Abstract
In this study, the expression of silencing RhoA gene in gastric MGC-803 Cells was investigated, in order to discuss the effect of RhoA gene on cell proliferation, cell cycles and tumor migration. SiRNA sequence of RhoA gene was designed and synthesized; MGC-803 cells were transfected by LipofectamineTM2000. The expression of RhoA gene in mRNA and protein after interference was detected by RT-PCR and Western blot; flow cytometry was used to detect the cell cycle; cell proliferation was detected by CCK-8 assay and cell migration was detected by scratch healing assay. RhoA expression in mRNA and protein of the experimental group was significantly lower than that of the control group and blank group, and the difference was statistically significant (P < 0.05). The growth rate significantly slowed down in experimental group; the cell cycle was arrested in the G0/G1 phase and the number of cells in S-phase reduced; there was a statistically significant difference (P < 0.05). Scratch healing assay showed that cell migration of the experimental group was significantly decreased, with a statistically significant difference (P < 0.05). Specific interference on RhoA gene expression could inhibit the proliferation and migration of MGC-803 cells; therefore, siRNA sequences of RhoA gene may be an effective target for the treatment of gastric cancer.
Keywords: Gastric cancer, RhoA, siRNA, cell proliferation
Introduction
Gastric cancer is one of the most common malignancies in clinical. Chemotherapy and neoadjuvant chemotherapy has been widely used in the treatment of gastric cancer. But the prognosis of gastric cancer is still not ideal, especially in patients with clinical metastasis and tumor recurrence. In recent years, Rho proteins, particularly RhoA, were discovered to play an important role in cancer incidence and development; the abnormal expression of RhoA may be associated with the proliferation and metastasis of malignant tumors [1-3]. In this study, RNA interference was used to silence RhoA gene of gastric MGC-803 cells and its impact on the biological behavior of gastric cancer cells was explored, in order to provide the experimental basis for targeted RhoA gene therapy against gastric cancer.
Materials and methods
The main materials
MGC-803 cells were provided by the laboratory; RPMI 1640 culture medium, trypsin and fetal bovine serum were purchased from Gibco Company, America; TRIzol and LipofectamineTM2000 liposomal transfection reagents were purchased from Invitrogen Corporation, America; RT-PCR two-step kit (Beijing Parkson gene Technology Co., Ltd.); AMV reverse transcription kit (Hangzhou Bioer); cDNA synthesis kit (Japan TOYOBO Corporation); siRNA gene fragments were synthesized by Zhuhai Ying Ping biotechnology Co., Ltd.; G418 was purchased from American Klontech company; CCK-8 (Shanghai Yan Bin Chemical Company); RhoA mouse anti-human monoclonal antibodies were purchased from Santa Cruz, USA; HRP-conjugated goat anti-mouse secondary antibody was purchased from Beijing Zhongshan company; cationic liposome reagent (Lipofectamine III 2000) were purchased from Invitrogen, USA; RIPA protein lysates and BCA protein quantification kit were purchased from Jiangsu Biyotime Company; ECL kit was purchased from American Thermo company.
Design, synthesis and transfection of siRNA
mRNA sequence NM_001664 of human RhoA gene was searched with the NCBI database, and according to the siRNA design principles, RhoA siRNA was designed. siRNA sequence was composed by 5’-TTITCTAAACTATCACGGCTG-3’ (sense strand) and 5’-GCCCTGATAGTVFAGAAAAT-3’ (antisense strand); the negative control siRNA sequence was composed of 5’-CCAGAAGAGCAATCTGTAC-3’ (sense strand) and 5’-GTACAGATTGCTC3TCTGG-3’ (antisense strand), both synthesized by Zhuhai Ying Ping biotechnology Co., Ltd. MGC-803 cells were cultured in RPMI 1640 medium containing 10% fetal calf serum (37°C, 5% CO2, saturated humidity). Passage was conducted every 2 d, and cells at logarithmic growth phase were used for the experiment. Gastric MGC-803 cells were seeded in 24-well plates, 1 × 105 cells/well; when the fusion rate was 70%, liposome transfection was performed. Transfections were divided into three groups: Cells transfected with pGenesil-1-RhoA-siRNA vector were the MGC-803/RhoA-siRNA group (experimental group); cells transfected with random control vector were MGC-803/control group (control group), and non-transfected gastric MGC-803 cells were the MGC-803 group (blank group). RNA interference effect was detected in 48 h after transfection.
