Abstract
Ginsenoside Rd (GsRd) reportedly protects the heart against ischemia-reperfusion (I/R) injury. Nrf2/HO-1 signaling plays a key role in attenuating oxidative stress. However, it remains unclear whether GsRd protects against myocardial I/R injury via Nrf2/HO-1 signaling. This study aimed to investigate the role of Nrf2/HO-1 signaling in the cardioprotective effect of GsRd. Rats received 30 min ischemia followed by 2 h reperfusion. Cardiac function, infarct size and serum CK, LDH, cTnI levels were detected. The expression of Nrf2 and HO-1 was detected by western blot. The results suggested that GsRd attenuated myocardial I/R injury as evidenced by improved cardiac function, decreased infarct size and decreased levels of serum CK, LDH and cTnI. In addition, GsRd administration enhanced the expression of Nrf2 and HO-1. In conclusion, the present study shows that GsRd protects against myocardial I/R injury via Nrf2/HO-1 signaling.
Keywords: Ginsenoside Rd, Nrf2/HO-1, myocardial ischemia-reperfusion injury
Introduction
Coronary heart disease exhibits a high morbidity and mortality, and it remains one of the major causes of death all around the world [1]. To give blood supply to the myocardium as soon as possible is the key to alleviate myocardial infarction [2]. However, reperfusion causes a lot of disorders, such as reactive oxygen species (ROS) overproduction, inflammation, apoptosis, calcium overload and mitochondrial dysfunction [3,4]. To date, much attention has been paid to the overproduction of ROS, and it is indicated that decreasing ROS may alleviated myocardial injury to a great extent [5]. However, there are still few effective therapies to alleviate ischemia-reperfusion injury.
The nuclear factor erythroid-2 related factor 2 (Nrf2), a transcription factor sensitive to the redox state in the cell, plays a key role in fighting against oxidative stress in the cells. Nrf2 activation has been suggested to be protective in many disorders, such as cerebral ischemia, retinal ischemia and myocardial ischemia [6-8]. Under physiological conditions, Nrf2 is located in the cytoplasm and it binds to Kelch-like ECH-associated protein 1 (Keap1). When threatened by oxidative stress and other stimuli, Nrf2 dissociates from Keap1 and then translocates from the cytoplasm to the nucleus [9,10]. In the nucleus, Nrf2 binds to the antioxidant response element (ARE) sequence, activating the transcription of antioxidative genes, including heme oxygenase-1 (HO-1) and NAD(P)H: quinine oxidoreductase 1 (NQO1) [11].
Ginseng, known as the root of Panax ginseng C.A. Meyer, has been widely used as a valuable medicinal herb for more than 20 centuries in China [12]. There have been more than 40 kinds of ginsenosides isolated from Ginseng, including ginsenoside Rd (GsRd) [13] (Figure 1). It has been suggested that GsRd exerts various pharmacological effects, such as removing free radicals [14-16], inhibiting calcium influx [17] and anti-apoptosis [18]. In addition, GsRd promotes neurogenesis in rat brain after transient focal cerebral ischemia and is protective to cerebral ischemia [13,19]. As for the cardiovascular system, GsRd has been suggested to be beneficial. GsRd is reported to attenuate myocardial ischemia-reperfusion injury through Akt/GSK-3βsignaling. However, whether GsRd protects the heart from ischemia-reperfusion via Nrf2/HO-1 signaling has not been elucidated. The present study, therefore, aims to testify the hypothesis that GsRd protects against myocardial I/R injury via Nrf2/HO-1 signaling pathway.
Figure 1.
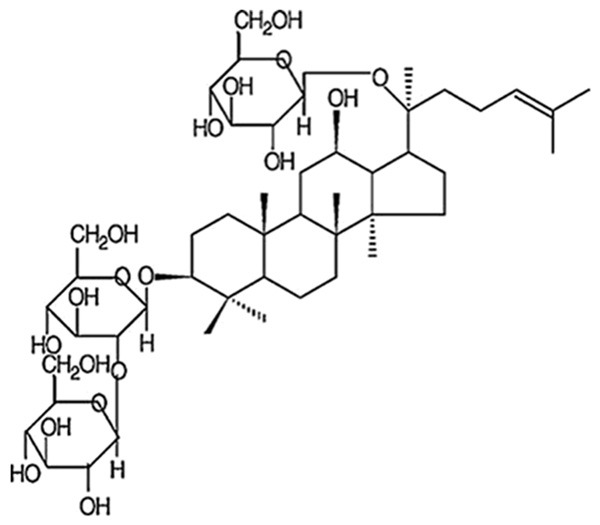
Structure of ginsenoside Rd.
