Abstract
The study was conducted to evaluate the toxicity and safety pharmacology of the traditional Chinese medicine, “Huhezi” granules. The results of acute toxicity test showed that the granules’ LD50 was more than 5000 mg/kg, which indicated that the “Huhezi” belonged to actually non-toxic drug. Subchronic toxicity study showed that non-toxic reaction were detected in high (1000 mg/kg), medium (500 mg/kg) and low dose (250 mg/kg) of “Huhezi” groups by measuring rat body weight, organ coefficient, blood physiological indexes and blood biochemical indexes. Pathological examination showed that no tissue lesions were observed in test organs except liver (mild granular degenerationand reversible vesicular degeneration), spleen (Langerhans cells infiltrating) and kidney (homogeneous red staining of renal tubule). Safety pharmacology study found that “Huhezi” had no effects on the central nervous system, respiratory system and cardiovascular system. These results suggested that the dose of “Huhezi” at or below 1000 mg/kg through oral administration is considered safe.
Keywords: “Huhezi” granules, acute toxicity, subchronic toxicity, safety pharmacology, rats
Introduction
Pullorosis is an infectious disease caused by salmonella pullorosis and has occurred in all parts of the world. The chicks of 2-3 weeks are most likely to have this disease [1,2]. When the disease occurred in chicks, clinical symptoms involved breathing hard, diarrhea and even blind on ill chicks [1-4]. The treatment of this disease around the world were given antibiotic drugs, but some researches showed it was easier to produce certain resistance if penicillin, spectinomycin and trimethoprim were used [3,4].
The traditional Chinese medicine “Huhezi” granules as a new formula was composed of extracts from PoLygonum Cuspidatum, Terminalia chebula and Violet yedoensis. It is showed that high, medium and low doses of “Huhezi” granules had certain protective effects on pullorosis salmonella infection [5]. Currently, there were no reports regarding the toxicity of “Huhezi”. The toxicity and safety pharmacology was evaluated in this study for the purpose of assessing the optimal dose of “Huhezi” for clinical use [6,7].
Materials and methods
The compound traditional Chinese medicine
Traditional Chinese medicine formula “Huhezi” granules (batch number: 130481) was made in Natural medicine research center, Sichuan agricultural university (Chengdu, China). “Huhezi” was composed of extracts of PoLygonum Cuspidatum, Terminalia chebula and Violet yedoensis (w/w/w, 3:2:1). The PoLygonum Cuspidatum (Sichuan, batch number: 081102), Terminalia chebula (Yunnan, batch number: 090401), Violet yedoensis (Sichuan, batch number: 110502) were bought in HuiMing hall pharmacy (Ya’an, Sichuan).
Experimental animals
Male and female Sprague-Dawley (SD) rats, 4- and 8-weeks old, were purchased from specific pathogen-free (SPF) facility at Chengdu Dossy Experimental Animals Co., Ltd. [License No. SCXK (Sichuan) 2008-24]. Based on the Guidelines of the International Committee on Laboratory Animals, they were maintained in environmentally controlled rooms at 20-25°C with a relative humidity of 55±5% and 12-15 air changes/h under a 12 h light-dark cycle (artifcial lighting from 08:00 to 20:00). Animals were separated according to gender and were housed in well ventilated sterile polypropylene cages with bedding throughout the study period. They were treated with standard rat chow from Nuvital Nutrients (Colombol/PR, Brazil) and given free access to distilled water ad libitum. All rats were allowed to adjust to the new environment for 7 days before the study started.
Oral acute toxicity
According to the previous reports [5], 16 healthy adult rats (male and female half, weight between 140-160 g) were administrated with “Huhezi” at a dose of 5000 mg/kg by gavage. The rats were fasted for 16 h prior to administration. Experimental animals were observed a week. The observation focused on mortality, behavioral neurologic, autonomic and toxic effects. At the end of the 7 days, mortality was expressed as an LD50 value estimated according to the Karber’s method [8].
30-day subchronic oral toxicity
Treatments
The determination of repeated dose 30-day oral toxicity was carried out according to OECD guideline 603. Forty SD rats were distributed in 4 groups of 10 animals each (5 female and 5 male). According to the acute toxicity and limit test, the four groups are as follows: saline control group (Group I), 250 mg/kg group (Group II) as the low-dose group, 500 mg/kg group (Group III) as the middle-dose group, 1000 mg/kg group (Group IV) as the high-dose group. Animals were treated daily at 6 p.m by gavage one a day for successive 30 days and observed once daily to detect signs of toxicity. The administered volume of “Huhezi” or saline was 1 mL/100 g body weight. Prior to administration, animals were marked, fasted overnight (animals had water but no food) and weighed. During the 30 days, the animals were monitored for clinical and behavioral symptoms such as diarrhea, immobility, and mortality. Twenty-four hours after the last administration, animals were euthanized under ether anesthesia after a 12 h overnight fasting.
Mortality and clinical signs
During the test, the condition and behavior of all animals was checked twice daily before and after dosing. The changes of animals’ fur, eyes, mucous membrane, respiratory system, central nervous system, physical activity, behavior, mortality (if any) were recorded. In order to reduce the residual interaction between the animals and postmortem tissue autolysis, the dead and endangered animals were dissected timely [9].
Bodyweight, food and water consumption
Animals were weighed on the frst day of dosing, once a week thereafter (with intervals of 7±1 days). Food consumption was also recorded once before onset of dosing and approximately once a week thereafter. Water intake was monitored daily during the experimental period.
