Abstract
The aim of the review is to highlight the current knowledge about established and new biologicals and to summarise recent advances by focusing on comparative efficacy, safety and possible discontinuation of treatment in patients with rheumatoid arthritis (RA). Up to now, comparative analyses showed only minor differences with respect to efficacy and safety among the established biologicals. Studies confirmed the excellent drug retention rate as well as efficacy and safety of approved biologicals including their use in monotherapy. Tapering and in some instances discontinuation of biologicals is possible in disease remission. In case of relapse, patients usually show full response after reintroduction of the same compound. The development of biologicals continues fast with several new biologicals targeting different or established cytokines or cellular subsets of the immune system. With several new biologicals in the pipeline and different formulations for established compounds, treatment options for RA will become even more versatile and sophisticated. Although we get closer to the aim of decreasing the proportion of refractory patients, many questions have to be addressed in the near future regarding emerging biosimilars and biologicals with new modes of action.
Keywords: Rheumatoid Arthritis, Treatment, DMARDs (biologic)
Key messages.
Biological disease-modifying antirheumatic drugs (DMARDs) have translated the knowledge on molecular pathways into targeted therapies and are increasingly used in patients with rheumatoid arthritis (RA) with excellent efficacy and acceptable safety.
Head-to-head studies confirm comparable efficacy of different biological DMARDs in RA treatment, however, with respect to adalimumab monotherapy the results are favourable for tocilizumab.
Discontinuation studies show that patients with RA in sustained remission can successfully taper and sometimes stop tumour necrosis factor inhibitor without functional or radiological deterioration and, in case of relapse, restarting of biologicals is possible and regularly leads to rapid improvement.
Several new biologicals are in developmental status with the potential to further decrease the proportion of refractory patients.
Introduction
Disease-modifying antirheumatic drugs (DMARDs) are the mainstay of rheumatoid arthritis (RA) therapy. Institution of conventional synthetic DMARDs (csDMARDs) in therapy was followed by the development of tumour necrosis factor (TNF) inhibitors (TNFi), the first biological DMARDs (bDMARDs) introduced into rheumatology. Today, five different TNFi (infliximab (IFX), adalimumab (ADA), etanercept (ETN), certolizumab (CZP) and golimumab (GLM)) are in use. Although distinct by structure, route of application and pharmacokinetics, they show overall excellent effects with respect to clinical and radiological outcomes especially when used in comedication with methotrexate (MTX). TNFi are effective in all stages of disease including MTX-naïve patients with early RA and in patients with an inadequate response to MTX (MTX-IR) or any csDMARDs (csDMARD-IR).
Following TNFi, new bDMARDs with different modes of action were developed. Abatacept (ABT), targeting the co-stimulation between T and B cells, rituximab (RTX), targeting CD20+ B cells and finally tocilizumab (TCZ), an interleukin 6 receptor (IL-6R) antagonist, confirmed their efficacy in active RA including patients with an inadequate response to TNFi (TNFi-IR).
Strategy trials
Clinical studies with a predefined strategy can teach us a lot about the best treatment approach by using different available compounds. Furthermore, they are usually designed to answer relevant issues of daily practice.
Add-on strategies
In a large phase IIIb trial (REALISTIC), patients with RA with an inadequate response to at least one DMARD were randomised to receive CZP or placebo plus current therapy.1 The primary end point (week 12 American College of Rheumatology 20 (ACR20)) was met, and differences were already evident at week 2. Of note, the disease activity and physical function improved both in patients with or without previous TNFi use, regardless of their baseline MTX use.
A recently performed, open-label, prospective study (GO-MORE) evaluated the efficacy and safety of subcutaneous GLM as add-on therapy in csDMARD-IR patients with active RA.2 In this large study with 3366 patients, 82.1% achieved good-to-moderate European League Against Rheumatism (EULAR) responses and 23.9% attained remission at month 6.
In the ACT-RAY study, MTX-IR patients with active RA were randomised to add-on TCZ or to switch to TCZ plus placebo.3 After 1 year there was a trend favouring add-on strategy, however, both strategies demonstrated meaningful clinical and radiographic responses.
The aforementioned studies confirm efficacy of add-on strategies with biologicals in csDMARD-IR patients with RA, however, for TCZ, monotherapy also has convincing results.
Early aggressive treatment
The TEAR study has been designed to answer whether early aggressive treatment is comparable to a step-up approach in early RA.4 Patients were randomised to receive ETN+MTX or triple therapy (TT) consisting of MTX, sulfasalazine and hydroxychloroquine, or MTX-monotherapy. If low disease activity (LDA) was not reached, patients with MTX-monotherapy were allowed to advance to one of the combination therapies. Finally, the clinical outcomes were similar between all groups, but only the ETN+MTX group showed a significant radiological benefit. Results of a similar study with IFX (Swefot) were also consistent with these findings.5 These two studies support the current strategy of a step-up therapy beginning with MTX in patients with early RA.
TT versus TNFi+MTX in MTX-IR patients with RA
The RACAT study was designed to answer the critical question of whether TT is equivalent to ETN+MTX in patients with RA after MTX failure.6 In this 48-week, double-blinded, non-inferiority trial, patients were randomised to one of the arms, and if patients did not improve at 24 weeks they were switched to the other arm. After 24 weeks, both groups of patients improved significantly (p=0.001) with a switch rate of 27%. Of note, the degree of significant improvement and response rates were similar between both groups after switching. The primary outcome, changes in disease activity score 28 (DAS28), at 48 weeks, was similar in both groups (−2.1 with TT and −2.3 with ETN+MTX, p=0.26) suggesting non-inferiority for TT compared with ETN+MTX. However, the higher hurdles such as ACR50, ACR70, LDA and radiological progression were in favour of ETN. The study can be criticised for several methodical issues including change of outcome parameters and potentially reduced power due to non-achieved recruitment goal.