Materials and methods
Reagents
GsRd (a purity of 98%) was purchased from Tai-He Biopharmaceutical Co. Ltd (Guangzhou, China). 2,3,5-triphenyltetrazolium chloride (TTC) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against Nrf2, HO-1, NQO1, lamin B and β-actin were purchased from Santa Cruz Biotechnology. CK assay kit, LDH assay kit and cTnI assay kit were purchased from Beyotime (Shanghai, China).
Animals
Male Sprague-Dawley (SD) rats (270-320 g) were purchased from the Experimental Animal Center of the Kunming Medical University. All animals were kept at 22-24°C under a 12 hour/12 hour light-dark cycle. And the rats had free access to food and water. All procedures were performed in adherence with the National Institutes of Health Guidelines for the Use of Laboratory Animals (NIH publication no. 85-23, revised 1996), and were approved by the Kunming Medical University Committee on Animal Care.
Rat myocardial ischemia/reperfusion model
Rats were anesthetized intraperitoneally via the administration of pentobarbital sodium at a dose of 50 mg/kg (Sigma, St. Louis, USA). The n, a left thoracic incision was made to expose the heart. Myocardial ischemia was triggered by occluding the left anterior descending coronary artery with a 6-0 silk slipknot around LAD. After ischemia for 30 min, the slipknot was relaxed allowing for 120 min of reperfusion. The sham group underwent the same procedures except for the coronary slipknot.
Experimental protocol
Rats were randomly assigned into three groups (n = 8 in each group): (1) sham group (2) I/R group: rats were administrated intraperitoneally with vehicle (0.9% NaCl); (3) I/R + GsRd group: GsRd (50 mg/kg, i.p.) was administered 30 min prior to reperfusion. The dose of GsRd was chosen according to previous studies [20].
Assessment of heart function
Rats were anesthetized with the intraperitoneal administration of sodium pentobarbital (50 mg/kg). A catheter was then inserted into the left ventricle through the right common carotid artery for assessing left ventricular ejection fraction (LVEF), left ventricular end-diastolic pressure (LVEDP) and the maximal rate of rise and decline of ventricular pressure (±dp/dt [max]). The data was obtained and analyzed with the AcqKnowledge 4.0 software.
Determination of myocardial infarct size
At the end of reperfusion, the slipknot was retied and Evans blue (2%, 4 ml) was administrated through the aorta into the body. The heart was quickly removed and was conserved in a -20°C refrigerator. The heart was then cut into 2 mm slices, and incubated in TTC solution for 20 min, and then in 4% formaldehyde solution overnight. Evans blue stained area indicates for non-I/R area (blue). TTC stained area indicates for the area at risk (AAR) red. Non-TTC stained area indicates for infarction (white).The size was analyzed with OPTIMAS software. Myocardial infarct area was calculated as follows: infarct area/area at risk% (INF/AAR%).
Evaluation of CK, LDH and cTnI
At the end of reperfusion, blood was collected from the heart to evaluate the levels CK, LDH and cTnI. The samples were centrifuged and the serum was collected to evaluate the levels of CK, LDH and cTnI according to manufacturer’s instruction.
Western blot
Protein extracts were prepared using the heart tissue by homogenization in a RIPA buffer. Protein was measured with the BCA Protein Assay kit (Beyotime, China). And proteins were separated by electrophoresis on SDS-PAGE (10%) and transferred to a nitrocellulose membrane. The membranes were blocked with TBST with 5% nonfat milk for 2 h at room temperature. The membranes were then incubated with the primary antibody rabbit anti-Nrf2, rabbit anti-NQO1 (1:500, Santa Cruz Biotechnology) rabbit anti-HO-1 (1:2000, Santa Cruz Biotechnology). Then, membranes were washed with TBST. The membranes were incubated for 1 h at room temperature with the corresponding secondary antibodies (1:3000, goat anti-rabbit; 1:5000, goat anti-mouse, Santa Cruz Biotechnology). Finally, western blots were detected using ECL Plus Detection kit (Millipore, USA). And the blots were visualized by a Bio-Rad Imaging system.
