Abstract
Berberine, an isoquinoline alkaloid originally isolated from the Chinese herb Coptischinensis, has been shown to display a wide range of pharmacological effects. The present study aims to investigate the effect of berberine on myocardial ischemia/reperfusion. Sixty male Sprague-Dawley rats were randomized equally into three groups: sham group, IR group, IR + berberine group. Rats were treated with berberine for 4 weeks and then I/R was performed. Myocardial infarction area was measured. Serum levels of creatine kinase isoenzyme (CK-MB), lactate dehydrogenase (LDH) and cardiac troponin I (cTnI) were assayed. Myocardial apoptosis was detected by terminal dexynucleotidyltransferase (TdT)-mediated dUTP nick end labeling (TUNEL). Mitochondrial function, including MMP and complex I activity, was assayed. Besides, the expression of Bcl-2, Bax and cytochrome c were detected by Western blot. Our results suggested that berberine decreased myocardial infarction area, and decreased serum levels of CK-MB, LDH and cTnI. Berberine attenuates myocardial apoptosis and improved mitochondrial dysfunction. Berberine up-regulates the expression of Bcl-2 and mitochondrial cytochrome c and down-regulates the expression of Bax and cytosolic cytochrome c. In conclusion, berberine protects the heart from ischemia/reperfusion injury via attenuating mitochondrial dysfunction and myocardial apoptosis.
Keywords: Berberine, myocardial ischemia/reperfusion injury, mitochondria dysfunction, apoptosis
Introduction
Ischemic heart disease (IHD) causes a high mortality and morbidity all around the world, and it will be the major cause of death in the world [1], and it is suggested that it will be the leading cause of death all around the world until 2020 [2]. During the process of IHD, ischemia reperfusion injury (IRI) is involved. And the effective treatment for IHD now is to reperfuse the heart. However, reperfusion leads to a serious of additional injuries, including myocardial apoptosis, mitochondrial dysfunction, calcium overload and reactive oxygen species (ROS) overproduction [3]. In addition, IRI is a pivotal cause of heart failure after myocardial infarction [4,5]. Mitochondria is the center of energy production. Ischemia triggers energy depletion, leading to mitochondrial dysfunction, which increases oxidative stress and induce apoptosis. In turn, oxidative stress aggravates IRI. The mitochondrial dysfunction causes a series of alterations in mitochondrial structure and function, such as mitochondrial permeability, the release of proapoptotic proteins, and uncoupling of mitochondrial oxidative phosphorylation [6]. Furthermore, this dysfunction causes myocardial apoptosis and necrosis. Therefore, attenuating mitochondrial dysfunction and apoptosis is of great clinical significance in the treatment of IRI.
Berberine (Ber), an isoquinoline derivative alkaloid isolated from Rhizoma Coptidis (Huanglian in Chinese), which has long been widely used in China and other Asian countries as a nonprescription oral drug to treat gastrointestinal infections [7]. It has been shown to exhibit a wide range of pharmacological activities, including antimicrobial, antidiarrheal, antidiabetic, antihyperlipidaemic, anti-inflammatory, and antiproliferative effects [8]. In addition, it is suggested that Ber has a wide array of beneficial effects on cardiovascular system [9]. Therefore, this study aims to investigate cardioprotective effect of Ber against IRI and to investigate its effect on mitochondrial dysfunction and apoptosis.
Materials and methods
Animals
Sixty adult male Sprague-Dawley rats (250-300 g) were purchased from the Center of Experimental Animal in the Hebei Medical University, China. Rats were fed under a pathogen-free condition at about 22°C on a 12 h light-dark cycle with free access to food and water. The experiments were performed in adherence with the National Institutes of Health Guidelines for the Use of Laboratory Animals and were approved by Hebei Medical University Committee on Animal Care.