Role of MTX dose in combination with bDMARDs
In the CONCERTO trial, biological and MTX-naïve patients with early RA were randomised to open-label ADA plus weekly blinded 2.5, 5, 10 or 20 mg MTX.7 With increasing doses of MTX, significant increasing trends were observed in the proportion of patients achieving the outcomes. Of note, differences comparing 10 and 20 mg MTX were minimal. ADA serum concentrations increased with ascending dose up to 10 mg MTX. The authors related this significant trend with an effect of MTX on ADA pharmacokinetic profile.
Head-to-head comparisons of biologicals
First eagerly awaited studies for head-to-head (H2H) comparisons of biologicals were performed recently. In the RED SEA trial, 125 adults with active RA despite treatment with two csDMARDs including MTX were randomised to add-on therapy with ADA or ETN.8 There was no significant difference between treatment arms.
The 2-year results of the AMPLE study, a H2H comparison of ABT and ADA in MTX-IR patients with RA, confirmed similar efficacy based on clinical, functional and radiographic outcome.9 Safety outcomes were comparable between both groups with fewer local injection site reactions (ISRs) and slightly lower discontinuation rate under ABT.
Another H2H trial, ADACTA, compared monotherapy with TCZ versus ADA in patients with RA who were intolerant or inappropriate candidates for MTX.10 Of note, mean DAS28 improvement was significantly higher in the TCZ (−3.3) than in the ADA group (−1.8) (difference −1.5, 95% CI −1.8 to −1.1; p<0.0001) from baseline to week 24, while safety findings were comparable between the treatment arms.
In summary, these studies confirm comparable efficacy of different bDMARDs in RA treatment. However, with respect to monotherapy, the results are favourable for TCZ.
Alternative routes of administration
Since different routes of administration allow for more flexibility, trials for subcutaneous versus intravenous biologicals were performed in recent years. The ACQUIRE trial compared efficacy and safety of subcutaneous ABT versus intravenous ABT in MTX-IR patients with RA with a non-inferiority design.11 At month 6, similar proportions of patients in both arms achieved the primary outcome of ACR20 response (estimated difference: 0.3%, 95% CI −4.2 to 4.8). Both formulations demonstrated equal efficacy and safety including similar patient retention rates (94.2% for subcutaneous ABT vs 93.8% for intravenous ABT).
In the GO-FURTHER trial, MTX-IR patients with active RA were randomised to receive GLM intravenously or placebo with background MTX.12 Significantly more patients on GLM achieved the efficacy outcomes compared with placebo. Adverse events (AEs) were similar, but serious AEs (most commonly infections) were reported more frequently under GLM+MTX (4.1%) than placebo plus MTX (2%).
The SUMMACTA study randomly assigned patients with RA with an inadequate response to DMARDs (DMARD-IR) (including TNFi in up to 20% of patients) to receive TCZ subcutaneously or TCZ intravenously in combination with csDMARDs.13 At week 24, non-inferiority of subcutaneous versus intravenous administration was confirmed by similar ACR20 response rates. The safety profile of subcutaneous TCZ was consistent with the known safety profile of the drug with the exception of a higher incidence of ISRs.
Thus, in addition to ABT, intravenous and subcutaneous forms of TCZ and GLM are already available.
Discontinuation trials
Discontinuation of TNFi in MTX-naïve patients with RA
In recent years, several studies investigated the possibility of TNFi tapering or discontinuation. In a subanalysis of the BeST study, 64% of patients with initial IFX treatment and 25% of patients with delayed IFX exposure were able to discontinue IFX.14 Median time without IFX treatment was 17 months, and about 60% of patients paused IFX for at least 1 year. Restarting IFX resulted in DAS≤2.4 in all patients without any progression of radiological damage. Presence of shared epitope, smoking and a long treatment with IFX were independent predictors for IFX restart.
In another study published recently (PRIZE), DMARD-naïve patients with early active RA received 50 mg of ETN+MTX for 52 weeks and in case of qualifying responses were randomly assigned to receive 25 mg of ETN+MTX, MTX alone, or placebo for an additional 39 weeks.15 Patients with maintained responses after this period stopped all DMARDs and were followed to week 65. As a result, continuing combination therapy at a reduced dose led to better disease control than switching to MTX alone or placebo. However, radiological progression did not differ between groups.
In the OPTIMA study, 44% of MTX-naïve patients with early RA achieved LDA under ADA+MTX and were rerandomised to receive placebo plus MTX or to continue ADA+MTX.16 After 1 year, more patients with continuous ADA treatment maintained LDA or remission compared with MTX alone (LDA 91% vs 81%, p=0·0361; remission 86% vs 66%, p=0·0014).
Interesting data also came from the HIT HARD study, where patients with early RA were treated with ADA or placebo with MTX.17 After 24 weeks, both groups continued with MTX for a second period of 24 weeks. As a result, 47% of ADA+MTX patients achieved DAS28 remission, and 44% of these patients were still in remission at week 48. Despite similar results at 48 weeks, patients on ADA+MTX reached good clinical efficacy significantly earlier and had better radiographic outcomes.
Discontinuation of TNFi in csDMARD-IR patients with RA
The RRR study included patients with RA with persistent LDA for >24 weeks under IFX treatment.18 After IFX withdrawal, 56 of 102 patients maintained LDA over 1 year. In case of relapse, the majority again reached LDA by re-treatment with IFX. In fact, this study was the first to demonstrate the possibility of biological-free remission in patients with longer disease duration (mean 4.8 years).
