Abstract
Background: Mild developmental dysplasia of hip (DDH) causes high morbidity of osteoarthritis (OA) on adult. It is thought that change of collagen and proteoglycans in cartilage may be the direct reasons for osteoarthritis. Objective: To detect the changes of the expressions of type II collagen of acetabular cartilage in early DDH and to investigate the relevance between type II collagen and the degeneration mechanism of the acetabular cartilage. Methods: The rabbit model of DDH was successfully established by applying the method of knee extending and fixing with cylinder cast in which left lower extremity as experimental group and right one as control group, checking with X-ray after 5 weeks. The stains of H&E and toluidine blue were applied on the samples of acetabular cartilage to observe the morphological changes of chondrocytes and extracellular matrix (ECM). The immunohistochemical staining and Western-blot were employed to respectively qualify and quantitate the expression of type II collagen. Results: Pathohistology observing indicated the signs of retrogressive changes of acetabular cartilage in experimental group. Also, the positive stained cells in type II collagen in experimental group was higher based on immunohistochemiscal staining. The quantitative amounts of type II collagen by Western-blot in experimental group was higher significant difference existed between two groups (t = 2.18, P < 0.05). Conclusions: The expression of type II collagen is correlated to a degeneration of acetabular cartilage and increase obviously in early DDH.
Keywords: Hip, bone disease, developmental, collagen, osteoarthritis
Introduction
Developmental dysplasia of the hip (DDH) refers to hip dysplasia in various forms on pediatric patients at different ages. This is the one of the most common diseases in pediatric orthopedics [1-3]. The reported morbidity of DDH throughout the world varies from 0.14% to 3.5%, averagely 3% [4-6], while the highest morbidity goes to Poland, 6.8% as reported in 2006 [7]. A lot of experiments showed that a perfect DDH model can be obtained with hip joint inflexion and with knee joint fixed and straightened [8-10]. The reason lies in that poses like sitting or squatting of rabbits are similar to the gesture of a fetus in uterus. The extended knee joint makes the hip joint bended and tight hamstring for long time, thus results in hip joint capsule and round ligament get stretched and gradually reaches subluxation or total dislocation.
Mild DDH causes high morbidity of osteoarthritis (OA) on adult. OA is the most common degenerative disease, characterized by cartilage degradation, loss of joint space, subchondral sclerosis, and formation of osteophytes [11,12]. Some long-term follow-up studies have shown that DDH patients are prone to OA at an earlier age than non-DDH controls, and the symptoms of OA in the DDH patients are more severe and require a higher rate of hip replacement surgery [13,14]. Pathology basis of OA is primarily degeneration and degradation of articular cartilage, including degradation of enzymes of extracellular matrix (ECM) and/or inhibition of new substrate synthesis, etc. At present, it is thought that change of quantity and quality of collagen and proteoglycans in cartilage are the direct reasons for OA losing its normal biomechanics features. During infancy and early childhood, there is increased turnover of type II collagen in the articular cartilage. Etiological study also holds the view that type II collagen gene variation may be related to development of DDH [15]. So far, researches on expression of macromolecule protein such as collagen in acetabular cartilage of DDH model only have few reports. In order to investigate the articular cartilage degenerative changes in early-stage DDH, this experiment detected the expression and changes of type II collagen in early acetabular cartilage of DDH model with immunohistochemical staining qualitative assessment and Western-blot quantitative testing.
Subjects and methods
Materials
This experiment introduced New Zealand albino rabbits of either gender with big ears, born after 4 to 5 weeks and weigh 0.5-0.8 kg (provided by department of laboratory animals from Fudan University). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Fudan University. Rabbits were intramscular injected with 5% ketamine hydrochloride 5-10 mg/kg; left lower extremities of rabbits were as experimental sides, make knee joints straightened and fixed with long leg cylinder cast; right lower extremities were as control sides which kept the way it is. Anteroposterior X-rays were taken for pelvis 5 weeks later and got 8 successful rabbit models of DDH. Specific indexes of X-ray for DDH diagnosis [8] were as follow: 1) enlarged inclination of acetabulum, Shenton’s line was continuous (Figure 1A, 1B); 2) femoral head moved outward, Shenton’s line was not continuous (Figure 2A, 2B). After euthanasia, obtained hip joints of both sides under asepsis conditions; take part of acetabular cartilage on each side as sample and kept them in liquid nitrogen for two usages: one was to detect protein expression with Western-blot immunoblotting; the rest was treated with routine histopathology staining and immunohistochemical staining.