Hematological assay
The blood samples, about 0.5 mL each for hematology assessments, were collected in a pre-calibrated tube containing sodium citrate. The hematological parameters included white blood cell count (WBC), red blood cell count (RBC), hemoglobin concentration (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), MCH concentration (MCHC), platelet count (PLT), and leukocyte differential count (lymphocytes, neutrophils, and monocytes).
Serum biochemistry
Blood samples were collected into non-heparinized tubes for separation of serum and biochemical analysis. The solidified blood samples in non-heparinized tubes were centrifuged at 3500 rpm (15 min at 4°C) and the supernatant (serum) was collected. The clinical chemistry parameters included Albumin (ALB), total protein (TP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), Alkaline phosphatase (ALP), urea nitrogen (BUN), creatinine (CRE), glucose (GLU), Calcium (Ca), Phosphorus (P), Total bilirubin (TBIL), Total cholesterol (CHO).
Organ coefficient
The absolute weight of the heart, liver, spleen, lung, kidney, stomach, intestines, ovary and testis were measured and their relative organ weight (percentage of body weight) was calculated.
Pathology
Terminal necropsy
In the end of the treatments, all animals were euthanized under ether anesthesia, following a detailed external and internal examination (i.e., the color and integrity of the skin and hair, the changes of all the tissues and the shape of abdomen). all tissues (i.e., Kidney, spleen, liver, heart, lung, testis and ovaries) from each animal were taken and weighed immediately. The tissues and organs were procured, preserved in 10% neutral buffered formalin, and processed for histopathological assessment.
Histopathological examination
The preserved organs and tissues of rats from each group were subjected to histological examination. They were pressed in a fixation medium of 10% solution of buffered formalin (pH 7.4) followed by dehydration and then enclosed in paraffin. The tissues were sectioned and stained with hematoxylin and eosin (H&E) prior to microscopic examination.
Safety pharmacology assay
Treatment
Forty SD rats were distributed in 4 groups of 10 animals each (5 female and 5 male). The four groups were as follows: saline control group (Group I), 250 mg/kg group (Group II), 500 mg/kg group (Group III), 1000 mg/kg group (Group IV). Each rat was smeared in the back skin with the volume of 0.3 mL for 5 days.
Central nervous system assay
At the last day of the treatments, the general behavior, posture, the changes of gait and pupil changes of every rat were closely observed within 4 hours after the last administration [10,11]. Rats with or without salivation, muscle trembling were also recorded. The rats’ situations were observed daily for 7 days after the last administration. The independent activities of rats were observed and recorded by the versatile recorder of rats’ locomotor activity at 0.5 h after the last administration. Each rat was also tested by the pole test at 0.5 h after the last administration. The rats were placed in the top of a rod which was fixed on the base. Then, each rat was crawling down and rated according to the following criteria:
0: Step by step to climb down; 1: Downward side; 2: Unable to grasp the stick; 3: Loss of righting reflex.
Heart rate and respiratory rate assay
The heart rate and respiratory rate of rats in each group were measured by using BL-420F (multi-channel physiological signal acquisition processing system) to record the electrocardiogram when the rats were anesthetized by ether before administration, at the end of the second administration and a week after the last administration [10,11].
Statistical analysis
Means and standard deviations were calculated for measurement data in each group, which contained body weight, food and water consumption, clinical pathological data, organ weights, heart rates and respiratory rates. The statistical significance was compared between control and experimental groups by one way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test. The software is a completely random design, individual difference is not considered. Just one factor is considered to find whether there are obvious difference between control and experimental groups.
Results
Acute toxicity study
After treatment with a single oral dose of “Huhezi” over 5000 mg/kg, the rats appeared lassitude temporarily, but back to normal after 1 h. During a week, drinking water, ingestion, general action, secretion and excreta were normal. The rats grew well, no symptoms of poisoning and death in rats, which means that LD50 was more than 5000 mg/kg and the granules is the actual non-toxic drug [8].
Subchronic toxicity study
Effect on body weight and Clinical observations
No groups had deaths. The color, appearance, behavior, feces, urine of rats were normal during the experimental period. The body weight of rats in each group increased to varying degrees. The results showed the male rats in the low-dose, medium-dose and high-dose groups were higher than the saline control group, indicating “Huhezi” granules may have an impact on growth in male. There were no statistical significance in weight gain of all the female rats. The results were shown in Figure 1. There were no significant differences in water consumption (Table 1) among all the groups. However, food consumption increased in male rats after treatment at a dose of 500 mg/kg bw∙day. In other groups, no significant differences of food consumption were detected.
Figure 1.
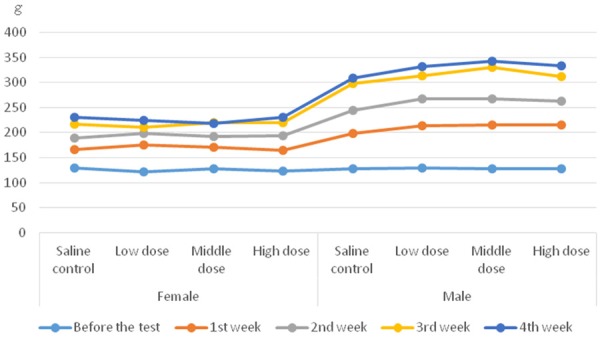
Effect of subchronic administration of “Huhezi” granules on body weight of male and female rats.
Table 1.