The HONOR study recruited patients treated with ADA+MTX who agreed to discontinue ADA after sustained remission for ≥6 months (DAS28<2.6).19 It is noteworthy that in patients with deep remission (DAS28—erythrocyte sedimentation rate ≤1.98) identified by receiver operating characteristics analysis following logistic analysis in the study, after 1 year the proportion of patients with sustained remission or LDA was not significantly different between patients who continued or stopped ADA. In case of relapse, restarting ADA was effective and safe.
In the CERTAIN study, patients with RA with low-to-moderate disease activity were randomised to CZP or placebo plus current csDMARD.20 Patients who achieved remission stopped study treatment and followed up. In the follow-up, only 3 of the 17 prior CZP patients and 2 of the 6 prior placebo patients maintained remission until week 52. However, re-treatment with CZP was effective in relapsing patients.
Dose reduction of TNFi in MTX-IR patients with RA
In the PRESERVE trial, moderately MTX-IR patients with active RA pretreated with 50 mg of ETN+MTX were randomised to receive 50 mg ETN+MTX, 25 mg ETN+MTX, or placebo plus MTX.21 After 1 year of follow-up, in both treatment groups on ETN a higher percentage remained in LDA (82.6% and 79.1%, respectively) compared with MTX monotherapy (42.6%).
Discontinuation of TCZ in MTX-IR patients with RA
In the ACT-RAY study, 50.4% of patients discontinued TCZ following sustained clinical remission after 1 year.22 Subsequently, 84% of those patients experienced a flare up with recurrent response to the reintroduced drug.
Discontinuation of ABT in very early active RA
The AVERT study included patients with very early active RA who were randomised to subcutaneous ABT 125 mg plus MTX, ABT 125 mg monotherapy, or MTX.23 Patients with LDA at month 12 entered a second 12-month period of withdrawal of all RA therapies. A small but significant number of patients sustained drug-free remission in the ABT+MTX group compared with MTX alone at both 12 and 18 months (14.8% vs 7.8%, respectively; p=0.045).
Discontinuation of any DMARDs following sustained remission
The recently published prospective study, RETRO, analysed the effect of continuing, tapering or stopping DMARDs in patients with sustained remission.24 In total, 82.2% of patients received MTX, 40.6% received bDMARDs and 9.9% received other DMARDs. Overall, 66.3% of patients remained in remission for 12 months, whereas 33.7% relapsed. Of note, anticitrullinated protein antibody positivity and treatment reduction predicted relapse.
In summary, the available results show that patients with greater depth of remission are more likely to successfully taper and in some instances to stop bDMARDs. This is valid for patients with early as well as for patients with longstanding RA. Owing to limited results for non-TNFi, it is unclear whether differences with respect to drug-free remission exist for the available bDMARDs. However, in patients with relapse, restarting of biologicals regularly leads to rapid improvement.
New biologicals
Biosimilars
The patents for the earliest antirheumatic biologicals are expiring, which has encouraged the development of biosimilars. The IFX biosimilar CT-P13 has been approved by the European Medicines Agency. The phase III trial of CT-P13, PLANETRA, demonstrated equivalent efficacy to original IFX with a comparable pharmacokinetic and safety profile including immunogenicity.25 Clinical trials are also ongoing for ETN, ADA and RTX biosimilars. Intended copies of ETN and RTX are already in use in some countries.
Agents targeting IL-6
Apart from the approved bDMARDs, new biologicals targeting different or established cytokines or cellular subsets of the immune system are currently in clinical development for treatment of RA (figure 1).
Figure 1.
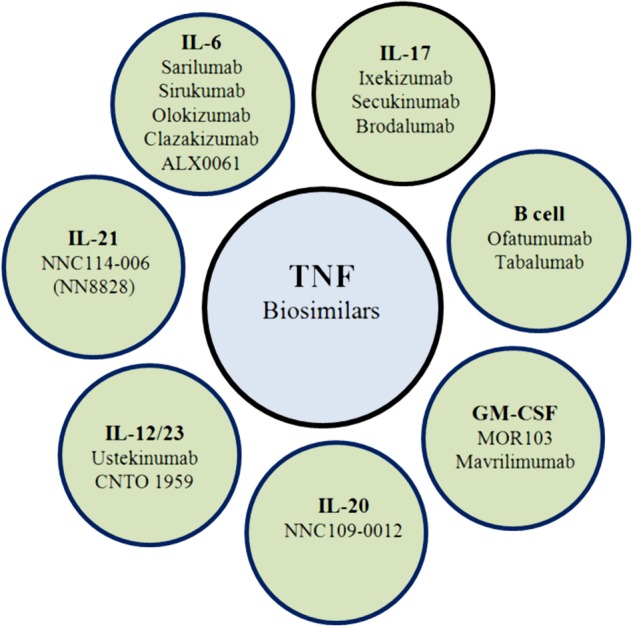
New agents on developmental procedures targeting different cytokines or cells (GM-CSF, granulocyte–macrophage colony-stimulating factor; IL, interleukin; TNF, tumour necrosis factor).