Figure 1.
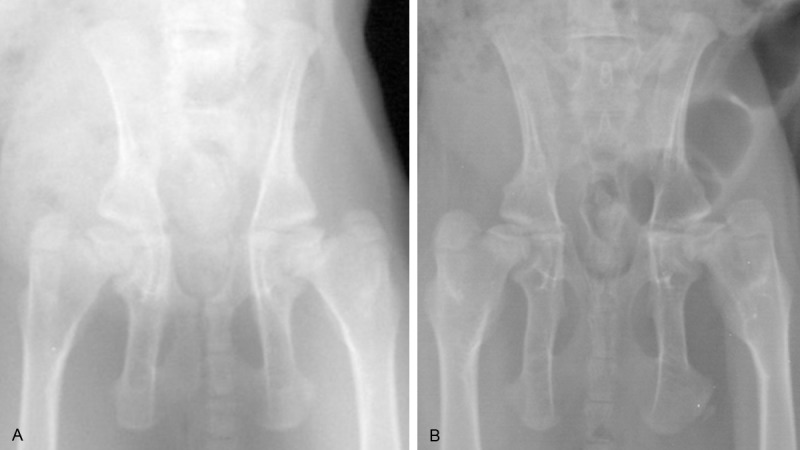
A: Anteroposterior X-ray before fixation; B: Anteroposterior X-ray 5 weeks after fixation: acetabulum dysplasia, enlarged inclination of acetabulum, Shenton’s line was continuous.
Figure 2.
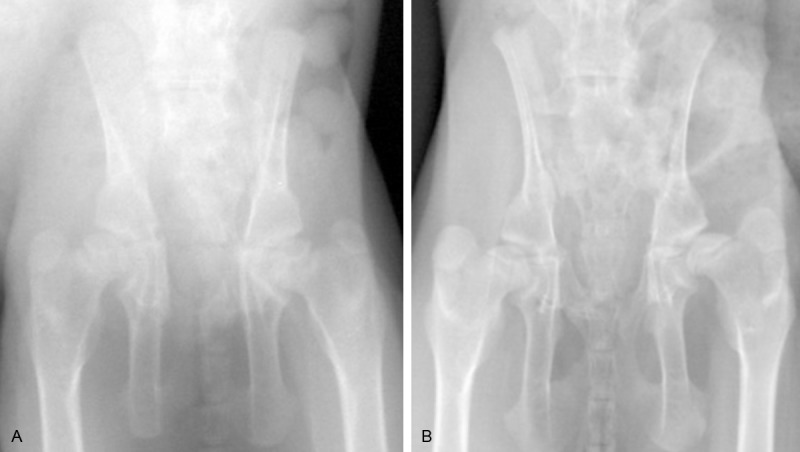
A: Anteroposterior X-ray before fixation; B: Anteroposterior X-ray 5 weeks after fixation: acetabulum dysplasia and subluxation, femoral head moved outward, Shenton’s line was not continuous.
Morphological observation
Samples were fixed with 4% paraformaldehyde and decalcified in EDTA decalcifying fluid for 3 to 4 weeks, then dehydrated, cleared and embedded with paraffin. Paraffin was cut into pieces and stained with H&E and toluidine blue. Observe morphous, size and arrangement of acetabular cartilage cells and changes of ECM under Leica-DMRA2 optical microscope.
Immunohistochemical staining
Here we introduced with immunohistochemical EnVision Two-Step Method. First antibodies was type II collagen antibody of 1:200 anti-mouse polyclone (product of Labvision company), second antibodies were Chem Mate TM EnVision kit of gene company. Both of them were observed under Leica-DMRA2 optical microscope. Replace first antibodies with PBS as the negative control. Determination index for the results: type II collagen presented positive if chondrocyte was surrounded with brown anachromasis, also with visible brown anachromasis in cytoplasm and wavy or irregular-shaped brown anachromasis in ECM.