Statistical analysis
All values are presented as means ± S.E.M. The significance of differences were evaluated by Dunnett’s t-test or ANOVAs. A value of P < 0.05 was considered statistically significant.
Results
GsRd improved cardiac function
As shown in the Figure 2, GsRd significantly increased LVEF and ±dp/dt max and lowered LVEDP in I/R + GsRd group compared with those in the I/R group (P < 0.05) (Figure 2).
Figure 2.

Effect of ginsenoside Rd on cardiac function. A. The effect of ginsenoside Rd on LVEDP. B. The effect of ginsenoside Rd on LVEF. C. The effect of ginsenoside Rd on +dp/dx max. D. The effect of ginsenoside Rd on -dp/dx max. LVEF, left ventricle ejection fraction; LVEDP, left ventricle end-diastolic pressure. Data were expressed as mean ± S.E.M. (n = 8 in each group). *P < 0.01 versus the sham group, #P < 0.05 versus I/R group.
GsRd attenuates myocardial infarction
As shown in the Figure 3, MI/R resulted in a dramatic infarction. GsRd reduced myocardial infarction area markedly compared with that the I/R group (P < 0.05) (Figure 3).
Figure 3.
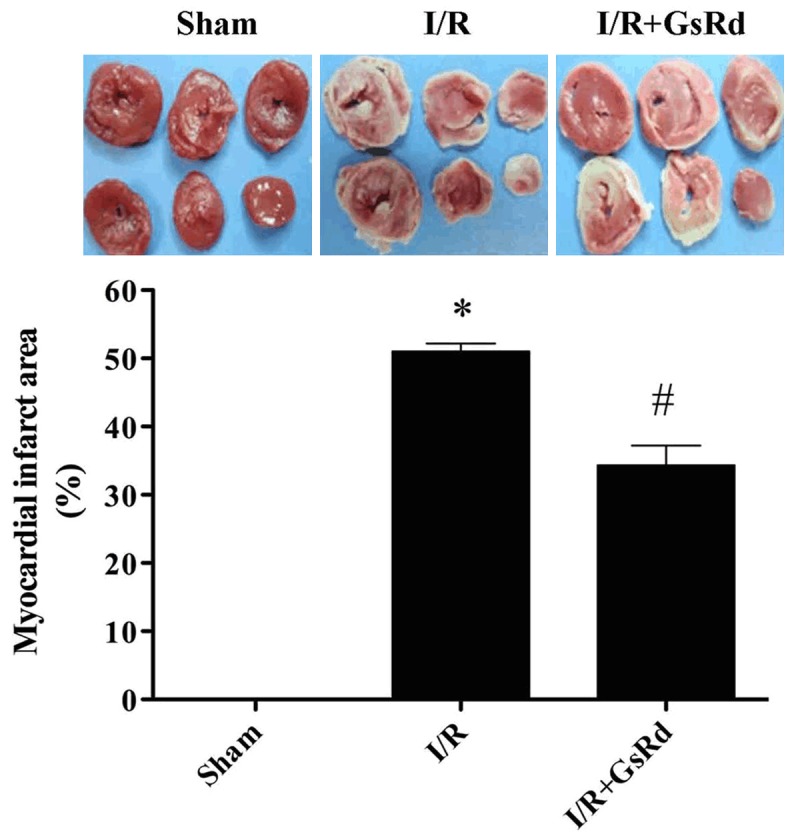
Effect of ginsenoside Rd on myocardial infarction size. Data were expressed as mean ± S.E.M. (n = 8 in each group). *P < 0.01 versus the sham group, #P < 0.05 versus I/R group.
Effect of GsRd on the activity of serum CK, LDH and cTnI
In the Figure 4, the activity of LDH, CK and cTnI increased dramatically in the I/R group compared with those levels in sham group (P < 0.05). However, GsRd treatment significantly decreased CK, LDH and cTnI activity compared with those in the I/R group (P < 0.05) (Figure 4).