Semi-quantitative RT-PCR
After abandoning the cell culture medium, cells was washed with PBS for three times; total RNA was extracted using TRIzol method, and cDNA was synthesized by reverse transcription using cDNA synthesis kit. RhoA and internal control GAPDH were amplified respectively. Upstream primer of RhoA was 5’-CGGGAGCTAGCCAAGATGAAG-3’, and downstream primer was 5’-GCTFGCAGAGCAGCTCTCGTA-3’; the amplified product was 258 bp. Upstream primer of GAPDH was: 5’-GAGTCAACGGATTTGGTCGT-3’, and downstream primer was: 5’-GACAAGCTTCCCGTTCTCAG-3’; the amplified product was 185 bp. PCR reaction conditions were: pre-degeneration at 95°C for 10 min, degeneration at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, totally 45 cycles. 2% agarose gel electrophoresis was conducted to identify PCR products. Gel imaging and analysis system was used to scan the absorbance and RhoA absorbance/that of GAPDH was taken as the relative intensity of mRNA expression.
The expression of RhoA detected by westem blot
After discarding the culture, cells were washed three times with PBS; 150 μL pre-cooled RIPA lysis buffer was added, and the RIPA lysis buffer was diluted by 1.5 μL PMSF beforehand to a final concentration of 1 mmol/L. Operations were conducted on ice. Cells were centrifuged under 4°C at 10 000 r/min (centrifugal radius of 4 cm) for 5 min; protein concentration of the supernatant was measured by BCA method before denaturation at 99°C for 10 min. 50 μg protein was used for 10% SDSPAGE electrophoresis, and then electrotransferred to a PVDF blotting membrane; after soaked in 5% skimmed milk for 2 h, it was incubated by RhoA monoclonal antibody (1:200) at 4°C overnight. After washed by TBS-T, it was incubated at room temperature for 2 h by horseradish peroxidase-labeled goat anti-mouse secondary antibody (1:5000), and then washed with PBS before being detected using ECL.
Cell cycle detection using flow cytometry
Cells were treated by 0.25% trypsin and centrifuged at 1000 r/min for 5 min (centrifuge radius of 17 cm) to remove the culture medium; then the remaining cells were washed with PBS once, and the supernatant was discarded and the cells were collected, which were washed two times with ice-cold PBS, and incubated in 80% ethanol overnight at 4°C; After being rinsed by PBS for three times, cells were fixed and washed with PBS, and then re-suspended in 0.1 mg/mL propidium iodide in the dark at room temperature, dyeing 30 min; cell cycle was detected by flow cytometry. The above experiment was repeated for three times.
Cell proliferation detected by CCK8 assay
Cells at logarithmic growth phase were collected and the density was adjusted to 1 × 103 cells/well, and then 200 μL DMEM/F12 culture containing 10% FBS was added, five wells each group. 20 μL CCK-8 was added after being cultured for 24 h; after incubation for 4 h, at the wavelength of 490 nm, absorbance (A [490]) of each well was detected using a microplate reader; The averages of cell absorbance were taken as the vertical axis to draw growth curve, with the time of 48, 72, 96 and 120 h as the abscissa.
Scratch healing assay
The above three kinds of cells in the logarithmic growth phase were seeded in 6-well plates at a density of 5 × 103 cells/well. After cell monolayer formation in each group, “-” shaped scratch was conducted with a pipette dropper along the bottom of the culture plate; after PBS gentle washing, each well was added 2 ml serum-free RPMI 1640 medium for continuous incubation. Scratches healing were observed after 48 h.
Statistical analysis
Data were analyzed by SPSS 15.0 statistical software; the results were presented as mean ± standard deviation (x̅ ± s); differences between the groups were compared using t-test and analysis of variance; P < 0.05 was considered statistically significant.
Results
RhoA siRNA inhibition of RhoA mRNA expression
Comparisons of RhoA mRNA expression detected by RT-PCR in MGC-803/RhoA-siRNA group (experimental group), MGC-803/control group (control group) and MGC-803 group (blank group) showed that the experimental group had significantly narrower bands, and there were statistically significant differences between the experimental group and the control group, and the observation group and blank group (P < 0.05). It was shown in Figure 1.
Figure 1.