Of these compounds, sarilumab, a fully human monoclonal antibody (mAb) against IL-6Rα was effective in MTX-IR patients with RA in phase II and III trials showing a safety profile similar to other IL-6 inhibitors.26 27 Published abstracts also confirmed a favourable efficacy–safety profile.28–30
Sirukumab, another new mAb targeting IL-6 also showed significant improvement in MTX-IR patients with active RA in a phase II trial.31 Safety results through 38 weeks were consistent with other IL-6 inhibitors and phase III trials are ongoing. Phase II trials with olokizumab and clazakizumab targeting IL-6 have also shown results consistent with other IL-6 inhibitors.32–34 However, with clazakizumab, no clear dose–response has been observed. Therefore, whether or not direct IL-6 inhibitors are really comparable to IL-6R antagonists has to be clarified by further studies. Another development in the field is the monovalent nanobody ALX-0061, also targeting the IL-6R. The data from a phase I/II study were promising with an 84% ACR20 response and 58% DAS28 remission rate.35
Agents targeting B cells
Beneficial results with RTX in RA have facilitated the development of several new drugs targeting B cells. Of these, two different humanised CD20 mAbs, ocrelizumab and ofatumumab, followed the same strategy of depleting B cells. Although ocrelizumab was shown to be effective, increased rate of serious infections led to termination of its development in RA.36–38 On the other hand, in a phase I/II study ofatumumab appeared to be effective and safe compared with placebo in DMARD-IR patients with RA.39 Subsequently, ofatumumab was also tested in biological-naïve MTX-IR patients with RA in a phase III study.40 ACR20 response rate was 50% versus 27% in the placebo group without any unexpected safety finding. A trial with subcutaneous ofatumumab in patients with RA on background MTX showed profound and prolonged B cell depletion without required glucocorticoid premedication.41
In addition to depleting strategies, neutralisation of B cell cytokines such as B cell-activating factor (BAFF) represents another alternative approach in RA. As an example, tabalumab is a fully human IgG4 mAb that neutralises membrane-bound and soluble BAFF. Although tabalumab showed some efficacy and acceptable safety in phase II trials,42–44 phase III trials could not confirm a clinical benefit.45–47
Agents targeting IL-17
In a phase I study, ixekizumab (LY2439821), a humanised IgG4 mAb against IL-17A, was effective in biological-naïve patients with RA, without significant safety signals.48 Recently published data of a phase II trial proved the same findings in patients with RA who were either biological-naïve or TNFi-IR.49 Furthermore, the published 1 year phase II data of another fully human IgG1k mAb against IL-17A, secukinumab, have also provided evidence that this treatment approach could be of benefit for csDMARD-IR and bDMARD-IR patients.50 The overall safety profile was acceptable with an increased rate of mostly mild infections of 31.9% and serious AEs in 8.9% of patients. Several phase III studies of secukinumab are ongoing, especially in TNFi-IR patients. In contrast, a phase Ib and a phase II study with brodalumab, a fully human IgG2 mAb against IL-17 receptor A (IL-17RA), showed good tolerance but no clinical relevant response.51 52
Agents targeting GM-CSF
Another cytokine with a close connection to the pathogenesis and clinical features of RA is granulocyte–macrophage colony-stimulating factor (GM-CSF). However, it was a long-time concern that targeted therapies against this cytokine could cause severe side effects such as neutropaenia or pulmonary alveolar proteinosis. Nevertheless, different compounds successfully entered clinical development for RA targeting the cytokine itself or its receptor. The phase I and phase IIa (EARTH) trials of mavrilimumab, a human mAb against GM-CSF receptor, showed profound and rapid onset of response, normalisation of acute phase reactants and an overall good safety profile.53 54 Moreover, the results of the phase IIb trial met the primary end points (DAS28 and ACR20) with a clear dose–response effect with excellent tolerability.55 Another compound is MOR103, a fully human mAb directed towards GM-CSF. This alternative approach was tested in a phase Ib/IIa study with promising results, including imaging data.56
Other agents
Ustekinumab, an anti-IL-12/23 p40 mAb, and CNTO 1959, a compound targeting IL-23 p19 were investigated in different regimes in patients with active RA on background MTX therapy in a phase II trial.57 The primary end point of ACR20 response was not reached; however, for improvement of DAS28 as a secondary end point, a significant difference was observed for patients receiving ustekinumab as well as for the higher dosage group of CNTO 1959.
Upregulation of IL-21 has been linked to increased disease activity and radiological damage in RA.58 Two phase I studies on safety, tolerability, pharmacokinetics and pharmacodynamics, and a phase II study regarding efficacy of a fully human anti-IL-21 mAb, NNC114-0006 (NN8828), have been completed, but results have not been presented so far.59–61
IL-20 and its receptors are present in RA synovial tissue and IL-20 is thought to play a role in the pathophysiology of RA.62 A novel human IgG4 mAb against IL-20, NNC0109-0012, was well tolerated in healthy respondents and patients with RA in phase I trials.63 64 Efficacy and safety results of phase IIa supported further trials.65 66 However, two phase II studies in MTX-IR and TNF-IR patients were terminated,67 68 and one phase II study investigating the mechanism of action through synovial biopsies was withdrawn prior to enrolment.69 Table 1 summarises selected new agents and their developmental phases.
Table 1.
