles from PubMed and 364 from the Web of Knowledge, and 52 articles were reviewed in full after reviewing the title/abstract. One article [15] reported the green tea consumption and thyroid cancer risk for females and males respectively, and one article [16] reported three varieties of tea consumption for thyroid cancer. So, 11 articles [15-25] with 14 studies (2 cohort studies and 12 case-control studies) involving 2,955 thyroid cancer cases and 106,447 participants were used in this meta-analysis after reviewed in full articles. The detailed steps of our literature search are shown in Figure 1. Four studies come from Greece, 3 from United States, 2 from Norway, 2 from Japan, 1 from Italy, 1 from Sweden and 1 from China. The characteristics of these studies are presented in Table 1.
Figure 1.
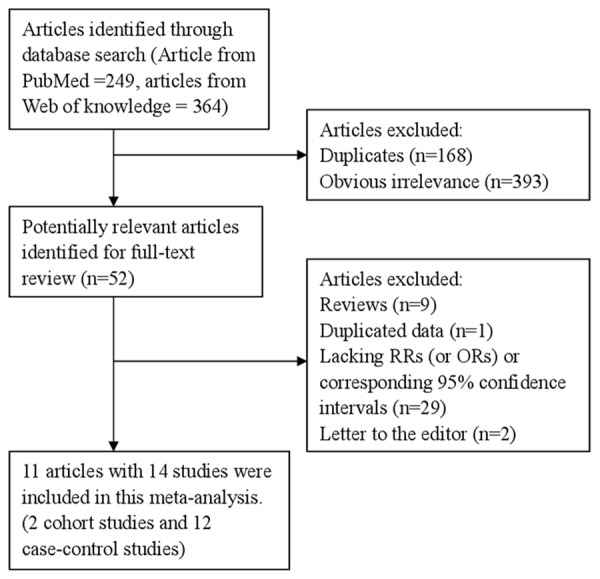
The flow diagram of screened, excluded, and analyzed publications.
Table 1.
Characteristics of studies between tea consumption and thyroid cancer risk
| Author (year) | Country | Study design | Quality score | Participants (case) | Age range | RR (95% CI) for highest vs. lowest category |
|---|---|---|---|---|---|---|
| D’Avanzo et al. (1995) | Italy | HCC | 6 | 1016 (399) | ≤ 75 | 1.13 (0.82-1.56) |
| Galanti et al. (1997) | Norway | PCC | 7 | 312 (116) | 18-75 | 1.16 (0.51-2.65) |
| Glattre et al. (1993) | Norway | HCC | 7 | 552 (92) | 20-59 | 1.33 (0.61-2.90) |
| Hallquist et al. (1993) | Sweden | PCC | 6 | 540 (180) | 20-70 | 0.93 (0.59-1.48) |
| Kolonel et al. (1990) | United States | PCC | 7 | 632 (191) | ≥18 | 0.75 (0.52-1.08) |
| Linos et al. (1989) | Greece | HCC | 7 | 141 (70) | Na | 0.76 (0.43-1.33) |
| Mack et al. (2002) | United States | PCC | 6 | 584 (292) | 15-54 | 0.3 (0.1-1.0) |
| Michikawa et al. (2011) | Japan | Cohort | 8 | 100507 (159) | 40-69 | Females: 0.91 (0.56-1.48) |
| Males: 0.71 (0.22-2.28) | ||||||
| Preston-Martin et al. (1987) | United States | PCC | 7 | 220 (110) | 15-40 | 0.51 (0.25-1.03) |
| Preston-Martin et al. (1993) | China | PCC | 7 | 414 (207) | 18-54 | 1.00 (0.25-4.03) |
| Riza et al. (2015) | Greece | HCC | 6 | 1529 (1139) | 42.8 | Chamomile tea |
| 0.25 (0.10-0.62) | ||||||
| Sage tea | ||||||
| 0.34 (0.09-1.21) | ||||||
| Mountain tea | ||||||
| 0.55 (0.21-1.41) |
Abbreviations: Na: not available; PCC: population-based case-control study; HCC: hospital-based case-control study; BMI: body mass index.