Figure 4.
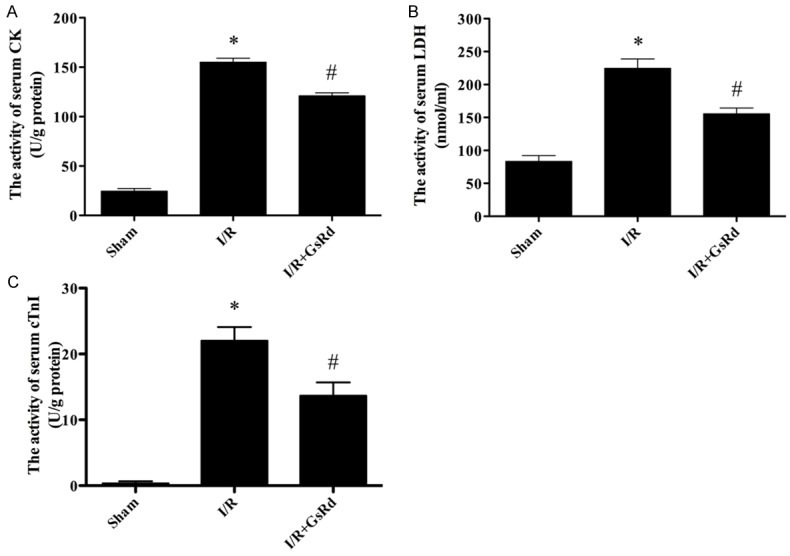
The comparison of CK, LDH, cTnI activity in each group. The CK, LDH, cTnI activity in the sham group was relatively lower, while the CK, LDH, cTnI activity in I/R group was dramatically increased, which was attenuated by ginsenoside Rd. Data were expressed as mean ± S.E.M (n = 8 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus I/R group.
Effect of GsRd on Nrf2/HO-1 signaling
As shown in the Figures 5, 6 the expression of Nrf2, HO-1 and NQO1 were detected by Western blot. The nucleus and total Nrf2, HO-1 and NQO1 were dramatically enhanced in I/R group. GsRd treatment significantly increased the expression of nucleus and total Nrf2, HO-1 and NQO1 compared with those in I/R group (P < 0.05) (Figures 5, 6).
Figure 5.
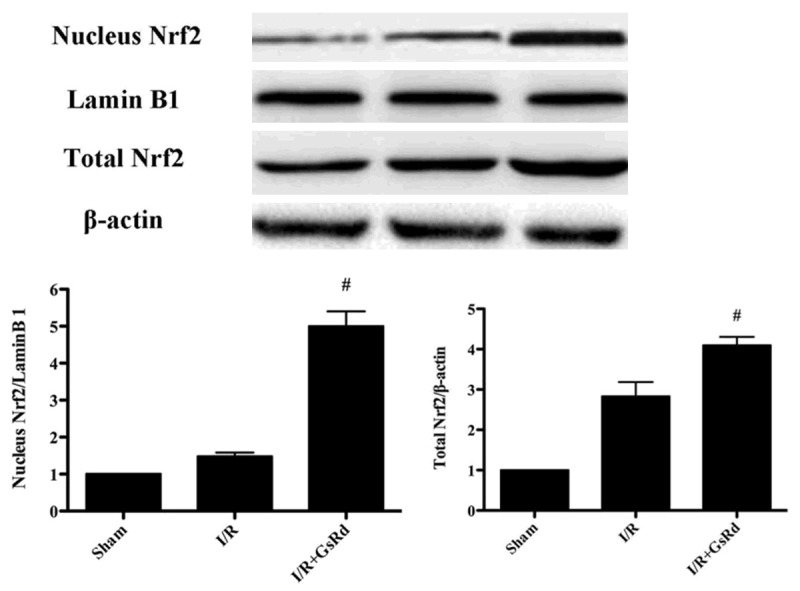
Effects of ginsenoside Rd on Nrf2 expression in rats subjected to myocardial I/R. The representative images of nucleusand total Nrf2 are shown. The results are expressed as the mean ± S.E.M. (n = 8 in each group). #P < 0.05 versus I/R group.