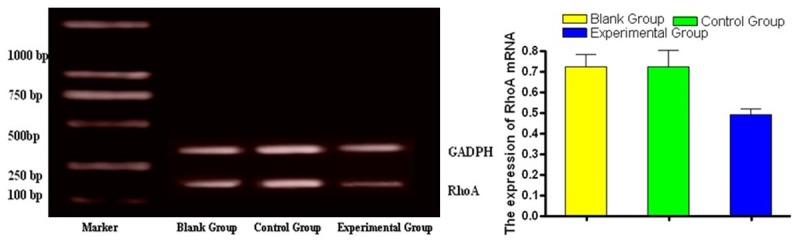
Detection of RhoA mRNA expression in MGC-803 cells by RT-PCR.
siRNA inhibition of the expression of RhoA proteins
Comparisons of RhoA protein expression detected by Western blot in MGC-803/RhoA-siRNA group (experimental group), MGC-803/control group (control group) and MGC-803 group (blank group) showed that the experimental group had significantly narrower bands, and there were statistically significant differences in Gray value (P < 0.05). It was shown in Figure 2.
Figure 2.
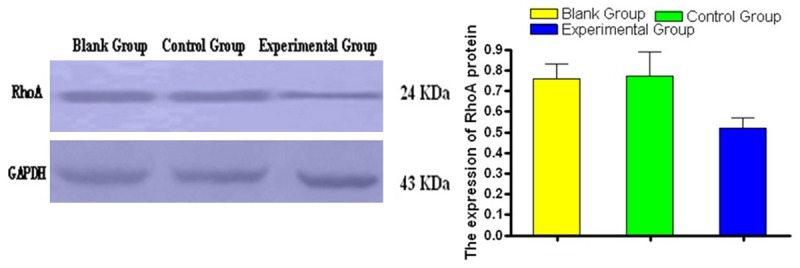
Detection of RhoA protein expression in MGC-803 cells by Western blot.
Cell cycle detection using flow cytometry
Flow cytometric analysis of cell cycle showed that compared with the control group and blank group, there was a slight increase in the G0/G1 phase and a slight decrease in S phase in the experimental group; There were significant differences between observation group and control group, and the observation group and the blank group (P < 0.05). Cells in M phase had no significant changes and the difference was not statistically significant (P > 0.05), indicating a clear S-phase arrest (Table 1).
Table 1.
siRNA inhibition of RhoA expression on MGC-803 cell cycle (x̅ ± s, n = 3)
| Group | The G0/G1 phase | Phase S | The G2/M phase |
|---|---|---|---|
| Blank group | 56.4 ± 3.8 | 35.1 ± 1.3 | 8.7 ± 0.8 |
| Control group | 56.4 ± 2.6 | 35.4 ± 2.6 | 9.4 ± 1.1 |
| Experimental group | 68.4 ± 3.4* | 21.9 ± 1.3* | 10.0 ± 1.2 |
And the control group, the control group;
P< 0.05.
Effect of siRNA on cell proliferation
The growth curve of CCK-8 assay showed that the curve of experimental group was significantly lower than that of the control group and the blank group (Figure 3), and differences were statistically significant (P < 0.05, Table 2).
Figure 3.
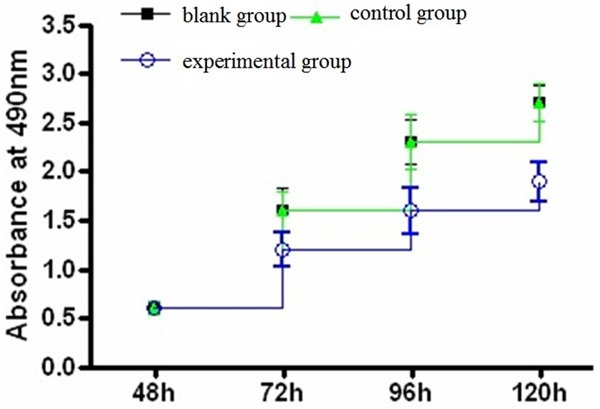
Growth curve of three groups.
Table 2.
Absorbance at 490 nm (x̅ ± s, n = 5)
| Group | 48 h | 72 h | 96 h | 120 h |
|---|---|---|---|---|
| Blank group | 0.6 ± 0.03 | 1.6 ± 0.22 | 2.3 ± 0.23 | 2.7 ± 0.18 |
| Control group | 0.6 ± 0.07 | 1.6 ± 0.20 | 2.3 ± 0.28 | 2.7 ± 0.19 |
| Experimental group | 0.6 ± 0.05 | 1.2 ± 0.17 | 1.6 ± 0.24 | 1.9 ± 0.20 |
Cell scratch healing results
After 48 hours, the MGC-803/RhoA-siRNA group (experimental group) has a slow healing of scratches, while scratches of MGC-803 group (blank group) and MGC-803/control group (control group) had been basically covered, shown in Figure 4.