Developmental status of new biologicals for RA treatment
| Target | Agent | Developmental status |
|---|---|---|
| IL-6 | Sarilumab (human mAb against IL-6Rα) | Phase II, phase III published26 27; several phase II and III trials ongoing (clinicaltrials.gov)(some presented with abstracts)28–30 |
| Sirukumab (human mAb against IL-6) | Phase II published;31 some of phase II presented with abstracts;70–72 several phase III trials ongoing (clinicaltrials.gov) | |
| Olokizumab (human mAb against IL-6) | Phase IIb published;32 3 phase II trials completed (no results posted)73–75 | |
| Clazakizumab (human mAb against IL-6) | Phase II trial published;33 1 phase IIb trial abstract presented;34 1 phase IIb trial, active not recruiting76 | |
| ALX-0061 (monovalent IL-6R targeting nanobody) | Phase I/II study abstract presented35 1 phase II combination therapy77 and 1 phase IIb monotherapy trials recruiting78 |
|
| B cells | Ofatumumab (human mAb against CD20) | Phase I/II studies and phase III trial published39–41 |
| Tabalumab (human mAb against BAFF) | Phase II trials published;42–44 several phase III trials ongoing, no results posted (clinicaltrials.gov), however some presented with abstracts45–47 | |
| IL-17 | Ixekizumab (human mAb against IL-17) | Phase I, phase II published48 49 |
| Secukinumab (human mAb against IL-17) | Phase II published;50 several phase III trials ongoing (clinicaltrials.gov) | |
| Brodalumab (human mAb against IL-17R) | Phase Ib published;51 1 phase II published;52 1 phase II terminated for lack of efficacy in RA79 | |
| IL-12/23 | Ustekinumab (human mAb against IL-12/23 p40) | Phase II completed, has results57 |
| CNTO 1959 (human mAb against IL23p19) | Phase II completed, has results57 | |
| GM-CSF | MOR103 (human mAb against, GM-CSF) | Phase Ib/IIa published56 |
| Mavrilimumab (human mAb against GM-CSFR) | Phase I and phase IIa published;53 54 phase IIb abstract presented;55 one phase II trial completed, no results posted;80 1 phase II trial recruiting81 | |
| IL-21 | NNC114-006 (NN8828) (human mAb against IL-21) | 2 phase I completed, no results posted;59 60 phase II completed, no results posted61 |
| IL-20 | NNC109-0012 (human mAb against IL-20) | 2 phase I abstracts presented;63 64 phase IIa abstracts presented;65 66 2 phase II trials terminated, no results posted;67 68 1 phase II trial withdrawn, no results posted69 |
BAFF, B cell-activating factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; GM-CSFR, GM-CSF receptor; IL-6R, interleukin 6 receptor; mAb, monoclonal antibody; RA, rheumatoid arthritis.
A combined blockade of TNFα and IL-17 by bispecific anti-TNFα/IL-17 antibodies was tested in human mesenchymal cells and in an animal model of arthritis and was more effective than single blockade in inhibiting cytokine, chemokine and matrix enzyme responses, and in blocking tissue destruction.82 This observation may encourage new studies for combined blockade of appropriate cytokines.
Conclusion
Biologicals offer a new dimension in the treatment of RA, allowing us to translate the knowledge on the molecular pathways into targeted therapies. Today, bDMARDs are increasingly used in patients with an inadequate response or intolerance to csDMARDs. Excellent efficacy and acceptable safety of bDMARDs have been established in the previous years. They also possess the chance of tapering and discontinuation in patients with sustained remission. Beyond approved biologicals, several new biologicals are in developmental status with the potential to fill the current treatment gap and help us to crack the hard nut of patients with refractory RA.
Footnotes
Contributors: All the authors contributed to the design of the work. ABA drafted the work, EF and G-RB revised it critically for important intellectual content. All the authors approved the submitted version of the manuscript.
Competing interests: ABA has received honoraria for consulting or lecturing from BMS and Actelion. EF has received honoraria for consulting or lecturing from Roche/Chugai, BMS, Novartis, Pfizer, AbbVie and MSD. EF is currently receiving a grant from Organisation BMS and Novartis. G-RB has received honoraria for consulting or lecturing from Roche/Chugai, BMS, Novartis, Pfizer, AbbVie, UCB and MSD.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Weinblatt ME, Fleischmann R, Huizinga TW et al. Efficacy and safety of certolizumab pegol in a broad population of patients with active rheumatoid arthritis: results from the REALISTIC phase IIIb study. Rheumatology (Oxford) 2012;51:2204–14. 10.1093/rheumatology/kes150 [DOI] [PubMed] [Google Scholar]
- 2.Combe B, Dasgupta B, Louw I et al. Efficacy and safety of golimumab as add-on therapy to disease-modifying antirheumatic drugs: results of the GO-MORE study. Ann Rheum Dis 2014;73:1477–86. 10.1136/annrheumdis-2013-203229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougados M, Kissel K, Conaghan PG et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study. Ann Rheum Dis 2014;73:803–9. 10.1136/annrheumdis-2013-204761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreland LW, O'Dell JR, Paulus HE et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum 2012;64:2824–35. 10.1002/art.34498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Vollenhoven RF, Geborek P, Forslind K et al. Conventional combination treatment versus biological treatment in methotrexate-refractory early rheumatoid arthritis: 2 year follow-up of the randomised, non-blinded, parallel-group Swefot trial. Lancet 2012;379:1712–20. 10.1016/S0140-6736(12)60027-0 [DOI] [PubMed] [Google Scholar]
- 6.O'Dell JR, Mikuls TR, Taylor TH et al. Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med 2013;369:307–18. 10.1056/NEJMoa1303006 [DOI] [PubMed] [Google Scholar]