Mean food and water consumption of SD rats orally administered “Huhezi” granules
| Group | Food Consumption (g/rat/day) | Water Consumption (ml/rat/day) | |||
|---|---|---|---|---|---|
|
|
|
||||
| Male | Female | Male | Female | ||
| Saline control | 28.27 | 21.3 | 35.76 | 28.32 | |
| Low dose | 27.11 | 20.93 | 32.49 | 19.89 | |
| Middle dose | 29.16 | 21.25 | 36.36 | 27.99 | |
| High dose | 30.41 | 22.59 | 37.05 | 32.39 | |
Organ coefficient
Twenty-four hours after the last administration, animals were euthanized under ether anesthesia after fasting for 12 h. The absolute weight of the heart, liver, spleen, lung, kidney, stomach, intestines, ovary and testis were measured and their relative organ weight (percentage of body weight) was calculated (Table 2). Compared with the saline control group, the organ coefficient of spleen in the middle-dose group was slightly higher, which indicated the medicine had effected on the immune system. There were no significant differences in other organ coefficients among all the groups.
Table 2.
Effect of subchronic administration of “Huhezi” granules on terminal body weight and organic coefficient (g/100 g) in grams of male and female rats
| Group | Gender | Heart (g/100 g) | Liver (g/100 g) | Spleen (g/100 g) | Lung (g/100 g) | Kidney (g/100 g) | Ovaries or Testes (g/100 g) |
|---|---|---|---|---|---|---|---|
| High dose | F | 0.38±0.081 | 3.25±0.433 | 0.25±0.049 | 0.60±0.075 | 0.76±0.079 | 0.07±0.009 |
| M | 0.43±0.073 | 3.63±0.318 | 0.25±0.008 | 0.57±0.044 | 0.82±0.023 | 1.01±0.207 | |
| Middle dose | F | 0.36±0.036 | 3.48±0.234 | 0.24±0.020 | 0.57±0.056 | 0.78±0.043 | 0.07±0.003 |
| M | 0.34±0.029 | 3.77±0.204 | 0.32±0.017* | 0.54±0.041 | 0.85±0.057 | 0.93±0.159 | |
| Low dose | F | 0.40±0.054 | 3.48±0.290 | 0.23±0.007 | 0.59±0.057 | 0.76±0.082 | 0.08±0.000* |
| Saline control | M | 0.39±0.043 | 3.61±0.238 | 0.26±0.012 | 0.56±0.039 | 0.84±0.026 | 1.11±0.018 |
| F | 0.43±0.061 | 3.56±0.170 | 0.26±0.017 | 0.53±0.007 | 0.80±0.010 | 0.06±0.008 |
Notice: The statistical significance is indicated with “*” in the column “high dose”, “middle dose” and “low dose” when comparing data of dose group with the saline group
means significant difference. P<0.05.
Hematology parameters
The hematological changes including HGB, RBC, WBC, GRA, LYM, MID and PLT were shown in Table 3. The three “Huhezi” groups in the PLT values were obviously higher than the saline control group, which suggested that “Huhezi” granules might make influence in the number of platelets in rats. HGB values were slightly decreased in the low-dose and middle-dose groups when compared with the saline control group. LYM values in the high-dose group were a little higher than the saline control group, indicating that there may be an inflammatory reaction. In other hematology parameters, there were no significant differences.
Table 3.
Effect of subchronic administration of “Huhezi” granules on Hematology parameters
| Group | High dose 1000 mg/kg | Middle dose 500 mg/kg | Low dose 250 mg/kg | Saline control |
|---|---|---|---|---|
| WBC, 109/L | 11.70±3.25 | 9.55±1.06 | 10.03±4.39 | 8.36±2.07 |
| RBC, 1012/L | 7.83±0.23 | 7.45±0.25 | 7.71±0.50 | 7.75±0.51 |
| HGB, g/L | 171.83±5.67 | 165.83±8.18* | 166.17±3.37* | 176.33±7.45 |
| PLT, 109/L | 931.67±110.41* | 875±141.89* | 942.17±73.94* | 649.33±99.91 |
| LYM, 109/L | 10.32±3.09* | 7.91±1.17 | 8.50±3.89 | 6.98±1.74 |
| MID, 109/L | 0.19±0.10 | 0.23±0.20 | 0.18±0.15 | 0.15±0.11 |
| GRA, 109/L | 1.19±0.50 | 1.42±0.53 | 1.34±0.67 | 1.22±0.39 |
Notice: The statistical significance is indicated with “*” in the column “high dose”, “middle dose” and “low dose” when comparing data of dose group with the saline group
means significant difference. P<0.05.
Serum biochemistry
The changes of serum biochemistry parameters in each group were shown in Table 4. The values of ALT in the three “Huhezi” groups were in the normal range, but differences were observed in comparison with the saline control group. The values of ALT may be related to liver function. Therefore, the “Huhezi” has certain influence on the liver.
Table 4.