Myocardial ischemia-reperfusion model and experimental protocol
Male Sprague-Dawley rats (250-300 g) were anesthetized i.p. with sodium pentobarbital (Sigma, St. Louis, USA, 40 mg/kg). Myocardial ischemia was produced by exteriorizing the heart with a left thoracic incision followed by a slipknot (4-0 silk) around the left anterior descending coronary artery (LAD). After 30 min of ischemia, the slipknot was released and the animal received 3 h of reperfusion. Sham-operated rats underwent the same surgical procedures with the exception of left anterior descending coronary artery occlusion.
The animals were orally gavaged with either saline or Ber (Sigma, St. Louis, MO, USA) at doses of 200 mg/kg body weight every day for 4 weeks. Rats were randomly assigned to three groups. There were 20 rats in each group: (1) Sham group (2) I/R group (3) I/R + Ber group.
Assay of myocardial infarct area
After reperfusion, coronary blood flow was again blocked and 1 ml of a 20 g/L Evans blue solution was injected by the rapid distribution of the right ventricle into the body. The heart was quickly removed to a -20°C refrigerator. The heart was cut into 1 mm slices, placed in 1% 2, 3, 5-triphenyltetrazolium chloride (TTC) solution, incubated for 15 min, and then fixed in 4% formaldehyde solution overnight. Evans blue stained area (blue staining, non-ischemic area), TTC stained area (red staining, ischemic area) and non-TTC stained area (white, infarct area) were analyzed with a digital imaging system by computer. Myocardial infarct area (infarct area/area at risk%, INF/AAR%) was then calculated.
Detection of creatine kinase-MB (CK-MB) and cardiac troponin I activity
After reperfusion, blood was taken from the carotid artery and was placed at room temperature for 30 min. Then, the serum was collected by centrifugation and placed at -70°C for preservation. According to manufacturer’s instruction, the CK-MB and cardiac troponinI assay kit were utilized to detect the serum CK-MB and cardiac troponinI activity.
Detection of lactate dehydrogenase (LDH) level
After reperfusion, blood was taken from the carotid artery and was placed at room temperature for 30 min. Then, the serum was collected by centrifugation and placed at -70°C for preservation. According to the manufacturer’s instruction, the CK assay kit was utilized to detect the serum LDH level.
Assay of myocardial apoptosis
Myocardial apoptosis was analyzed by terminal deoxynucleotidyltransferase UTP nick end labeling (TUNEL) assay. Terminal deoxynucleotidyl transferase UTP nick end labeling was performed using an in situ cell death detection kit (Roche Molecular Biochemicals, Mannheim, Germany). Briefly, 50 μL TUNEL reaction mixture were added on each sample, and the slides were incubated in humidified atmosphere for 60 min at 37°C in the dark and then rinsed with PBS (pH 7.4) three times with 5 min for each time. To detect the nuclei, the slides were incubated with DAPI for 5 min at room temperature in the dark, rinsed with PBS three times with 5 min for each time, and observed with a fluorescence microscopy. Apoptosis was determined as the number of TUNEL-positive cells/the total number of cells × 100%.
Isolation of mitochondria and cytosolic fraction
The samples were collected and homogenized in an ice-cold isolation buffer (0.25 mM sucrose, 1 mM K-EDTA, 10 mM Tris-HCl, pH 7.4). The homogenate was immediately centrifuged at 2000 g for 3 min, then the supernatant was centrifuged again at 2000 g for 3 min, and the second supernatant was decanted and centrifuged at 12,000 g for 8 min. The supernatant was discarded, and the pellet was resuspended in isolation buffer without K-EDTA. Then, the suspension was centrifuged at 12,000 g for 10 min. The resulting brown mitochondrial pellet was resuspended in the same buffer. The supernatant was the cytosolic fraction.
Measurement of mitochondrial membrane potential (MMP)
As described previously [10], MMP was determined using JC-1. Mitochondria samples (0.5 mg/mL, 1 mL) were incubated with 19 mL JC-1 staining buffer according to the manufacturer’s instructions (Sigma-Aldrich). At the end of the experiments, valinomycin was added as a negative control. Fluorescent intensity was determined at 37°C in a fluorescence spectrophotometer (Tecan, Mannedorf, Switzerland). The ratio of aggregates (red in the Web version, 590 nm) to monomer (green in the Web version, 525 nm) was calculated as an indicator of MMP.