High versus low analyses
Data from 14 studies including 2,955 thyroid cancer cases were used in this meta-analysis. Only 2 studies reported that tea consumption could reduce the risk of thyroid cancer, while no significant association was reported in 12 studies. Pooled results suggested that highest tea consumption versus lowest amount was significantly associated with reduced the risk of thyroid cancer [summary RR=0.774, 95% CI = 0.619-0.967, I2 = 36.5%] (Figure 2).
Figure 2.
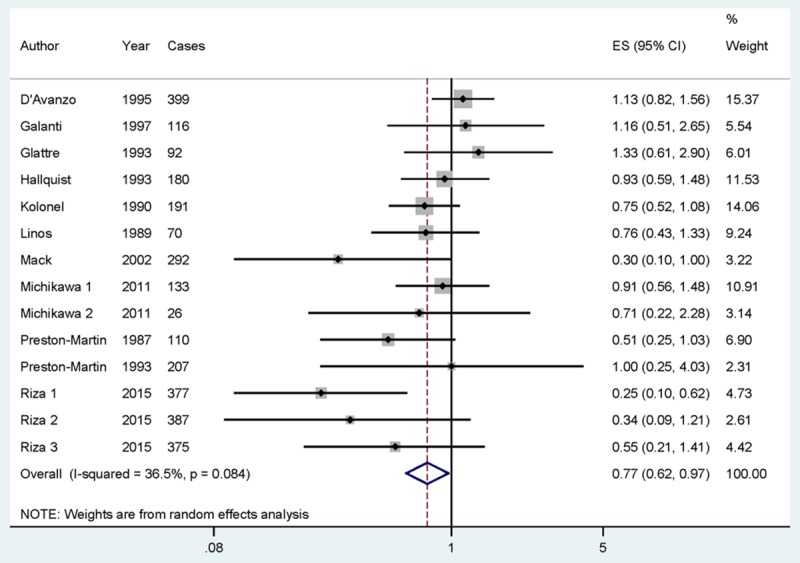
The forest plot between highest versus lowest categories of tea consumption and thyroid cancer risk.
In stratified analysis by study design, the association was found in the case-control studies [summary RR = 0.747, 95% CI = 0.574-0.972], but not in the cohort studies. In subgroup analyses for geographic locations, highest tea consumption level versus lowest level was significantly associated with reduced the risk of thyroid cancer in Europe [summary RR = 0.765, 95% CI = 0.575-0.981] and America [summary RR = 0.602, 95% CI = 0.390-0.928], but not in the Asia. When we conducted the subgroup analysis by sources of control, the association was significant in the population-based controls [summary RR = 0.761, 95% CI = 0.580-0.998], but not in the hospital-based controls. The detailed results are summarized in Table 2.
Table 2.
Summary risk estimates of the association between tea consumption and thyroid cancer risk
| Subgroups | No. (cases) | No. studies | Risk estimate (95% CI) | Heterogeneity test I2 (%) P-value | |
|---|---|---|---|---|---|
| All studies | 2955 | 14 | 0.774 (0.619-0.967) | 36.5 | 0.084 |
| Study design | |||||
| Prospective | 159 | 2 | 0.877 (0.560-1.374) | 0.0 | 0.701 |
| Case-control | 2796 | 12 | 0.747 (0.574-0.972) | 45.7 | 0.042 |
| Geographic locations | |||||
| Europe | 1996 | 8 | 0.805 (0.575-1.127) | 51.3 | 0.045 |
| America | 593 | 3 | 0.602 (0.390-0.928) | 27.9 | 0.250 |
| Asia | 366 | 3 | 0.888 (0.580-1.361) | 0.0 | 0.915 |
| Sources of control | |||||
| Population-based | 1096 | 6 | 0.761 (0.580-0.998) | 11.1 | 0.344 |
| Hospital-based | 1700 | 6 | 0.699 (0.431-1.133) | 64.2 | 0.016 |
Sources of heterogeneity and meta-regression
As seen in the pooled results, low to moderate heterogeneity (I2 = 36.5%, P heterogeneity = 0.084) was found in the analysis. In order to explore the low to moderate between-study heterogeneity founded in the pooled results, univariate meta-regression with the covariates of publication year, location where the study was conducted, study design (case-control or cohort), sources of control (population-based or hospital-based) and number of cases were performed. No significant findings were found in the above-mentioned analysis.