Effect of subchronic administration of “Huhezi” on serum biochemistry parameters
| Group | High dose 1000 mg/kg | Middle dose 500 mg/kg | Low dose 250 mg/kg | Saline control |
|---|---|---|---|---|
| ALB (g/L) | 30.53±2.43 | 31.10±1.99 | 26.68±9.48 | 30.53±2.20 |
| ALP (U/L) | 227.00±59.85 | 202.33±79.56 | 214.00±68.96 | 199.83±21.01 |
| ALT (U/L) | 34.67±4.08* | 36.17±12.97* | 37.00±3.74* | 25.17±6.62 |
| AST (U/L) | 126.00±16.64 | 114.67±22.61 | 126.67±12.85 | 134.67±13.81 |
| BUN (mmol/L) | 5.29±1.44 | 5.92±0.68 | 3.83±1.47 | 4.74±0.77 |
| Ca (mmol/L) | 2.30±0.20 | 2.27±0.26 | 7.12±7.92* | 2.24±0.10 |
| CHO (mmol/L) | 2.07±0.27 | 2.04±0.47 | 3.77±1.65* | 2.02±0.36 |
| CRE (umol/L) | 48.50±8.64 | 48.50±3.99 | 53.17±26.29 | 44.50±4.93 |
| GLU (mmol/L) | 5.54±1.25 | 6.22±0.81 | 6.86±2.09 | 5.32±0.96 |
| P (mmol/L) | 2.29±0.18 | 2.39±0.21 | 1.63±0.56* | 2.15±0.10 |
| TBIL (umol/L) | 4.83±0.69 | 4.49±1.60 | 6.51±2.33 | 7.78±4.70 |
| TP (g/L) | 62.12±5.63 | 62.35±4.81 | 63.48±5.71 | 60.47±3.27 |
Notice: The statistical significance is indicated with “*” in the column “high dose”, “middle dose” and “low dose” when comparing data of dose group with the saline group
means significant difference. P<0.05.
Pathological examination
Anatomy
After the animals were euthanized and dissected, compared with the normal groups, no lesions were observed except some bleeding points in lungs. 10% neutral buffered formalin was used to preserve the tissues.
Histopathological analysis
The results of histopathological studies including heart, liver, spleen, lung, kidney, stomach, intestine, ovary and testis were shown in Figures 2, 3, 4 and 5.
Figure 2.
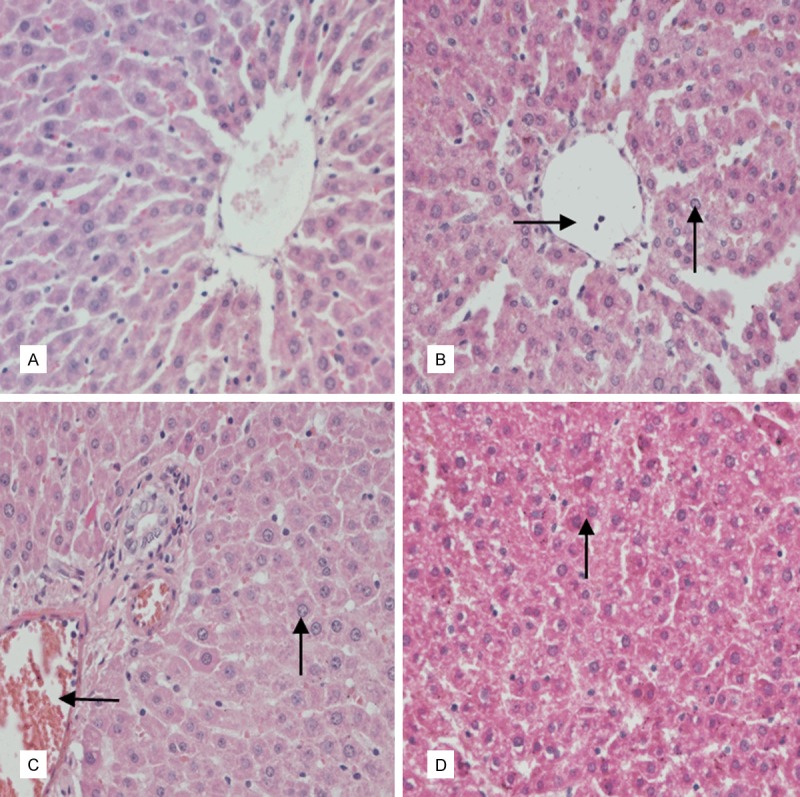
According to the (A) saline control group of liver, structure of liver tissue was normal. (B) Low-dose group of the liver: There was vacuolar degeneration and granular degeneration (black up arrow), and inflammatory cells appeared in the central vein (black rightward arrow). (C) Medium-dose group of the liver: There was mild vacuoles degeneration (black up arrow), and slight hemolysis phenomenon (black leftward arrow). (D) High-dose group of the liver: There was vesicular degeneration (black up arrow). (HE, 400×).
Figure 3.