Mitochondrial complex I activity assay
As described previously, mitochondrial complex I was measured spectrophotometrically [11]. The reaction mixture contained 0.2 M glycyl glycine buffer pH 8.5, 6 mM NADH in 2 mM glycyl glycine buffer, and 10.5 mM cytochrome c. The reaction was triggered with the adding of requisite amount of solubilized mitochondrial sample and followed absorbance change at 550 nm for 2 min.
Western blot
Myocardial samples were lysed in lysis buffer on ice for 30 min, and the lysates were clarified by centrifugation at 4°C for 20 min at 10,000 rpm. After quantitation of protein concentration, 30 μg of total protein was separated by 10% SDS-PAGE and then transferred to nitrocellulose membranes (Millipore, USA). The membranes were blocked for 30 min at 37°C with 5% non-fat dry milk, then incubated with primary antibody including Bcl-2, Bax, cytochrome c, β-actin, (Cell Signaling Technology, USA) (1:1,000) over night at 4°C. After three washings with TBST, the membranes were incubated with secondary antibody in TBST solution for 30 min at 37°C, then, washed as above. The positive protein bands were developed using a chemiluminescent system, and the bands were scanned and quantified by densitometric analysis using an image analyzer Quantity One System (Bio-Rad, Richmond, CA, USA).
Statistical analysis
Data is presented as means ± SD. The significance of differences among groups was evaluated by a Student’s t-test for unpaired data or Dunnett’s t-test for multiple comparisons preceded by one-way analysis of variance (ANOVA) (SPSS 13.0, Chicago, IL, USA). For all test, P < 0.05 was considered as statistically significant.
Results
Effect of berberine on the myocardial infarction area
I/R induced a dramatic infarction area. Compared with the I/R group, berberine reduced myocardial infarction area significantly (P < 0.05) (Figure 1).
Figure 1.
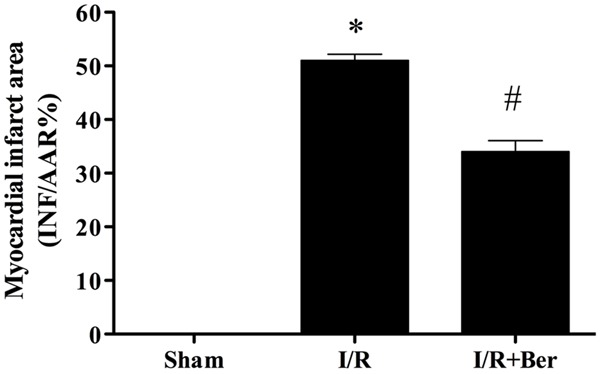
Effect of berberine on the myocardial infarction area. Data were expressed as mean ± SD (n = 10 in each group). *P < 0.01 versus the sham group, #P < 0.05 versus I/R group.
Effect of berberine on the activity of serum CK, LDH and cTnI activity
As shown in Figure 2, the activity of CK, LDH and cTnI increased significantly in the I/R group compared with the sham group. CK, LDH and cTnI activity decreased significantly in the I/R + berberine group compared with the I/R group (P < 0.05).
Figure 2.