Influence analysis and publication bias
Influence analysis showed that no individual study had excessive influence on the association of tea consumption and thyroid cancer risk (Figure 3). Egger’s test (P = 0.148) showed no evidence of significant publication bias between tea consumption and thyroid cancer risk.
Figure 3.
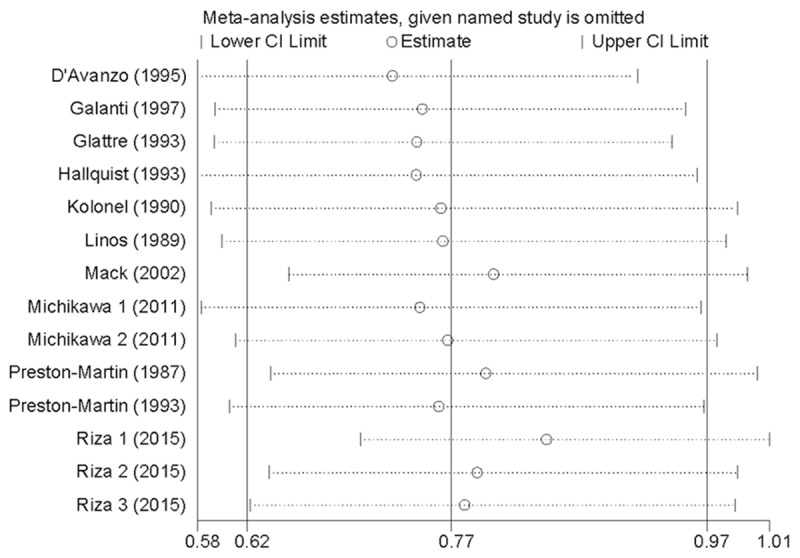
Analysis of influence of individual study on the pooled estimate in tea consumption and thyroid cancer risk. Open circle, the pooled RR, given named study is omitted. Horizontal lines represent the 95% CIs.
Discussion
Finding from this meta-analysis suggested that the higher tea consumption could reduce the risk of thyroid cancer. The associations were also found in Europe and America for tea consumption and thyroid cancer risk.
Previous research in vitro and in vivo has suggested that tea polyphenols provide protective effects against several types of cancer [26-28]. Meta-analysis has suggested that a favorable effect of green tea consumption and risk of lung cancer [29]. Significant associations were also found in esophageal cancer among female with green tea consumption [30,31]. A meta-analysis had reported that tea consumption might be the protective factor for prostate cancer [32]. Furthermore, tea consumption could reduce the oral cancer risk [33]. In our study, the results are consistent with these meta-analyses.