Figure 6.
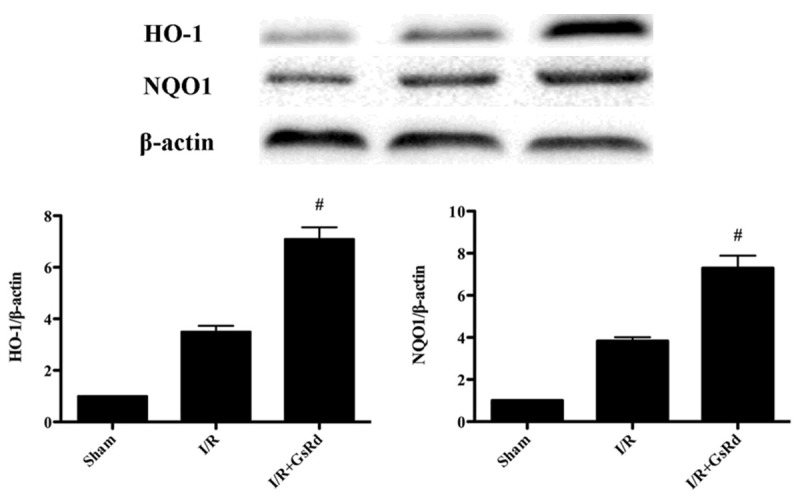
Effects of ginsenoside Rd on HO-1 and NQO1 expression in rats subjected to myocardial I/R. The representative images of HO-1 and NQO1 are shown. The results are expressed as the mean ± S.E.M. (n = 8 in each group). #P < 0.05 versus I/R group.
Discussion
The important observations in our present study are: (1) GsRd attenuates myocardial I/R injury through alleviating cardiac dysfunction. (2) GsRd attenuates myocardial I/R injury by decreasing serum CK, LDH and cTnI activities. (3) The cardioprotective effect of GsRd is associated with the activation of Nrf2/HO-1 signaling.
The ginseng has been used worldwide for a long time, especially in China. Ginsenosides Rd is one of the major ginseng components. In addition, more than 40 ginsenosides have been identified [13]. Various biological effects of ginsenosides have been reported in recent researches. Xie et al. reported that ginsenoside-Rg1 protects against cerebral ischemia/reperfusion injury in rats by downregulating the expression of protease-activated receptor-1 [21]. Zhu et al. reported that ginsenoside Rd ameliorates experimental autoimmune encephalomyelitis in C57BL/6 mice [22]. Wu et al. reported that ginsenoside Rb1 protects rats against sepsis [23]. In addition, previous studies have demonstrated that ginsenosides have a huge protective effect against cardiovascular disorders [24-27]. Wang et al. reported that ginsenoside Rb1 preconditioning protects against myocardial infarction after ischemia and reperfusion via activating phosphatidylinositol-3-kinase (PI3K) signaling [28]. In the present study, we found that GsRd improved cardiac function, increasing ±dP/dt max and LVEF and decreasing LVEDP, GsRd also decreased myocardial infarct size and lowered the activity of CK, LDH and cTnI, which stands for the severity of myocardial injury.
Ischemia-reperfusion induces the dissociation of Nrf2 from Keap1, leading to the translocation to the nucleus, then binding to the ARE, and activating phase 2 antioxidant and detoxifying genes, such as HO-1 and NQO1 [29]. HO-1 is a rate-limiting enzyme, catalyzing the degradation of heme into biliverdin, carbon monoxide and ferritin [30]. NQO1 is generally regarded as a detoxifying enzyme, since it is able to remove reactive quinones and quinone imines and to diminish toxic hydroquinones [31]. As a result, the up-regulation of HO-1 and NQO1 protects the heart against oxidative stress induced by I/R injury. Moreover, Nrf2/HO-1 signaling pathway have effect on cell survival via various substrates, such as Bcl-2 and Bax [32]. Our results suggest that GsRd attenuates myocardial ischemia reperfusion injury by activating Nrf-2/HO-1 signaling.