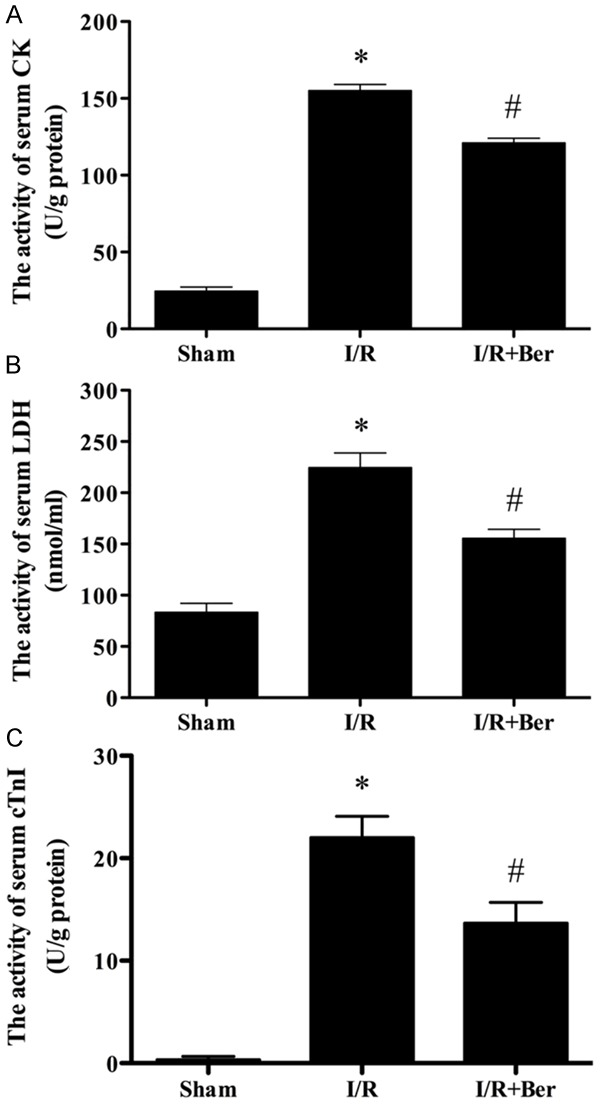
The comparison of CK, LDH and cTnIactivity in each group. The activity of CK, LDH and cTnI in the sham group was relatively lower, while the activity of them in I/R group was significantly increased, which was attenuated by berberine. Data were expressed as mean ± SD (n = 10 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus I/R group.
Effect of berberine on myocardial apoptosis following I/R
As shown in Figure 3, I/R triggered a significant increase of TUNEL positive nuclei in the hearts compared with the sham group (P < 0.05). Berberine treatment dramatically attenuated apoptosis.
Figure 3.
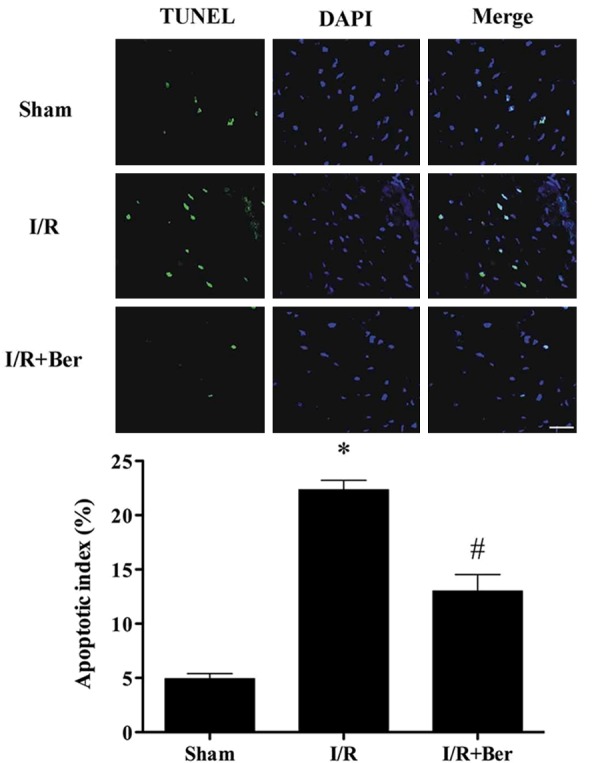
Berberine attenuates myocardial apoptosis following I/R. Representative photomicrographs of in situ detection of apoptosis by terminal deoxynucleotidyltransferasedUTP nick end labeling (TUNEL) staining. Data was expressed as mean ± SD (n = 6 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus I/R group.