- 7.Burmester GR, Kivitz AJ, Kupper H et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis 2015;74:1037–44. 10.1136/annrheumdis-2013-204769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jobanputra P, Maggs F, Deeming A et al. A randomised efficacy and discontinuation study of etanercept versus adalimumab (RED SEA) for rheumatoid arthritis: a pragmatic, unblinded, non-inferiority study of first TNF inhibitor use: outcomes over 2 years. BMJ open 2012;2:e001395 10.1136/bmjopen-2012-001395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiff M, Weinblatt ME, Valente R et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis 2014;73:86–94. 10.1136/annrheumdis-2013-203843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabay C, Emery P, van Vollenhoven R et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 2013;381:1541–50. 10.1016/S0140-6736(13)60250-0 [DOI] [PubMed] [Google Scholar]
- 11.Genovese MC, Covarrubias A, Leon G et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum 2011;63:2854–64. 10.1002/art.30463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinblatt ME, Bingham CO III, Mendelsohn AM et al. Intravenous golimumab is effective in patients with active rheumatoid arthritis despite methotrexate therapy with responses as early as week 2: results of the phase 3, randomised, multicentre, double-blind, placebo-controlled GO-FURTHER trial. Ann Rheum Dis 2013;72:381–9. 10.1136/annrheumdis-2012-201411 [DOI] [PubMed] [Google Scholar]
- 13.Burmester GR, Rubbert-Roth A, Cantagrel A et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis 2014;73:69–74. 10.1136/annrheumdis-2013-203523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allaart CF, Lems WF, Huizinga TW. The BeSt way of withdrawing biologic agents. Clin Exp Rheumatol 2013;31(4 Suppl 78):S14–18. [PubMed] [Google Scholar]
- 15.Emery P, Hammoudeh M, FitzGerald O et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med 2014;371:1781–92. 10.1056/NEJMoa1316133 [DOI] [PubMed] [Google Scholar]
- 16.Smolen JS, Emery P, Fleischmann R et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 2014;383:321–32. 10.1016/S0140-6736(13)61751-1 [DOI] [PubMed] [Google Scholar]
- 17.Detert J, Bastian H, Listing J et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis 2013;72:844–50. 10.1136/annrheumdis-2012-201612 [DOI] [PubMed] [Google Scholar]
- 18.Tanaka Y, Takeuchi T, Mimori T et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis 2010;69:1286–91. 10.1136/ard.2009.121491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka Y, Hirata S, Kubo S et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study. Ann Rheum Dis 2015;74:389–95. 10.1136/annrheumdis-2013-204016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolen JS, Emery P, Ferraccioli GF et al. Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double-blind, randomised, placebo-controlled trial. Ann Rheum Dis 2015;74:843–50. 10.1136/annrheumdis-2013-204632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolen JS, Nash P, Durez P et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381:918–29. 10.1016/S0140-6736(12)61811-X [DOI] [PubMed] [Google Scholar]
- 22.Huizinga TW, Conaghan PG, Martin-Mola E et al. Clinical and radiographic outcomes at 2 years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT-RAY study. Ann Rheum Dis 2015;74:35–43. 10.1136/annrheumdis-2014-205752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery P, Burmester GR, Bykerk VP et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis 2015;74:19–26. 10.1136/annrheumdis-2014-206106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haschka J, Englbrecht M, Hueber AJ et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy: interim results from the prospective randomised controlled RETRO study. Ann Rheum Dis 2015. Published Online First 6 Feb 2015. doi:10.1136/annrheumdis-2014-206439. 10.1136/annrheumdis-2014-206439 [DOI] [PubMed] [Google Scholar]
- 25.Yoo DH, Hrycaj P, Miranda P et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013;72:1613–20. 10.1136/annrheumdis-2012-203090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huizinga TW, Fleischmann RM, Jasson M et al. Sarilumab, a fully human monoclonal antibody against IL-6Ralpha in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis 2014;73:1626–34. 10.1136/annrheumdis-2013-204405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genovese M, Fleischmann RM, Kivitz AJ et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: Results of a phase III study. Arthritis Rheum 2015;67:1424–37. 10.1002/art.39093 [DOI] [PubMed] [Google Scholar]
- 28.Fleischmann R, Decktor DL, Fan C et al. Comparable efficacy with sarilumab plus methotrexate in biologic-experienced and biologic-naïve patients with moderate-to-severe rheumatoid arthritis from a phase 3, randomized, double-blind, placebo-controlled, international study. [2823]. ACR2014.
- 29.Kavanaugh A, Decktor DL, Fan C et al. A profile of the efficacy of sarilumab plus methotrexate in rheumatoid arthritis patients: results of a 52-week, phase 3, randomized, double-blind, placebo-controlled, international study. [2824]. ACR2014.
- 30.Huizinga TW, Kivitz AJ, Rell-Bakalarska M et al. Sarilumab for the treatment of moderate-to-severe rheumatoid arthritis: results of a phase 2, randomized, double-blind, placebo-controlled, international study. [OP0023]. EULAR2012.
- 31.Smolen JS, Weinblatt ME, Sheng S et al. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2014;73:1616–25. 10.1136/annrheumdis-2013-205137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genovese MC, Fleischmann R, Furst D et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised phase IIb study. Ann Rheum Dis 2014;73:1607–15. 10.1136/annrheumdis-2013-204760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mease P, Strand V, Shalamberidze L et al. A phase II, double-blind, randomised, placebo-controlled study of BMS945429 (ALD518) in patients with rheumatoid arthritis with an inadequate response to methotrexate. Ann Rheum Dis 2012;71:1183–9. 10.1136/annrheumdis-2011-200704 [DOI] [PubMed] [Google Scholar]
- 34.Weinblatt M, Mease P, Mysler E et al. A phase IIb study of the efficacy and safety of subcutaneous clazakizumab (anti-IL-6 monoclonal antibody) with or without methotrexate in adults with moderate-to-severe active rheumatoid arthritis and an inadequate response to methotrexate. [SAT0244]. EULAR2014.
- 35.Holz JB, Sargentini-Maier L, De Bruyn S et al. Twenty-four weeks of treatment with a novel anti-IL-6 receptor Nanobody® (ALX-0061) resulted in 84% ACR20 improvement and 58% DAS28 remission in a phase I/II study in RA. [OP0043]. EULAR2013.