Western blot analysis
Here we set beta-actin as internal reference (products of Santa Cruz). Take tissue samples which were kept in liquid nitrogen and added extracting solution of total sample protein, homogenate were iced bath and total protein was extracted. After centrifugation, detected protein density in supernatant fluid under ultraviolet spectrometer. Take 300 μg of total protein and added buffer solution of samples then degenerate 3 min with 100°C. Apply protein electrophoresis separately on 10% (beta-actin) and 8% (type II collagen) PAGE gelatin. After electrophoresis was done, protein transferred to cellulose acetate membrane and sealed with TTBS which include 0.5% skimmed milk powder. Then added first antibodies (working concentration of type II collagen was 1:200; working concentration of beta-actin antibody was 1:200) and react 2 hours at room temperature. Washed the extra first antibodies and added second antibodies (Gene Company, working concentration of 1:1000) then react for 1 hour at room temperature and visualized with luminescence substrate. Then the film was scanned and the strap analyzed with photodensitometry. Divided grey scale value of interest protein by grey scale value of internal reference and used the result to correct the error, the results embodied relative amount of interest protein.
Statistical analyses
All data was expressed with mean ± standard error, and processed the collected experimental data with SPSS12.0 statistical package and run t-test of two groups samples. The difference has statistical significance if P < 0.05.
Results
Morphologic observation under microscope
Experimental group: Fibrosis were visible in many chondrocytes of surface layer with H&E staining; arrangement of cells in middle layer was in disorder; cells in columnar and cell layer were decreased with irregular arrangement and a few clustered chondrocytes (Figure 3); ECM stained by toluidine blue suffered with uneven staining on columnar cell layer (Figure 4A).
Figure 3.
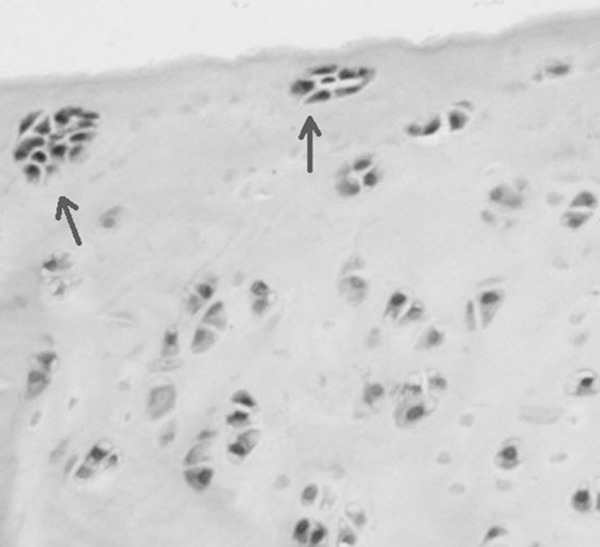
Clustered chondrocytes were seen in experimental group with H&E staining (indicated with arrows). Magnification × 200.
Figure 4.
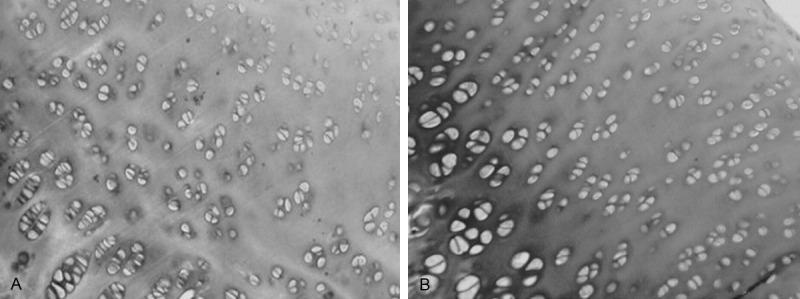
A: ECM stained by toluidine blue suffered with uneven staining on columnar cell layer. Magnification × 200. B: ECM stained with toluidine blue had not obvious staining loss. Magnification × 200.