In conclusion, our findings suggest that GsRd mitigates myocardial ischemia/reperfusion injury as evidenced by elevated cardiac function and decreased CK, LDH and cTnI activities. The cardioprotective effect of GsRd is tightly related toNrf2/HO-1 signaling pathway.
Acknowledgements
This study was supported by the grants from Applied Basic Research Project of Kunming Medical University and Science and Technology Department of Yunnan Province (2013FB127) and Applied Basic Research Project of the Education Department of Yunnan Province (2012Z086).
Disclosure of conflict of interest
None.
References
- 1.Lu MJ, Chen YS, Huang HS, Ma MC. Hypoxic preconditioning protects rat hearts against ischemia-reperfusion injury via the arachidonate12-lipoxygenase/transient receptor potential vanilloid 1 pathway. Basic Res Cardiol. 2014;109:414. doi: 10.1007/s00395-014-0414-0. [DOI] [PubMed] [Google Scholar]
- 2.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 3.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Wang Y, Ye J, Lu X, Cheng Y, Xiang L, Chen L, Feng W, Shi H, Yu X, Lin L, Zhang H, Xiao J, Li X. bFGF attenuates endoplasmic reticulum stress and mitochondrial injury on myocardial ischaemia/reperfusion via activation of PI3K/Akt/ERK1/2 pathway. J Cell Mol Med. 2015;19:595–607. doi: 10.1111/jcmm.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan J, Wang N, Deng C, Zhang S, Li Y, Chen W, Yu S, Yi D, Jin Z. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med. 2013;65:667–679. doi: 10.1016/j.freeradbiomed.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Han J, Wang M, Jing X, Shi H, Ren M, Lou H. (-)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem Res. 2014;39:1292–1299. doi: 10.1007/s11064-014-1311-5. [DOI] [PubMed] [Google Scholar]
- 7.He M, Pan H, Chang RC, So KF, Brecha NC, Pu M. Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage. PLoS One. 2014;9:e84800. doi: 10.1371/journal.pone.0084800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Wu M, Tang L, Pan Y, Liu Z, Zeng C, Wang J, Wei T, Liang G. Novel curcumin analogue 14p protects against myocardial ischemia reperfusion injury through Nrf2-activating anti-oxidative activity. Toxicol Appl Pharmacol. 2015;282:175–183. doi: 10.1016/j.taap.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia L, Zhao Y. Current evaluation of the millennium phytomedicine--ginseng (I): etymology, pharmacognosy, phytochemistry, market and regulations. Curr Med Chem. 2009;16:2475–2484. doi: 10.2174/092986709788682146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu XY, Zhou XY, Hou JC, Zhu H, Wang Z, Liu JX, Zheng YQ. Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Aktpathway. Acta Pharmacol Sin. 2015;36:421–428. doi: 10.1038/aps.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye R, Li N, Han J, Kong X, Cao R, Rao Z, Zhao G. Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci Res. 2009;64:306–310. doi: 10.1016/j.neures.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Ye R, Han J, Kong X, Zhao L, Cao R, Rao Z, Zhao G. Protective effects of ginsenoside Rd on PC12 cells against hydrogen peroxide. Biol Pharm Bull. 2008;31:1923–1927. doi: 10.1248/bpb.31.1923. [DOI] [PubMed] [Google Scholar]
- 16.Zhang YX, Wang L, Xiao EL, Li SJ, Chen JJ, Gao B, Min GN, Wang ZP, Wu YJ. Ginsenoside-Rd exhibits anti-inflammatory activities through elevation of antioxidant enzyme activities and inhibition of JNK and ERK activation in vivo. Int Immunopharmacol. 2013;17:1094–1100. doi: 10.1016/j.intimp.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Guan YY, Zhou JG, Zhang Z, Wang GL, Cai BX, Hong L, Qiu QY, He H. Ginsenoside-Rd from panax notoginseng blocks Ca2+ influx through receptor- and store-operated Ca2+ channels in vascular smooth muscle cells. Eur J Pharmacol. 2006;548:129–136. doi: 10.1016/j.ejphar.