Figure 4.
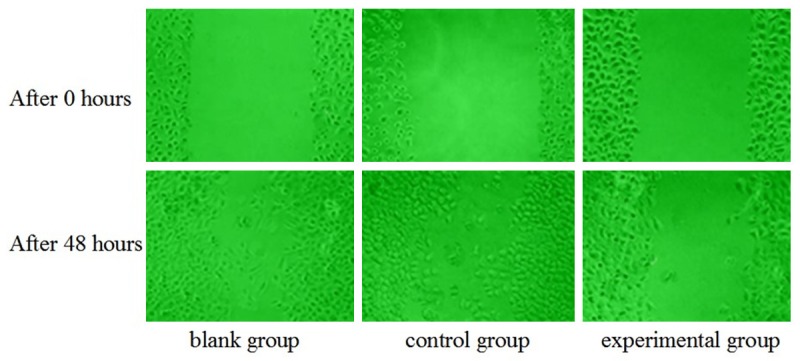
Cell scratch healing results.
Discussion
Rho family is a member of Ras superfamily small G binding protein, which is an important molecule in cellular signal transduction pathway. In recent years, studies have shown that Rho plays an extremely important role in cancer development. By regulating the reconstruction of the actin cytoskeleton skeleton, the biological behavior, such as cell morphology, adhesion, polarity, apoptosis, cytokinesis are adjusted accordingly, and therefore these caused tumor invasion and metastasis [4,5]. Rho subfamily includes three members: RhoA, RhoB and RhoC. The RhoA gene is located on chromosome 3p21.3, with a total length of 582bp and encoded 193 amino acids containing protein RhoA. Previous studies show that RhoA protein was only expressed in the cell membrane and cytoplasm. But in recent years, studies have shown that Rho proteins were expressed in the cell membrane, pulp and nuclear in varying degrees [6], and the increased expression and activity of RhoA protein in S-phase cells suggested that RhoA activation in the nucleus may be involved in cell mitosis. Research showed that RhoA protein and mRNA expression had significantly increased in tissues of liver cancer, breast cancer, lung cancer, kidney cancer and colon cancer [7,8]. Recent studies showed that RhoA expression in gastric carcinoma was significantly increased [9]. Pan et al [10] determined the RhoA in 53 cases of Gastric cancer tissues and corresponding adjacent tissues, and the results showed that RhoA mRNA levels of gastric cancer tissues were significantly higher than that of the adjacent tissues and the expression of of RhoA mRNA was higher in poorly differentiated gastric cancer than that in highly differentiated gastric cancer. Cai et al [11] measured the RhoA expression in 60 cases of gastric carcinoma and 10 cases of normal gastric mucosa by immunohistochemical method, the results showed that RhoA expression in gastric cancer tissues was significantly higher than that in normal gastric mucosa.
The main biological function of RhoA is to change the cell skeleton structure and be involved in the adhesion, shrinkage and movement of tumor cells, releasing adhesion, matrix degradation and regulating the invasion of blood vessels and lymphatic vasculature [12], which was considered to have enhanced effect on tumor invasion and metastasis [13,14]. In the research of IL-6 promotion in gastric cancer development, Lin et al [15] found that IL-6 could activate downstream signals by serine phosphorylation to increment RhoA expression and the activated RhoA caused actin cytoskeletal rearrangement, resulting in gastric cancer cell migration. In this study, the expression of RhoA gene in gastric MGC-803 cells was inhibited by RNAi technology, in order to compare the cell cycle, apoptosis, cell proliferation and migration in the experimental and control groups. The results showed that the growth rate of the experimental group significantly slowed down and the migration significantly decreased, with statistically significant differences (P < 0.05). After the RhoA gene silencing, cell cycle was arrested in the G0/G1 phase, and the number of cells in S phase was decreased; the difference was statistically significant (P < 0.05). This suggested that specific interference of RhoA gene expression in gastric MGC-803 cells can inhibit the migration and proliferation of tumor cells.