- 36.Rigby W, Tony HP, Oelke K et al. Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum 2012;64:350–9. 10.1002/art.33317 [DOI] [PubMed] [Google Scholar]
- 37.Stohl W, Gomez-Reino J, Olech E et al. Safety and efficacy of ocrelizumab in combination with methotrexate in MTX-naive subjects with rheumatoid arthritis: the phase III FILM trial. Ann Rheum Dis 2012;71:1289–96. 10.1136/annrheumdis-2011-200706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tak PP, Mease PJ, Genovese MC et al. Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to at least one tumor necrosis factor inhibitor: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum 2012;64:360–70. 10.1002/art.33353 [DOI] [PubMed] [Google Scholar]
- 39.Ostergaard M, Baslund B, Rigby W et al. Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying antirheumatic drugs: results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum 2010;62:2227–38. 10.1002/art.27524 [DOI] [PubMed] [Google Scholar]
- 40.Taylor PC, Quattrocchi E, Mallett S et al. Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann Rheum Dis 2011;70:2119–25. 10.1136/ard.2011.151522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurrasch R, Brown JC, Chu M et al. Subcutaneously administered ofatumumab in rheumatoid arthritis: a phase I/II study of safety, tolerability, pharmacokinetics, and pharmacodynamics. J Rheumatol 2013;40:1089–96. 10.3899/jrheum.121118 [DOI] [PubMed] [Google Scholar]
- 42.Genovese MC, Bojin S, Biagini IM et al. Tabalumab in rheumatoid arthritis patients with an inadequate response to methotrexate and naive to biological therapy: a phase II, randomized, placebo-controlled trial. Arthritis Rheum 2013;65:880–9. 10.1002/art.37820 [DOI] [PubMed] [Google Scholar]
- 43.Genovese MC, Fleischmann RM, Greenwald M et al. Tabalumab, an anti-BAFF monoclonal antibody, in patients with active rheumatoid arthritis with an inadequate response to TNF inhibitors. Ann Rheum Dis 2013;72:1461–8. 10.1136/annrheumdis-2012-202775 [DOI] [PubMed] [Google Scholar]
- 44.Genovese MC, Lee E, Satterwhite J et al. A phase 2 dose-ranging study of subcutaneous tabalumab for the treatment of patients with active rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 2013;72:1453–60. 10.1136/annrheumdis-2012-202864 [DOI] [PubMed] [Google Scholar]
- 45.Genovese MC, Silverman GJ, Emery P et al. Efficacy and safety of subcutaneous administration of tabalumab, an anti-B cell activating factor monoclonal antibody, in rheumatoid arthritis: results from a phase 3 multicenter, randomized, double-blind study. [1732]. ACR2013.
- 46.Smolen JS, Weinblatt ME, van der Heijde D et al. Efficacy and safety of tabalumab, an anti-B-cell-activating factor monoclonal antibody, in patients with rheumatoid arthritis who had an inadequate response to methotrexate therapy: results from a phase III multicenter, randomised, double-blind study. Ann Rheum Dis 2015;74:1567–70. 10.1136/annrheumdis-2014-207090 [DOI] [PubMed] [Google Scholar]
- 47.Schiff M, Combe B, Dörner T et al. Efficacy and safety of tabalumab, an anti-B cell activating factor monoclonal antibody, in patients with rheumatoid arthritis who had an inadequate response to TNF-alpha inhibitors: results from a phase 3 multicenter, randomized, double-blind study. [AB0438]. EULAR2014.
- 48.Genovese MC, Van den Bosch F, Roberson SA et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum 2010;62:929–39. 10.1002/art.27334 [DOI] [PubMed] [Google Scholar]
- 49.Genovese MC, Greenwald M, Cho CS et al. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheum 2014;66:1693–704. 10.1002/art.38617 [DOI] [PubMed] [Google Scholar]
- 50.Genovese MC, Durez P, Richards HB et al. One-year efficacy and safety results of secukinumab in patients with rheumatoid arthritis: phase II, dose-finding, double-blind, randomized, placebo-controlled study. J Rheumatol 2014;41:414–21. 10.3899/jrheum.130637 [DOI] [PubMed] [Google Scholar]
- 51.Martin DA, Churchill M, Flores-Suarez L et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther 2013;15:R164 10.1186/ar4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pavelka K, Chon Y, Newmark R et al. A study to evaluate the safety, tolerability, and efficacy of brodalumab in subjects with rheumatoid arthritis and an inadequate response to methotrexate. J Rheumatol 2015;42:912–19. 10.3899/jrheum.141271 [DOI] [PubMed] [Google Scholar]
- 53.Burmester GR, Feist E, Sleeman MA et al. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-alpha, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann Rheum Dis 2011;70:1542–9. 10.1136/ard.2010.146225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burmester GR, Weinblatt ME, McInnes IB et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann Rheum Dis 2013;72:1445–52. 10.1136/annrheumdis-2012-202450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burmester G, McInnes IB, Kremer JM et al. Efficacy and safety/tolerability of mavrilimumab, a human GM-CSFRa monoclonal antibody in patients with rheumatoid arthritis. [2821]. ACR2014.
- 56.Behrens F, Tak PP, Østergaard M et al. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann Rheum Dis 2015;74:1058–64. 10.1136/annrheumdis-2013-204816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.A Study of the Effectiveness and Safety of Ustekinumab (STELARA) and CNTO 1959 administered under the skin of patients with active rheumatoid arthritis, despite existing methotrexate therapy. ClinicalTrials.gov NCT01645280 (accessed 19 Apr 2015).