Control group: Cell sizes were even with H&E staining, cells in columnar cell layer were arranged in column and perpendicular to the chondrocytes surface, no clustered chondrocytes; ECM stained with toluidine blue had not obvious staining loss (Figure 4B).
Immunohistochemical staining
Experimental group: Type II collagen expressed unevenly in ECM of cartilage layer, cells were surrounded with hyperchromatic, wavy or irregular-shaped collagen in ECM, and obvious hyperchromatic cytoplasm showed in chondrocyte. The positive cells were higher than those of control group (Figure 5A). Some cells suffered olistherozone with blooming-effect (Figure 6).
Figure 5.
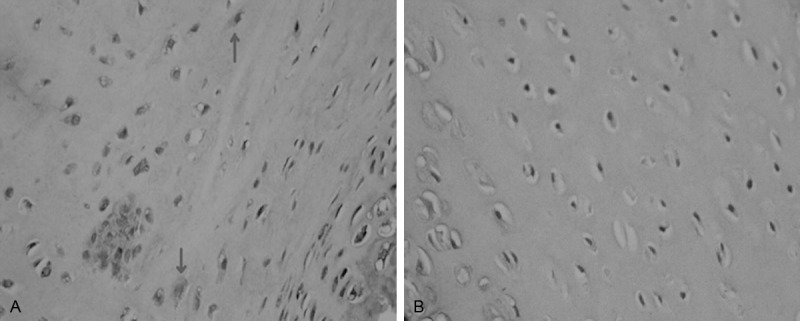
A: Uneven expression of type II collagen in the ECM in experimental group, cells were surrounded with hyperchromatic, wavy or irregular-shaped collagen in ECM, and obvious hyperchromatic cytoplasm showed in chondrocyte (indicated with arrow); Magnification × 400. B: lower staining of ECM of normal chondrocytes in control group, lighter staining in cytoplasm and fewer stained cells. Magnification × 400.
Figure 6.
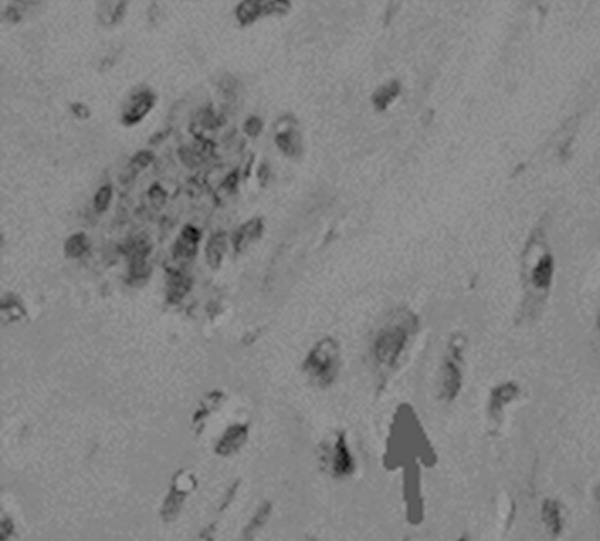
Olistherozone with blooming-effect surrounding of chondrocytes (indicated with arrow). Magnification × 400.
Control group: Type II collagen expresses evenly in ECM of normal acetabular cartilage and staining level was low and no staining in cytoplasm of chondrocytes generally (Figure 5B).
Western blot
Visualization of expression of type II collagen protein: Strap of relative molecular mass of 129 × 103 was type II collagen, strap of relative molecular mass of 43 × 103 was β-actin protein (Figure 7).
Figure 7.
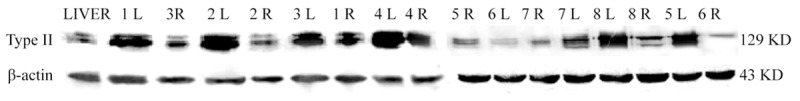
Visualization results of expression of type II collagen protein in both groups under Western-blot.
Relative quantity of protein expression: Divide gray value of interest protein of type II collagen protein by gray value of internal reference β-actin protein and used the result to correct the error and it also presented the relative amount of interest protein.
The result showed that there were type II collagen expression in control group, meanwhile expression of type II collagen in the experimental group were higher, so there was a significant difference between two groups (t = 2.18, P = 0.047).