Effect of berberine on mitochondrial dysfunction following I/R
Compared with the I/R group, berberine treatment significantly increased the MMP (Figure 4A, P < 0.05), complex I activity (Figure 4B, P < 0.05), and mitochondrial cytochrome c expression (Figure 4C, P < 0.05). Berberine also decreased cytosolic cytochrome c expression (Figure 4C, P < 0.05).
Figure 4.
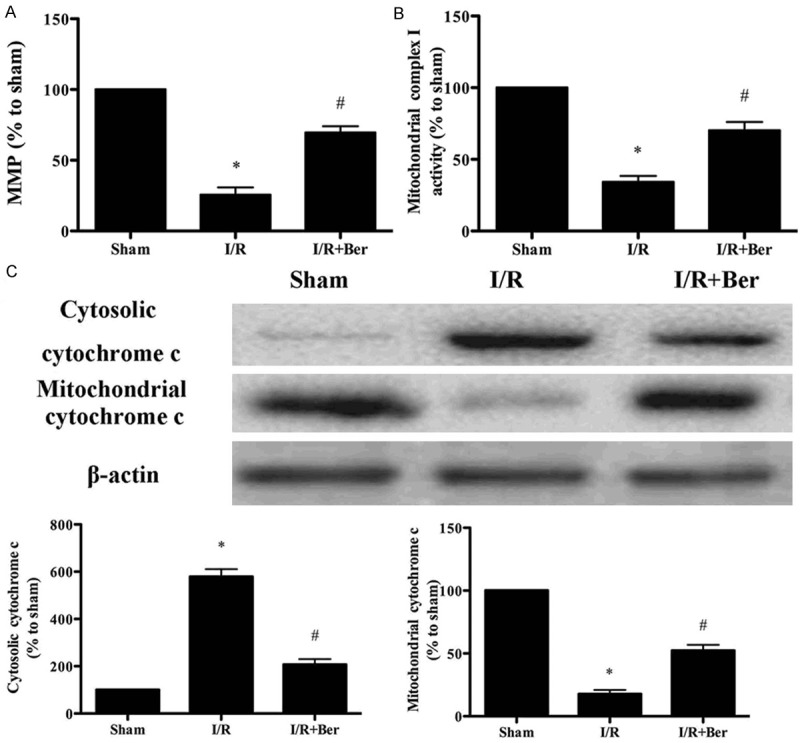
Berberine improved mitochondrial dysfunction following I/R. A. MMP level. B. Mitochondrial complex I activity. C. The expression of mitochondrial cytochrome c and cytosolic cytochrome c detected by Western blot. Data was expressed as mean ± SD (n = 10 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus I/R group. MMP, mitochondrial membrane potential.
Effect of berberine on the expression of Bax and Bcl-2
As shown in Figure 5, berberine treatment dramatically decreased Bax expression and increased Bcl-2 expression compared with the I/R group (P < 0.05).
Figure 5.
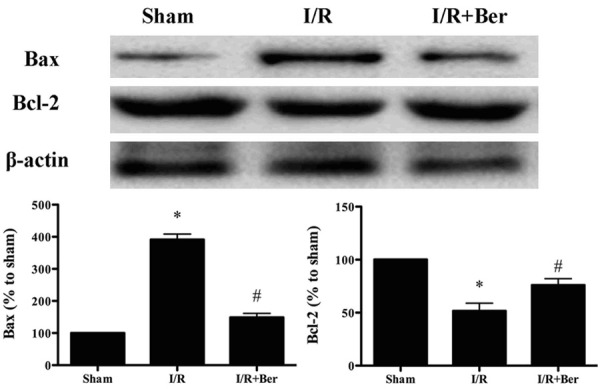
Effects of berberine on the expression of Bcl-2 and Bax. Data was expressed as mean ± SD (n = 10 in each group). *P < 0.05 versus the sham group, #P < 0.05 versus I/R group.