As a meta-analysis of published studies, our findings showed some advantages. First, large number of cases and participants were included, allowing a much greater possibility of reaching reasonable conclusions between tea consumption and thyroid cancer risk. Second, no significant publication bias was found, indicating that our results are stable. However, there were some limitations in this meta-analysis. First, as a meta-analysis of observational studies, it was prone to biases (eg, recall and selection bias) inherent in the original studies. Cohort studies are less susceptible to bias than case-control studies because, in the prospective design, information on exposures is collected before the diagnosis of the cancer. Although the results of the meta-regression showed no evidence of significant heterogeneity between subgroups, summary association estimates was different in subgroup analyses by study design. It is possible that a small number of cohort studies were included and the relations reported by case-control studies may have been overstated as a result of recall or interviewer bias. Thus, more large studies, especially prospective studies, are warranted in the future. Second, for the subgroups of geographic locations, the associations were significant only in the Europe and America, but not in the Asia. And only 3 studies with 366 cases were conducted in Asia. Due to this limitation, the results are applicable to Europe and America, but cannot be extended to populations elsewhere. More studies originating in other countries are required to investigate the association between tea consumption and thyroid cancer risk. Third, low to moderate between-study heterogeneity was found in the pooled analysis, but the between-study heterogeneity was not successfully explained by the subgroup analysis and meta-regression. However, other genetic and environment variables, as well as their possible interaction may be potential contributors to this disease-effect unconformity.
In summary, results from this meta-analysis suggested that the higher tea consumption might reduce the risk of thyroid cancer, especially in Europe and America.
Disclosure of conflict of interest
None.
References
- 1.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–11. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Groves FD, McLaughlin CC, Jemal A, Martin J, Chen VW. Cancer incidence patterns among adolescents and young adults in the United States. Cancer Causes Control. 2005;16:309–20. doi: 10.1007/s10552-004-4026-0. [DOI] [PubMed] [Google Scholar]
- 3.Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20:75–86. doi: 10.1007/s10552-008-9219-5. [DOI] [PubMed] [Google Scholar]
- 4.Preston-Martin S, Franceschi S, Ron E, Negri E. Thyroid cancer pooled analysis from 14 case-control studies: what have we learned? Cancer Causes Control. 2003;14:787–9. doi: 10.1023/a:1026312203045. [DOI] [PubMed] [Google Scholar]
- 5.Mack WJ, Preston-Martin S, Dal Maso L, Galanti R, Xiang M, Franceschi S, Hallquist A, Jin F, Kolonel L, La Vecchia C, Levi F, Linos A, Lund E, McTiernan A, Mabuchi K, Negri E, Wingren G, Ron E. A pooled analysis of case-control studies of thyroid cancer: cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control. 2003;14:773–85. doi: 10.1023/a:1026349702909. [DOI] [PubMed] [Google Scholar]
- 6.Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol Res. 2011;64:113–22. doi: 10.1016/j.phrs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rong Y, Chen L, Zhu T, Song Y, Yu M, Shan Z, Sands A, Hu FB, Liu L. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ. 2013;346:e8539. doi: 10.1136/bmj.e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–82. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobias A. Assessing the in fluence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15–7. [Google Scholar]
- 15.Michikawa T, Inoue M, Shimazu T, Sasazuki S, Iwasaki M, Sawada N, Yamaji T, Tsugane S. Green tea and coffee consumption and its association with thyroid cancer risk: a population-based cohort study in Japan. Cancer Causes Control. 2011;22:985–93. doi: 10.1007/s10552-011-9771-2. [DOI] [PubMed] [Google Scholar]