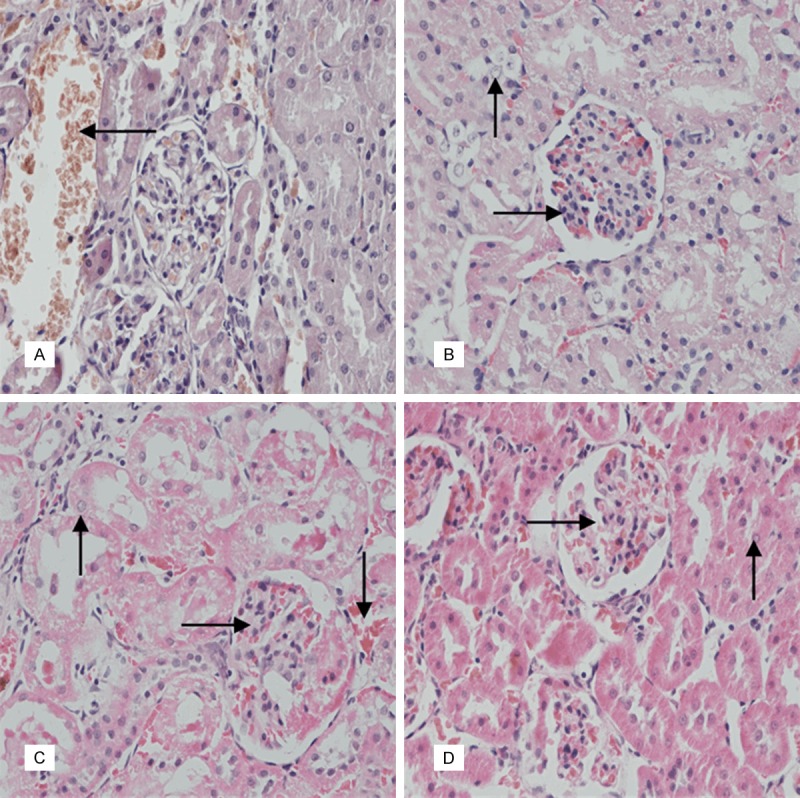
According to the (A) saline control group of kidney, It’s normal, hemolysis phenomenon exists in the interstitial capillary (→). (B) Low-dose group of kidneys: Congestion occurred in the glomerular capillary, renal tubular epithelial cells vacuoles degeneration (→). (C) Medium-dose group of the kidney: Congestion occurred in renal tissue capillary, renal epithelial cells appeared granular degeneration, renal tubular was in homogeneous red staining (→). (D) High-dose group of kidney There was granular degeneration in epithelial cells of the glomerulus, congestion occurred in the glomerular capillary (→). (HE, 400×).
Figure 4.
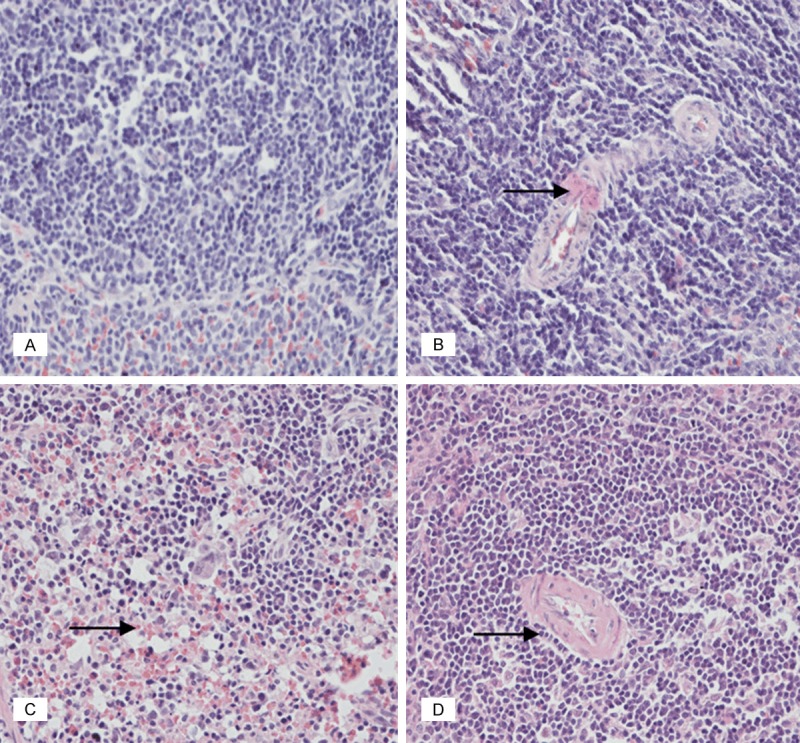
According to the (A) saline control group of spleen, there was normal lymph node structure, macrolymphocyte and small lymphocyte. (B) Low-dose group of spleen. (C) Medium-dose group of spleen. (D) High-dose group of the spleen had normal lymph node structure, and with the increase of drug concentration, there were more Langerhans cells (black rightward arrow). (HE, 400×).
Figure 5.
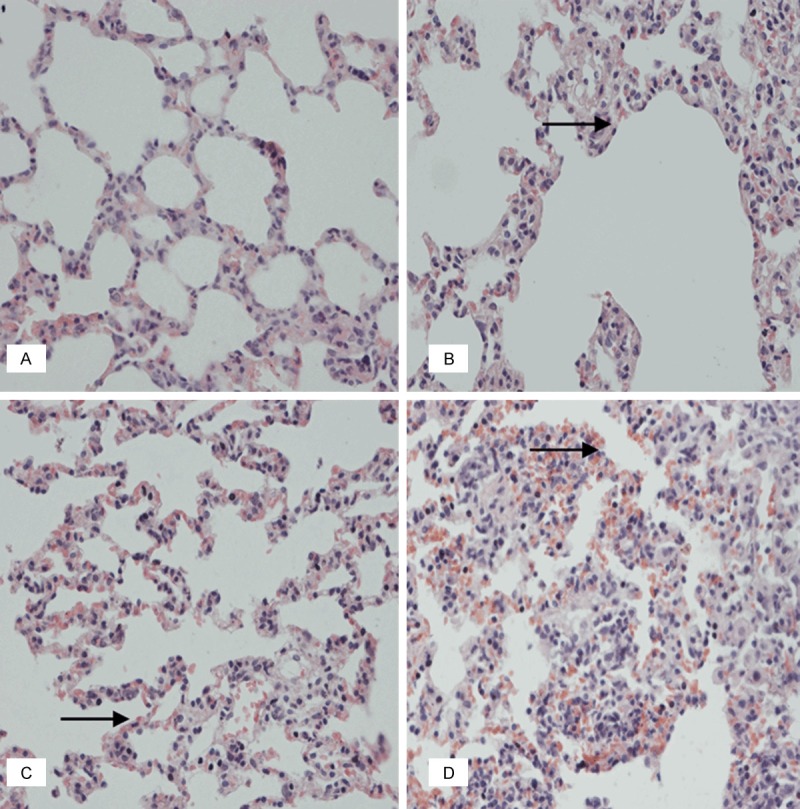
According to the (A) saline control group of lungs, there was normal lungs structure. And there were different levels of the alveolar capillary hyperemia and thickening of alveolar walls in (B) low-dose of the lungs, (C) medium-dose of the lungs, (D) high-dose of the lungs (black rightward arrow). (HE, 400×).