Discussion
In early period, Wilkinson [16] pointed out that human acetabulum shares similarity with rabbit. Originally, people would drag lower extremity with excessive physical exertion to get DDH animal models while now it has been evolved into using plastic cannula, intramedullary needle and percutaneous fixation to straighten and fix the knee joint [8]. In this experiment, these young rabbits were fixed with plaster fixation and with extended knee joint, so their hips were under mechanical stress of muscle skeleton under a long time and finally got the DDH models successfully, this has been provided a theoretical foundation on the importance of the acquired environment effect on this disease as well as proved that DDH is a “developmental” disease to some extent. 21 rabbits were involved and 16 rabbit models were obtained (8 were applied for this experiment), so the teratogenic rate was 76.2% (16/21) and 3 got dead (two were infected; one accidently falled). To sum up, special attention should be paid to improve success ratio of DDH model: 1) Plaster fixation should be long enough to wrap the whole lower extremity. Because there are large connected aliform skin between the hind limb and gaster-skin, so plaster could not be fixed in groin region completely but just fixed at the middle and lower part of thigh, meanwhile rabbits grow very quick thus make plaster easily peeled off and then knee joint will recover to the flexion mode, so the model establish will be a failure. Therefore, we should frequently observe during model establish and add plaster length at proximal to keep knee joint extended continuously. 2) Proper plaster tightness. Thigh diameter of rabbit is 3 to 4 times of crus diameter. Plaster is cone-shaped and inversed, if plaster is not fixed tightly it will get loosed easily, if it is too tight the lower extremity will be swelling and necrosis. Therefore, after fixation with plaster, open the slot is a good way to reduce stress and adjust plaster tightness. 3) Apply polyamine polymer plaster. Rabbits are rodents and common calcium sulfate plaster can be bitten off and destroyed easily while polyamine polymer plaster with strong intensity and high hardness can better prevent this issue.
DDH refers to children of different ages present dysplasia of hip joint in various forms, which is the most common disease of pediatric orthopedics. Mild DDH causes high morbidity of osteoarthritis for grown up. It is reported that 76% hip joint arthritis is caused by DDH, most of them have to take total hip replacement before the age of 50 [17]. Normal chondrocytes secrete proteoglycan and show prunosus after staining with toluidine blue. After compressed, the invisible layer of articular cartilage surface having immunologic barrier to type II collagen antibody will be destroyed, then collagen fibers expose in synovial and cause chondrocytes damages as well as dysfunction and secret abnormal proteoglycans, therefore toluidine blue fails staining or with unevenly staining. In this experiment, it is observed that more visible fibrosis in many chondrocytes of surface layer, chaotic arrangement of cells in the middle layer, cells in columnar cell layer reduction as well as disordered, uneven toluidine blue staining and failed staining on matrix, which indicates that chondrocytes became degenerated due to compression in early DDH and then caused ECM elements changes in quantity and quality such as proteoglycans. This may be the pathological basis of osteoarthritis in prophase.
A lot of clustered chondrocytes in articular cartilage is the pathological feature for osteoarthritis suffers cartilaginous degeneration; as for mild osteoarthritis, metabolism of chondrocyte is active thus cells will be clustered. Proliferation of chondrocyte induces chondrocyte close to surface layer to cluster in early stage of osteoarthritis [18]. Local clustered chondrocytes in this experiment verifies that uneven stress of cartilage indifferent areas in early stage of DDH will affect the mechanical biological environment of chondrocytes.
Aigner et al [19] found that there was an obvious proliferation of chondrocytes in osteoarthritis; and presumed that cartilage degeneration in osteoarthritis started from macromolecule protein synthesis changes, which reflected as synthesis changes of type II collagen protein and proteolytic enzyme, etc. In 1990, the first genetic mutation responsible for OA was reported after researchers found a high rate of OA with a mild chondrodysplasia in the family of a 44-year-old patient with degenerative changes of both hips. The mutation responsible for OA in this family occurred in the type II collagen gene, COL2A1 (collagen, type II, alpha 1.), which encodes a protein expressed almost exclusively in cartilage [20,21]. This mutation weakens the matrix and leads to premature degeneration of the cartilage [22].