Discussion
In the present study, we provide evidence that berberine protects the heart against I/R injury as evidenced by its improving cardiac function, reducing myocardial apoptosis and attenuating mitochondrial dysfunction.
Berberine is an alkaloid from the coptischinensis species (Huanglian), and it has a long history for treating diarrhea in traditional Chinese medicine. An accumulating number of studies suggest that berberine has a wide variety of biological effects, including anti-tumor and cardiovascular-protective actions [9,12]. It is suggested that berberine improved cardiac function in patients with severe congestive heart failure [13,14]. In addition, Lv, et al. found that berberine could inhibit doxorubicin-triggered cardiomyocyte apoptosis [15]. Our results suggest that berberine treatment for four weeks improved the myocardial infarction and the injury of the cardiomyocytes, as indicated by the amelioration of CK-MB, LDH and cTnI in the serum.
The mechanisms underlying I/R injury is multifactorial, and apoptosis is one of the most important mechanisms. Recent studies have shown that inhibiting myocardial apoptosis will slow down or even prevent heart failure induced by I/R injury [16,17]. Apoptosis is positively and negatively regulated by the Bcl-2 protein family. Proapoptotic proteins include Bax, Bak, Bcl-XS, Bad, Bid, Bik, and Bim, etc., whereas antiapoptotic proteins include Bcl-2, Bcl-XL, Bcl-w, Mcl-1 and A1/Bfl-1 [18]. It has been suggested that Bax translocation from the cytosol to mitochondria results in cytochrome c release from the mitochondria. While Bcl-2 can interfere with mitochondrial cytochrome c release and suppress apoptosis progression [19]. In this study, we found that berberine inhibited apoptosis in ischemic/reperfused heart, which is supported by TUNEL assay, up-regulated Bcl-2 expression and down-regulated Bax expression.
In addition, mitochondrial dysfunction is involved in myocardial I/R injury [20]. And we tested the mitochondrial function in our study. The results indicate that the mitochondrial MMP is improved due to berberine pretreatment, which is accompanied by significantly elevated mitochondrial complex I activity, suggesting that the mitochondrial dysfunction caused by I/R was significantly attenuated. Furthermore, we assay the expression of cytochrome c both in the mitochondria and cytoplasm. The results suggest that berberine increases mitochondrial cytochrome c and decreases cytosolic cytochrome c. Cytochrome c released from the mitochondria can trigger apoptosis via mitochondrial pathway [18]. Our results indicate that berberine can prevent the release of cytochrome c from the mitochondria, leading to the reduction of following apoptosis.
In summary, our results demonstrate that berberine exerts cardioprotection via preventing apoptosis and ameliorating mitochondrial dysfunction following I/R. The findings suggest that berberine might be a potential drug in the treatment and prevention of ischemic heart disease.
Disclosure of conflict of interest
None.