In liver, compared with the saline control group (Figure 2A), vacuolar and granular degenerations were observed and inflammatory cells appeared in the central vein in the low-dose group (Figure 2B). There was mild vacuoles degeneration and slight hemolysis in the middle dose group (Figure 2C). There was vesicular degeneration in the high-dose group (Figure 2D).
In kidney, Congestion occurred in the glomerular capillary was found in the three “Huhezi” groups. The saline control group was normal (Figure 3A). Renal tubular epithelial cells appeared vacuoles degeneration in the low-dose group (Figure 3B). In the middle-dose group, renal epithelial cells appeared granular degeneration and renal tubular was in homogeneous red staining (Figure 3C). In the high-dose group, there was granular degeneration in epithelial cells of the glomerulus and congestion occurred in the glomerular capillary (Figure 3D).
In spleen, there was normal lymph node structure in these four groups. Compared with the saline control group, with the increase of drug concentration, there were more Langerhans cells (Figure 4).
In lung, all the group showed normal structure. There were different levels of alveolar capillary hyperemia and thickening of alveolar walls in low-dose (Figure 5B), middle-dose (Figure 5C and high-dose (Figure 5D) groups when compared with the saline control group (Figure 5A).
Safety pharmacology study
The central nervous system
There were no abnormalities observed on the behavior performance, posture, gait and pupil change; no bizarre behaviors such as salivation and muscle trembling were observed. In the climbing pole test, there were no changes between the experimental groups and the saline control group. The levels of all the groups were “0”.
Cardiovascular and Respiratory system
The result was shown in the Tables 5 and 6. By measuring heart and respiratory rates of all the rats, there were no significant changes (P>0.05) found in the cardiovascular system between the experimental groups and the saline control group.
Table 5.
The rats of different groups at different time points of heart rate (Unit: times)
| Group | Low dose | Middle dose | High dose | Saline control |
|---|---|---|---|---|
| Before the test | 325.17±4.62 | 327.83±1.33 | 332.67±3.45 | 332.83±7.63 |
| 1 day after the tset | 325±4.98 | 329.33±4.41 | 334.17±2.71 | 334±8.51 |
| 1 weekend after the test | 329.17±2.23 | 329.83±3.49 | 333±4.38 | 331.83±4.58 |
Table 6.
The rats of different groups at different time points of Respiratory frequency (Unit: times)
| Group | Low dose | Middle dose | High dose | Saline control |
|---|---|---|---|---|
| Before the test | 88.83±7.11 | 95.17±3.66 | 91.17±7.93 | 91.33±7.69 |
| 1 day after the test | 85.83±4.17 | 94.83±3.31 | 91.17±5.91 | 90±6.97 |
| 1 weekend after the test | 83.67±1.97 | 92.5±3.51 | 89.17±5.00 | 88.5±5.72 |
Discussion
Acute toxicity
In acute toxicity, a single oral administration of 5000 mg/kg body weight of “Huhezi” did neither induce mortality nor any toxicological symptoms in animals [12,13]. Thus, according to the acute toxicity grading standards [11], if the LD50 is more than 5000 mg/kg, the drug is considered as practically non-toxic and its LD50is estimated higher than 5000 mg/kg.
Subchornic toxicity
The growth change
The changes of body weight are the most basic index to reflect toxicity to organs and systems [13,14] and also reflect the combined effects of xenobiotics on the body. There were no rats died during the test, and no abnormalities observed on the behavior performance. A week after the Administration, the male rats’ weight in the medium-dose group showed higher than the saline control group. Other male rats’ also were a little higher than the control for a while. The reason for this phenomenon might be the increase of food and water. There were no significant differences in female rats. Therefore, the “Huhezi” has certain influence on growth of male rats in the medium-dose group.
Hematology parameters
The blood system performs important functions, for example, delivering of oxygen to all body tissues, maintaining vascular integrity, providing necessary immune factors for host defense reaction and so on [15,16]. The system is highly value-added differentiation, which make it susceptibility to toxicants. It is an important target organ for toxicity of drugs. The results showed a little difference between the “Huhezi” and the saline control groups in a few indexes. PLT values in every “Huhezi” groups’ were higher than that in the saline control group, but all these changes were in normal range [17-19].
Serum biochemistry
The changes of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) contents is a sensitive index to reflect the degree of liver cell damage [19,20]. When the chronic liver injury happened, ALT and AST would be released from the injury of the liver cells, resulting in the increase in the content of serum [21,22]. This study showed ALT was higher in the “Huhezi” groups than that in the saline control, but all in the normal range. In addition, Serum urea nitrogen (BUN) reflect glomerular filtration function, when renal parenchymal was damaged, BUN could increase [23,24]. The content of BUN in all the test groups were in the normal range. “Huhezi” granules had little effect on the liver and kidney.