In short, RhoA gene not only was closely related to the changes of the adhesion of tumor cells, but also played an important role in promotion of tumor cell proliferation. Specific interference of RhoA gene expression in gastric MGC-803 cells can inhibit the migration and proliferation of tumor cells; therefore, siRNA sequences of RhoA may be an effective target for the treatment of gastric cancer.
Disclosure of conflict of interest
None.
References
- 1.Li Y, Chen Y, Tao Y, Wang Y, Chen Y, Xu W. Fibronectin increases RhoA activity through inhibition of PKA in the human gastric cancer cell line SGC-7901. Mol Med Rep. 2011;4:65–69. doi: 10.3892/mmr.2010.396. [DOI] [PubMed] [Google Scholar]
- 2.Lee YC, Cheng TH, Lee JS, Chen JH, Liao YC, Fong Y, Wu CH, Shih YW. Nobiletin, a citrus flavonoid, suppresses invasion and migration involving FAK/PI3K/Akt and small GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell Biochem. 2011;347:103–115. doi: 10.1007/s11010-010-0618-z. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Chen Y, Tao Y, Xu J, Chen M. RhoA protein is generally distributed in the nuclei of cancer cells. Oncol Rep. 2010;24:1005–1009. doi: 10.3892/or.2010.1005. [DOI] [PubMed] [Google Scholar]
- 4.De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cell lead the way. Arterioscler Thromb Vasc Biol. 2009;29:639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- 5.Yang KY, Chen DJ, Li XR, Wen YG. Construction and identification of shRNA expressing vectors targeting RhoA gene. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010;26:707–8. [PubMed] [Google Scholar]
- 6.Tao Y, Chen YC, Li YY, Yang SQ, Xu WR. Localization and translocation of RhoA Protein in the human gastric cancer cell line SGC-7901. World J Gastroenterol. 2008;14:1175–1181. doi: 10.3748/wjg.14.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong W, Dou KF, Yang XK, Zhang FQ, Wang DS. Effect of RhoA gene silencing on proliferation and migration of HepG2 cells. Chinese Journal of Digestive Surgery. 2010;9:216–219. [Google Scholar]
- 8.Tsumuraya M, Kato H, Miyachi K, Sasaki K, Tsubaki M, Akimoto K, Sunagawa M. Comprehensive analysis of genes involved in the malignancy of gastrointestinal stromal tumors. Anticancer Res. 2010;30:2705–2715. [PubMed] [Google Scholar]
- 9.Tian XL, Jiang ZQ, Luo QW. Expression and clinical significance of RhoA and Ezrin in gastric carcinoma. Journal of Clinical Medicine in Practice. 2011;15:111–114. [Google Scholar]
- 10.Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y, Fan D. Expression of seven main Rho family members in gastric eareinoma. Biochem Biophys Res Commun. 2004;315:686–691. doi: 10.1016/j.bbrc.2004.01.108. [DOI] [PubMed] [Google Scholar]
- 11.Cai J, Niu X, Chen Y, Hu Q, Shi G, Wu H, Wang J, Yi J. Emodin-induced generation of reactive oxygen species inhibits RhoA activation to sensitize gastric carcinoma cells to anoikis. Neoplasia. 2008;10:41–45. doi: 10.1593/neo.07754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun YQ, Xiong JX, Liu Y, Zhu YL. Acceleration of invasion and metastasis of gastric cancer by RhoA and Ki-67 overexpression. Heilongjiang Medicine and Pharmacy. 2009;32:1–2. [Google Scholar]
- 13.Liu XP, Wang HB, Yang K, Sui AH, Shi Q, Qu S. Inhibitory effects of adenovirus mediated tandem expression of RhoA and RhoC shRNAs in HCTll6 cells. J Exp Clin Cancer Res. 2009;28:52. doi: 10.1186/1756-9966-28-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X, Lu L, Pokhriyal N, Ma H, Duan L, Lin S, Jafari N, Band H, Band V. Overexpression of Rho induces preneoplastic transformation of primary manlnlary epithelial cells. Cancer Res. 2009;69:483–91. doi: 10.1158/0008-5472.CAN-08-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin MT, Lin BR, Chang CC, Chu CY, Su HJ, Chen ST, Jeng YM, Kuo ML. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int J Cancer. 2007;120:2600–2608. doi: 10.1002/ijc.22599. [DOI] [PubMed] [Google Scholar]