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Li XY, Liang J, Tang YB, Zhou JG, Guan YY. Ginsenoside Rd prevents glutamate-induced apoptosis in rat cortical neurons. Clin Exp Pharmacol Physiol. 2010;37:199–204. doi: 10.1111/j.1440-1681.2009.05286.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Shi M, Ye R, Wang W, Liu X, Zhang G, Han J, Zhang Y, Wang B, Zhao J, Hui J, Xiong L, Zhao G. Ginsenoside Rd attenuates tau protein phosphorylation via the PI3K/AKT/GSK-3beta pathway after transient forebrain ischemia. Neurochem Res. 2014;39:1363–1373. doi: 10.1007/s11064-014-1321-3. [DOI] [PubMed] [Google Scholar]
- 20.Sun D, Wang B, Shi M, Zhang YX, Zhou LF, Liu ZR, Wu ZL, Jiang W, Han JL, Xiong LZ, Zhao G. Pharmacokinetic, tissue distribution and excretion of ginsenoside-Rd in rodents. Phytomedicine. 2012;19:369–373. doi: 10.1016/j.phymed.2011.08.061. [DOI] [PubMed] [Google Scholar]
- 21.Xie CL, Li JH, Wang WW, Zheng GQ, Wang LX. Neuroprotective effect of ginsenoside-Rg1 on cerebral ischemia/reperfusion injury in rats by downregulating protease-activated receptor-1 expression. Life Sci. 2015;121:145–151. doi: 10.1016/j.lfs.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhu D, Liu M, Yang Y, Ma L, Jiang Y, Zhou L, Huang Q, Pi R, Chen X. Ginsenoside Rd ameliorates experimental autoimmune encephalomyelitis in C57BL/6 mice. J Neurosci Res. 2014;92:1217–1226. doi: 10.1002/jnr.23397. [DOI] [PubMed] [Google Scholar]
- 23.Wu LL, Jia BH, Sun J, Chen JX, Liu ZY, Liu Y. Protective effects of ginsenoside Rb1 on septic rats and its mechanism. Biomed Environ Sci. 2014;27:300–303. doi: 10.3967/bes2014.053. [DOI] [PubMed] [Google Scholar]
- 24.Ma L, Liu H, Xie Z, Yang S, Xu W, Hou J, Yu B. Ginsenoside Rb3 protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of JNK-mediated NF-kappaB pathway: a mouse cardiomyocyte model. PLoS One. 2014;9:e103628. doi: 10.1371/journal.pone.0103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y, Han B, Yu X, Qu S, Sui D. Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury in rats. Pharm Biol. 2011;49:900–906. doi: 10.3109/13880209.2011.554845. [DOI] [PubMed] [Google Scholar]
- 26.Peng L, Sun S, Xie LH, Wicks SM, Xie JT. Ginsenoside Re: pharmacological effects on cardiovascular system. Cardiovasc Ther. 2012;30:e183–188. doi: 10.1111/j.1755-5922.2011.00271.x. [DOI] [PubMed] [Google Scholar]
- 27.Xia R, Zhao B, Wu Y, Hou JB, Zhang L, Xu JJ, Xia ZY. Ginsenoside Rb1 preconditioning enhances eNOS expression and attenuates myocardial ischemia/reperfusion injury in diabetic rats. J Biomed Biotechnol. 2011;2011:767930. doi: 10.1155/2011/767930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Li M, Wu WK, Tan HM, Geng DF. Ginsenoside Rb1 preconditioning protects against myocardial infarction after regional ischemia and reperfusion by activation of phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs Ther. 2008;22:443–452. doi: 10.1007/s10557-008-6129-4. [DOI] [PubMed] [Google Scholar]
- 29.Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Dore S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147:53–59. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siow RC, Sato H, Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bi- lirubin and carbon monoxide? Cardiovasc Res. 1999;41:385–394. doi: 10.1016/s0008-6363(98)00278-8. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Li Q, Wang X, Yu S, Li L, Wu X, Chen Y, Zhao J, Zhao Y. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One. 2013;8:e59843. doi: 10.1371/journal.pone.0059843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niture SK, Jaiswal AK. INrf2 (Keap1) targets Bcl-2 degradation and controls cellular apoptosis. Cell Death Differ. 2011;18:439–451. doi: 10.1038/cdd.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