Organ coefficient
Organ coefficient is providing important clues for the target organ of drugs. Although abnormal organ coefficient cannot reflect the nature of the lesion, it can provide circumstantial evidence for pathological diagnosis [11]. Organ coefficient of rats are no obvious differences in all the groups. The spleen coefficient of male rats in the medium-dose groups was more higher than that in the control group. The reason may be due to the immune enhancement.
Histopathological analysis
In various organs, liver and kidney are strong for drug’s affinity and conducive to the elimination of the drug, but also have a certain role in the accumulation [16,25]. The histopathological studies showed that the damages were mainly found in the liver and kidney. Congestion and bleeding were found in the different organs to varying degrees, especially in the lung. These lesions may be the reasons for rats’ death [16]. The liver in the “Huhezi” groups showed liver cells swelled to varying degrees and some liver cells appeared granular degeneration, and degeneration. According to the Table 4, ALT was higher in the “Huhezi” groups, indicating there may be damages in liver. These damages all were reversible. In the kidney, renal tubules in the high-dose group exuded red homogeneous (protein tube), which suggested that there may be a chronic glomerulonephritis to the rats [26,27]. Epithelial cells appeared slightly granular degeneration. In the low-dose group, interstitial capillary appeared hemolysis. In the spleen, the red pulp in the “Huhezi” groups had many Langerhans cells. Langerhans cell is an immature dendritic cell [28]. The increase of Langerhans cells suggested the corresponding organs of immune function enhanced. These results suggested “Huhezi” could enhance immune function [28,29]. Other organs were normal, cell was dyed uniformly, no significant changes were observed.
Safety pharmacology
Safety pharmacology is a subdivision of pharmacology which focuses on identification and characterization of pharmacological activities that affect the clinical safety of a drug. The veterinary medicine and natural medicine guideline for conducting safety pharmacology studies was established by China Institute of Veterinary Drugs Control [10-11,30]. The guideline recommends assessing effects on functions of cardiovascular, central nervous and respiratory systems, which are referred as the core test battery of safety pharmacology. Additionally, safety pharmacology studies satisfy a key requirement in the process of drug development. Using a variety of in vivo and in vitro experimental models, safety pharmacologists carefully design and execute tightly controlled studies to evaluate or predict the harmful and beneficial effects of new drugs on animals or humans. To supplement the toxicity tests and provide a basis for a comprehensive understanding of the toxicity of “Huhezi”, we conducted the safety pharmacology study. The results suggested that “Huhezi” had no effect on the central nervous system, respiratory system and cardiovascular system.
Conclusion
The acute toxicity test showed “Huhezi” granules is a safe and non-toxic formulation. In the subchornic toxicity, the medium-dose (500 mg/kg) “Huhezi” granules can promote the growth of male rats. “Huhezi” granules make no significant effects on blood system in rats. The “Huhezi” granules could produce slightly subchronic toxicity, mainly caused reversible changes to varying degrees. “Huhezi” granules could enchance the immune function. The safety pharmacology showed “Huhezi” had no effect on the central nervous system, respiratory system and cardiovascular system. “Huhezi”, as an oral granule preparation, is considered safe when the clinical dose is no more than 1000 mg/kg.
Acknowledgements
These studies were supported by National Science & Technology Program in Rural Areas During the 12th Five Year Plan Period (2011BAD34B03-4).
Disclosure of conflict of interest
None.
References
- 1.Courtecuisse C, Japiot F, Bloch N, Diallo I. Serological survey on Newcastle and Gumboro diseases, pasteurellosis and pullorosis in local hens in Niger. Rev Elev Med Vet Pays Trop. 1990;43:27–29. [PubMed] [Google Scholar]
- 2.Gadzhiev KS. Species and age resistance of chickens to pullorosis-typhus. Veterinariia. 1971;6:41–44. [PubMed] [Google Scholar]
- 3.Marchenko NS. [Effectiveness of ampicillin in pullorosis-typhoid of chickens] . Veterinariia. 1976:42–44. [PubMed] [Google Scholar]
- 4.Gadzhiev KS, Samedov AG, Tagiev SG. Measures for controlling avian pullorosis-typhoid. Veterinariia. 1969;46:37–39. [PubMed] [Google Scholar]
- 5.Dai RY, Ying ZQ, Jia RY. An acute toxicity test of compound traditional Chinese medicine “Huhezi” and its efficacy in chickens experimentally infected with Salmonella pullorum. Journal of South China Agricultural University. 2015;36:14–17. [Google Scholar]