Although a healthy adult’s articular cartilage has rich type II collagen, only little type II collagen mRNA expression in chondrocytes, which proves that anabolism of type II collagen in normal articular chondrocytes is weak. Because the expression of type II collagen mRNA in articular chondrocytes of osteoarthritis significantly increases, it indicates that injured chondrocytes may repair ECM with the extra synthesis of collagen [23]. Cellular metabolism is active in minor injured articular cartilage, gene expression of type II collagen and proteoglycans is higher than that in the control group. With the increment of gene expression, the synthesis of type II collagen increase also and meanwhile the deliquescence and denaturation of collagen increase obviously. Functions of chondrocytes will change and compound more matrix metalloproteinases to participate in deliquescence and denaturation of type II collagen, which will destroy the balance of ECM maintained by matrix components including type II collagen. Collagen total amount in cartilage during early and middle stage of osteoarthritis is up; the increment of synthesis of type II collagen in early stage shows that type II collagen is stained darker than the normal situation, especially around the chondrocyte cells and cytoplasm, the increment of collagen is larger than that of destroyed before cartilage reach the certain damage degree [24].
In this experiment, anachromasis of type II collagen in ECM of dysplastic acetabular cartilage suggested that more collagen in ECM; anachromasis of collagen in chondrocytes suggested that stronger synthesis of collagen. The Western-blot detected that the expression of type II collagen protein rose significantly, further explained that when DDH acetabular cartilage under continuous abnormal stress, chondrocytes were activated and cartilaginous degeneration started then synthesized many extra cellular matrix like collagen and other macromolecule protein to adapt this kind of stress changes. Olistherozones with blooming-effect were also visible around chondrocytes in this experiment, the cause of it lay in the changes of chondrocytes function and synthesis of matrix metalloproteinases would destroy matrix components like type II collagen around cells.
Morphology change of acetabular cartilage and abnormal expression of type II collagen in early stage of DDH model which generated under mechanical stress were observed in this experiment, suggesting that these degeneration changes may be a compensatory repair reaction of chondrocytes to environmental factors (such as the mechanical changes, cytokine, etc.). Once this compensatory repair reaction is decompensated, cartilage may appear inconvertible degeneration changes and present the syndrome of osteoarthritis. Therefore, the amount and intensity of II type collagen are probably interrelated with different cartilage degeneration degrees, furthermore, as a biological parameter that reflects cartilaginous degeneration to which degree osteoarthritis will occur has not yet studied.
Acknowledgements
We thank the pathological staff of Children’s Hospital of Fudan University, especially Pro. Chen Lain, for all their help completing this project.
Disclosure of conflict of interest
None.
References
- 1.Peled E, Eidelman M, Katzman A, Bialik V. Neonatal incidence of hip dysplasia: ten years of experience. Clin Orthop Relat Res. 2008;466:771–775. doi: 10.1007/s11999-008-0132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahan ST, Kasser JR. Does swaddling influence developmental dysplasia of the hip? Pediatrics. 2008;121:177–178. doi: 10.1542/peds.2007-1618. [DOI] [PubMed] [Google Scholar]
- 3.Kokavec M, Bialik V. Developmental dysplasia of the hip prevention and real incidence. Bratisl Lek Listy. 2007;108:251–254. [PubMed] [Google Scholar]
- 4.Yiv BC, Saidin R, Cundy PJ, Tgetgel JD, Aguilar J, McCaul KA, Keane RJ, Chan A, Scott H. Developmental dysplasia of the hip in South Australia in 1991: prevalence and risk factors. J Paediatr Child Health. 1997;33:151–156. doi: 10.1111/j.1440-1754.1997.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 5.Chan A, McCaul K, Cundy P, Haan E, Byron-Scott R. Perinatal risk factors for developmental dysplasia of the hip. Arch Dis Child Fetal Neonatal Ed. 1997;76:F94–F100. doi: 10.1136/fn.76.2.f94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel H Canadian Task Force on Preventive Health Care. Preventive health care, 2001 update: Screening and managementof developmental dysplasia of the hip in newborns. CMAJ. 2001;164:1669–1677. [PMC free article] [PubMed] [Google Scholar]