References
- 1.Booth EA, Lucchesi BR. Estrogen-mediated protection in myocardial ischemia-reperfusion injury. Cardiovasc Toxicol. 2008;8:101–113. doi: 10.1007/s12012-008-9022-2. [DOI] [PubMed] [Google Scholar]
- 2.Lu MJ, Chen YS, Huang HS, Ma MC. Hypoxic preconditioning protects rat hearts against ischemia-reperfusion injury via the arachidonate12-lipoxygenase/transient receptor potential vanilloid 1 pathway. Basic Res Cardiol. 2014;109:414. doi: 10.1007/s00395-014-0414-0. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Wang Y, Wei C, Bai S, Zhao Y, Li H, Wu B, Wang R, Wu L, Xu C. Mediation of exogenous hydrogen sulfide in recovery of ischemic post-conditioning-induced cardioprotection via down-regulating oxidative stress and up-regulating PI3K/Akt/GSK-3beta pathway in isolated aging rat hearts. Cell Biosci. 2015;5:11. doi: 10.1186/s13578-015-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105:151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 5.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 6.Li T, Zhang P, Liu J, Zhou R, Li Q, You Z, Dian K. Protective effects of hemoglobin-based oxygen carrier given to isolated heart during ischemia via attenuation of mitochondrial oxidative damage. Free Radic Biol Med. 2010;48:1079–1089. doi: 10.1016/j.freeradbiomed.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Wang N, Zhao L, Lu F. Berberine in the treatment of type 2 diabetes mellitus: a systemic review and meta-analysis. Evid Based Complement Alternat Med. 2012;2012:591654. doi: 10.1155/2012/591654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao X, Zhang J, Tong N, Liao X, Wang E, Li Z, Luo Y, Zuo H. Berberine attenuates doxorubicin-induced cardiotoxicity in mice. J Int Med Res. 2011;39:1720–1727. doi: 10.1177/147323001103900514. [DOI] [PubMed] [Google Scholar]
- 9.Affuso F, Mercurio V, Fazio V, Fazio S. Cardiovascular and metabolic effects of Berberine. World J Cardiol. 2010;2:71–77. doi: 10.4330/wjc.v2.i4.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye R, Kong X, Yang Q, Zhang Y, Han J, Zhao G. Ginsenoside Rd attenuates redox imbalance and improves stroke outcome after focal cerebral ischemia in aged mice. Neuropharmacology. 2011;61:815–824. doi: 10.1016/j.neuropharm.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Gaur V, Aggarwal A, Kumar A. Protective effect of naringin against ischemic reperfusion cerebral injury: possible neurobehavioral, biochemical and cellular alterations in rat brain. Eur J Pharmacol. 2009;616:147–154. doi: 10.1016/j.ejphar.2009.06.056. [DOI] [PubMed] [Google Scholar]
- 12.Lau CW, Yao XQ, Chen ZY, Ko WH, Huang Y. Cardiovascular actions of berberine. Cardiovasc Drug Rev. 2001;19:234–244. doi: 10.1111/j.1527-3466.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 13.Marin-Neto JA, Maciel BC, Secches AL, Gallo Júnior L. Cardiovascular effects of berberine in patients with severe congestive heart failure. Clin Cardiol. 1988;11:253–260. doi: 10.1002/clc.4960110411. [DOI] [PubMed] [Google Scholar]
- 14.Zeng XH, Zeng XJ, Li YY. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;92:173–176. doi: 10.1016/s0002-9149(03)00533-2. [DOI] [PubMed] [Google Scholar]
- 15.Lv X, Yu X, Wang Y, Wang F, Li H, Wang Y, Lu D, Qi R, Wang H. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PLoS One. 2012;7:e47351. doi: 10.1371/journal.pone.0047351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 2007;568:213–221. doi: 10.1016/j.ejphar.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 17.Song JQ, Teng X, Cai Y, Tang CS, Qi YF. Activation of Akt/GSK-3beta signaling pathway is involved in intermedin (1-53) protection against myocardial apoptosis induced by ischemia/reperfusion. Apoptosis. 2009;14:1061–1069. doi: 10.1007/s10495-009-0382-2. [DOI] [PubMed] [Google Scholar]
- 18.Shen M, Wu RX, Zhao L, Li J, Guo HT, Fan R, Cui Y, Wang YM, Yue SQ, Pei JM. Resveratrol attenuates ischemia/reperfusion injury in neonatal cardiomyocytes and its underlying mechanism. PLoS One. 2012;7:e51223. doi: 10.1371/journal.pone.0051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res. 2002;62:4592–4598. [PubMed] [Google Scholar]
- 20.Bulteau AL, Lundberg KC, Ikeda-Saito M, Isaya G, Szweda LI. Reversible redox-dependent modulation of mitochondrial aconitase and proteolytic activity during in vivo cardiac ischemia/reperfusion. Proc Natl Acad Sci U S A. 2005;102:5987–5991. doi: 10.1073/pnas.0501519102. [DOI] [PMC free article] [PubMed] [Google Scholar]