- 6.Mirghazanfari SM, Hosseinzadeh L, Shokoohinia Y, Aslany M, Kamali-Nejad M. Acute and subchronic toxicological evaluation of Echinophora platyloba DC (Apiaceae) total extract in Wistar rats. Clinics (Sao Paulo) 2012;67:497–502. doi: 10.6061/clinics/2012(05)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang K, Chen C, Lee S, Shen L, Wang H. Development of acute and subacute toxicity with the serotonin transporter radiopharmaceutical, ADAM. Drug Chem Toxicol. 2010;33:393–402. doi: 10.3109/01480540903530753. [DOI] [PubMed] [Google Scholar]
- 8.Gu B, Zhang Z, Li YP, Yu RY, Wang XR. Summary of median lethal dose and its calculation methods. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2009;6:507–511. [Google Scholar]
- 9.Schneeman BO. Dietary fiber and gastrointestinal function. Nutr Rev. 1987;45:129–132. doi: 10.1111/j.1753-4887.1987.tb06343.x. [DOI] [PubMed] [Google Scholar]
- 10.China Institute of Veterinary Drugs Control. Safety pharmacology research technical guidelines of veterinary medicine, natural medicine. 2011. [Google Scholar]
- 11.Duan WL, Liang XM. Technical guidelines assembly of veterinary medicine research. Beijing: Chemical Industry Press; 2011. [Google Scholar]
- 12.Kapoor U, Srivastava MK, Trivedi P, Garg V, Srivastava LP. Disposition and acute toxicity of imidacloprid in female rats after single exposure. Food Chem Toxicol. 2014;68:190–195. doi: 10.1016/j.fct.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Thinkratok A, Suwannaprapha P, Srisawat R. Safety assessment of hydroethanolic rambutan rind extract: acute and sub-chronic toxicity studies. Indian J Exp Biol. 2014;52:989–995. [PubMed] [Google Scholar]
- 14.Lee M, Seo C, Cha S, Shin H. Safety assessment of So-cheong-ryong-tang: subchronic toxicity study in Crl: CD Sprague Dawley rats. Mol Med Rep. 2014;9:2273–2282. doi: 10.3892/mmr.2014.2114. [DOI] [PubMed] [Google Scholar]
- 15.Rahman MF, Siddiqui MK, Jamil K. Effects of vepacide (Azadirachta indica) on aspartate and alanine aminotransferase profiles in a subchronic study with rats. Hum Exp Toxicol. 2001;20:243–249. doi: 10.1191/096032701678227730. [DOI] [PubMed] [Google Scholar]
- 16.Lou YJ. Toxicology of drug. 3rd edition. Beijing: People’s Medical Publishing House; 2013. [Google Scholar]
- 17.Traesel GK, de Souza JC, de Barros AL, Souza MA, Schmitz WO, Muzzi RM, Arena AC. Acute and subacute (28 days) oral toxicity assessment of the oil extracted from Acrocomia aculeata pulp in rats. Food Chem Toxicol. 2014;74:320–325. doi: 10.1016/j.fct.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Nidhi B, Baskaran V. Acute and subacute toxicity assessment of lutein in lutein-deficient mice. J Food Sci. 2013;78:T1636–T1642. doi: 10.1111/1750-3841.12256. [DOI] [PubMed] [Google Scholar]
- 19.Shaluei F, Hedayati A, Jahanbakhshi A, Kolangi H, Fotovat M. Effect of subacute exposure to silver nanoparticle on some hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix) Hum Exp Toxicol. 2013;32:1270–1277. doi: 10.1177/0960327113485258. [DOI] [PubMed] [Google Scholar]
- 20.Ramaiah SK. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol. 2007;45:1551–1557. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Han YD, Song SY, Lee JH, Lee DS, Yoon HC. Multienzyme-modified biosensing surface for the electrochemical analysis of aspartate transaminase and alanine transaminase in human plasma. Anal and Bioanal Chem. 2011;400:797–805. doi: 10.1007/s00216-011-4797-6. [DOI] [PubMed] [Google Scholar]
- 22.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol. 2012;40:1031–1048. doi: 10.1177/0192623312444618. [DOI] [PubMed] [Google Scholar]
- 24.Hosseinzadeh H, Sadeghi Shakib S, Khadem Sameni A, Taghiabadi E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran J Pharm Res. 2013;12:93–99. [PMC free article] [PubMed] [Google Scholar]
- 25.Benko I, Nagy G, Tanczos B, Ungvari E, Sztrik A, Eszenyi P, Banfalvi G. Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ Toxicol Chem. 2012;31:2812–2820. doi: 10.1002/etc.1995. [DOI] [PubMed] [Google Scholar]
- 26.Wen Y, Wen K. Rapidly progressive glomerulonephritis as a presenting feature of chronic lymphocytic leukemia. Int Urol Nephrol. 2014;46:217–221. doi: 10.1007/s11255-012-0352-4. [DOI] [PubMed] [Google Scholar]
- 27.Chiu HY, Huang HL, Li CH, Yin YJ, Chen HA, Hsu ST, Lin SJ, Tsai TF, Ho SY. Increased risk of glomerulonephritis and chronic kidney disease in relation to the severity of psoriasis, concomitant medication, and comorbidity: A nationwide population-based cohort study. Br J Dermatol. 2015;173:146–54. doi: 10.1111/bjd.13599. [DOI] [PubMed] [Google Scholar]
- 28.Romani N, Schuler G. The immunologic properties of epidermal Langerhans cells as a part of the dendritic cell system. Springer Semin Immunopathol. 1992;13:265–279. doi: 10.1007/BF00200527. [DOI] [PubMed] [Google Scholar]
- 29.Kim YS, Yang SH, Kang HG, Seong EY, Lee SH, Gao W, Strom TB. Distinctive role of donor strain immature dendritic cells in the creation of allograft tolerance. Int Immunol. 2006;18:1771–1777. doi: 10.1093/intimm/dxl111. [DOI] [PubMed] [Google Scholar]
- 30.CFDA. Test No. GPT2-1: Acute toxicity studies technical guidelines of traditional Chinese medicine and natural medicine. CFDA Publishing; 2004. [Google Scholar]