- 16.Riza E, Linos A, Petralias A, de Martinis L, Duntas L, Linos D. The effect of Greek herbal tea consumption on thyroid cancer: a case-control study. Eur J Public Health. 2015 doi: 10.1093/eurpub/ckv063. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.D’Avanzo B, La Vecchia C, Franceschi S, Negri E, Talamini R. History of thyroid diseases and subsequent thyroid cancer risk. Cancer Epidemiol Biomarkers Prev. 1995;4:193–9. [PubMed] [Google Scholar]
- 18.Galanti MR, Hansson L, Bergstrom R, Wolk A, Hjartaker A, Lund E, Grimelius L, Ekbom A. Diet and the risk of papillary and follicular thyroid carcinoma: a population-based case-control study in Sweden and Norway. Cancer Causes Control. 1997;8:205–14. doi: 10.1023/a:1018424430711. [DOI] [PubMed] [Google Scholar]
- 19.Glattre E, Haldorsen T, Berg JP, Stensvold I, Solvoll K. Norwegian case-control study testing the hypothesis that seafood increases the risk of thyroid cancer. Cancer Causes Control. 1993;4:11–6. doi: 10.1007/BF00051708. [DOI] [PubMed] [Google Scholar]
- 20.Hallquist A, Hardell L, Degerman A, Boquist L. Occupational exposures and thyroid cancer: results of a case-control study. Eur J Cancer Prev. 1993;2:345–9. doi: 10.1097/00008469-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Kolonel LN, Hankin JH, Wilkens LR, Fukunaga FH, Hinds MW. An epidemiologic study of thyroid cancer in Hawaii. Cancer Causes Control. 1990;1:223–34. doi: 10.1007/BF00117474. [DOI] [PubMed] [Google Scholar]
- 22.Linos A, Linos DA, Vgotza N, Souvatzoglou A, Koutras DA. Does coffee consumption protect against thyroid disease? Acta Chir Scand. 1989;155:317–20. [PubMed] [Google Scholar]
- 23.Mack WJ, Preston-Martin S, Bernstein L, Qian D. Lifestyle and other risk factors for thyroid cancer in Los Angeles County females. Ann Epidemiol. 2002;12:395–401. doi: 10.1016/s1047-2797(01)00281-2. [DOI] [PubMed] [Google Scholar]
- 24.Preston-Martin S, Bernstein L, Pike MC, Maldonado AA, Henderson BE. Thyroid cancer among young women related to prior thyroid disease and pregnancy history. Br J Cancer. 1987;55:191–5. doi: 10.1038/bjc.1987.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preston-Martin S, Jin F, Duda MJ, Mack WJ. A case-control study of thyroid cancer in women under age 55 in Shanghai (People’s Republic of China) Cancer Causes Control. 1993;4:431–40. doi: 10.1007/BF00050862. [DOI] [PubMed] [Google Scholar]
- 26.Qin J, Xie LP, Zheng XY, Wang YB, Bai Y, Shen HF, Li LC, Dahiya R. A component of green tea, (-)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem Biophys Res Commun. 2007;354:852–7. doi: 10.1016/j.bbrc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Adhami VM, Ahmad N, Mukhtar H. Molecular targets for green tea in prostate cancer prevention. J Nutr. 2003;133:2417S–24S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- 28.Sartippour MR, Heber D, Ma J, Lu Q, Go VL, Nguyen M. Green tea and its catechins inhibit breast cancer xenografts. Nutr Cancer. 2001;40:149–56. doi: 10.1207/S15327914NC402_11. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Yu X, Wu Y, Zhang D. Coffee and tea consumption and risk of lung cancer: a dose-response analysis of observational studies. Lung Cancer. 2012;78:169–70. doi: 10.1016/j.lungcan.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Zheng P, Zheng HM, Deng XM, Zhang YD. Green tea consumption and risk of esophageal cancer: a meta-analysis of epidemiologic studies. BMC Gastroenterol. 2012;12:165. doi: 10.1186/1471-230X-12-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sang LX, Chang B, Li XH, Jiang M. Green tea consumption and risk of esophageal cancer: a meta-analysis of published epidemiological studies. Nutr Cancer. 2013;65:802–12. doi: 10.1080/01635581.2013.805423. [DOI] [PubMed] [Google Scholar]
- 32.Fei X, Shen Y, Li X, Guo H. The association of tea consumption and the risk and progression of prostate cancer: a meta-analysis. Int J Clin Exp Med. 2014;7:3881–91. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Yang Y, Zhang W, Wu W. Association of tea consumption and the risk of oral cancer: a meta-analysis. Oral Oncol. 2014;50:276–81. doi: 10.1016/j.oraloncology.2013.12.014. [DOI] [PubMed] [Google Scholar]