- 7.Synder M, Harcke HT, Domzalski M. Role of ultrasound in the diagnosis and management of developmental dysplasia of the hip: an international perspective. Orthop Clin North Am. 2006;37:141–147. doi: 10.1016/j.ocl.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Shimogaki K, Yasunaga Y, Ochi M. A histological study of articular cartilage after rotational acetabular osteotomy for hip dysplasia. J Bone Joint Surg Br. 2005;87:1019–1023. doi: 10.1302/0301-620X.87B7.15589. [DOI] [PubMed] [Google Scholar]
- 9.Greenhill BJ, Hainau B, Ellis RD, el-Sayed RM. Acetabular changes in an experimental model of developmental dysplasia of the hip (DDH) J Pediatr Orthop. 1995;15:789–793. doi: 10.1097/01241398-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Bo N, Peng W, Xinghong P, Ma R. Early cartilage degeneration in a rat experimental model of developmental dysplasia of the hip. Connect Tissue Res. 2012;53:513–520. doi: 10.3109/03008207.2012.700346. [DOI] [PubMed] [Google Scholar]
- 11.Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22:351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93:1–24. xv. doi: 10.1016/j.mcna.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Dudkiewicz I, Salai M, Ganel A, Blankstein A, Chechik A. Total hip arthroplasty in patients younger than 30 years of age following developmental dysplasia of hip (DDH) in infancy. Arch Orthop Trauma Surg. 2002;122:139–142. doi: 10.1007/s004020100307. [DOI] [PubMed] [Google Scholar]
- 14.Thomas SR, Wedge JH, Salter RB. Outcome at forty-five years after open reduction and innominate osteotomy for late-presenting developmental dislocation of the hip. J Bone Joint Surg Am. 2007;89:2341–2350. doi: 10.2106/JBJS.F.00857. [DOI] [PubMed] [Google Scholar]
- 15.Rubini M, Cavallaro A, Calzolari E, Bighetti G, Sollazzo V. Exclusion of COL2A1 and VDR as developmental dysplasia of the hip genes. Clin Orthop Relat Res. 2008;466:878–883. doi: 10.1007/s11999-008-0120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson JA. Prime factors in the etiology of congenital dislocation of the hip. J Bone Joint Surg (Br) 1963;49-B:268–283. [Google Scholar]
- 17.Michaeli DA, Murphy SB, Hipp JA. Comparison of predicted and measured contact pressures in normal and dysplastic hips. Med Eng Phys. 1997;19:180–186. doi: 10.1016/s1350-4533(96)00051-3. [DOI] [PubMed] [Google Scholar]
- 18.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2011;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, McKenna L. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2011;44:1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Alba-Kokko L, Baldwin CT, Moskowitz RW, Prockop DJ. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990;87:6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knowlton RG, Katzenstein PL, Moskowitz RW, Weaver EJ, Malemud CJ, Pathria MN, Jimenez SA, Prockop DJ. Genetic linkage and polymorphism in the type II procollagen gene (COL2A1) to primary osteoarthritis associated with mild chondrodysplasia. N Engl J Med. 1990;322:526–530. doi: 10.1056/NEJM199002223220807. [DOI] [PubMed] [Google Scholar]
- 22.Sandell LJ. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol. 2012;8:77–89. doi: 10.1038/nrrheum.2011.199. [DOI] [PubMed] [Google Scholar]
- 23.Aigner T, Stöss H, Weseloh G, Zeiler G, von der Mark K. Activation of collagen type II expression in osteoarthritic and rheumatoid cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:337–345. doi: 10.1007/BF02899701. [DOI] [PubMed] [Google Scholar]
- 24.Stoop R, Buma P, van der Kraan PM, Hollander AP, Billinghurst RC, Meijers TH, Poole AR, van den Berg WB. Type II collagen degradation in articular cartilage fibrillation after anterior cruciate ligament transection in rats. Osteoarthritis Cartilage. 2001;9:308–315. doi: 10.1053/joca.2000.0390. [DOI] [PubMed] [Google Scholar]