- 58.Rasmussen TK, Andersen T, Hvid M et al. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J Rheumatol 2010;37:2014–20. 10.3899/jrheum.100259 [DOI] [PubMed] [Google Scholar]
- 59.First-in-Man Trial of NNC114–0005 in healthy subjects and subjects with rheumatoid arthritis. ClinicalTrials.gov NCT01208506; (accessed 19 Apr 2015).
- 60.Safety and tolerability of NNC0114–0006 at increasing dose levels in subjects with rheumatoid arthritis. ClinicalTrials.gov NCT01565408; (accessed 19 Apr 2015).
- 61.A randomised, double-blind, placebo-controlled, parallel-group trial to assess clinical efficacy of NNC0114–0006 in subjects with active rheumatoid arthritis. ClinicalTrials.gov NCT01647451; (accessed 19 Apr 2015).
- 62.Hsu YH, Li HH, Hsieh MY et al. Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum 2006;54:2722–33. 10.1002/art.22039 [DOI] [PubMed] [Google Scholar]
- 63.Kotbi NA, Jensen L, Graff LB. NNC0109–0012 (anti-IL-20 mAb), well tolerated in healthy subjects and patients wıth rheumatoid arthritis. [FRI0196]. EULAR2012.
- 64.Leszczynski P, Eshof MK, Stegmann HVB et al. NNC0109–0012 (anti-IL-20 mAb), well tolerated in patients wıth rheumatoid arthritis. [FRI0197]. EULAR2012.
- 65.Senolt L, Göthberg M, Valencia X et al. Efficacy and safety of NNC0109–0012 (anti-IL-20 mAb) in patients with rheumatoid arthritis: results from a phase 2a trial. [LB0004]. EULAR2012.
- 66.Senolt L, Hansen BB, Strandberg-Larsen M et al. Improvements in patient-reported physical function, pain and global disease activity in patients with rheumatoid arthritis after treatment with NNC0109–0012 (antı-IL-20 mAb) in a phase 2a trial. [SAT0112]. EULAR2013.
- 67.A trial of NNC0109–0012, an anti-IL-20 biologic, in patients with active rheumatoid arthritis who are inadequate responders to methotrexate. ClinicalTrials.gov NCT01636843; (accessed 19 Apr 2015).
- 68.A trial of NNC0109–0012, an anti-IL-20 biologic, in patients with active rheumatoid arthritis who are inadequate responders to anti-TNFa biologics. ClinicalTrials.gov NCT01636817; (accessed 19 Apr 2015).
- 69.A trial investigating the mechanism of action of NNC0109–0012 (Anti-IL-20 mAb) through synovial biopsies in subjects with rheumatoid arthritis and an inadequate response to methotrexate. ClinicalTrials.gov NCT02097264; (accessed 19 Apr 2015).
- 70.Hsu B, Chiou CF, Sheng S et al. Sirukumab, a human anti-IL-6 monoclonal antibody, improves physical function in patients with active RA despite methotrexate therapy: results from a 2-part, proof-of-concept, dose-ranging, randomized, double-blind, placebo-controlled, phase 2 study. [FRI0181]. EULAR 2012.
- 71.Hsu B, Sheng S, Weinblatt ME et al. Results from a multicenter, international, randomized, double-blind, placebo-controlled, phase 2 study of sirukumab, a human anti-IL-6 monoclonal antibody, in patients with active rheumatoid arthritis despite methotrexate therapy. [OP0025]. EULAR2012.
- 72.Hsu B, Sheng S, Smolen JS et al. Results from a 2-part, proof-of-concept, dose-ranging, randomized, double-blind, placebo-controlled, phase 2 study of sirukumab, a human anti-IL-6 monoclonal antibody, in patients with active rheumatoid arthritis despite methotrexate therapy. [THU0100]. EULAR2012. [DOI] [PMC free article] [PubMed]
- 73.The long-term safety and efficacy of olokizumab (CDP6038) with active rheumatoid arthritis. ClinicalTrials.gov NCT01533714; (accessed 19 Apr 2015).
- 74.Efficacy and safety of olokizumab with rheumatoid arthritis with previously failed to anti-tumor necrosis factor (anti-TNF) therapy. ClinicalTrials.gov NCT01463059; (accessed 19 Apr 2015).
- 75.Open-label study to assess the safety and efficacy of CDP6038 in patients who completed RA0056. ClinicalTrials.gov. NCT01296711; (accessed 19 Apr 2015).
- 76.Phase IIB dose ranging study in subjects with moderate to severe rheumatoid arthritis. ClinicalTrials.gov NCT02015520; (accessed 19 Apr 2015).
- 77.A dose-range finding study for ALX-0061 combination therapy in subjects with rheumatoid arthritis. ClinicalTrials.gov NCT02309359; (accessed 19 Apr 2015).
- 78.A phase IIb study for ALX-0061 monotherapy in subjects with rheumatoid arthritis. ClinicalTrials.gov NCT02287922; (accessed 19 Apr 2015).
- 79.Safety and efficacy of AMG 827 in subjects with RA. ClinicalTrials.gov NCT01059448; (accessed 19 Apr 2015).
- 80.A study of mavrilimumab versus anti tumor necrosis factor in subjects with rheumatoid arthritis. ClinicalTrials.gov NCT01715896; (accessed 19 Apr 2015).
- 81.A long term safety study of mavrilimumab in adult subjects with rheumatoid arthritis. ClinicalTrials.gov NCT01712399; (accessed 19 Apr 2015).
- 82.Fischer JA, Hueber AJ, Wilson S et al. Combined inhibition of tumor necrosis factor alpha and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: development and characterization of a novel bispecific antibody. Arthritis Rheum 2015;67:51–62. 10.1002/art.38896 [DOI] [PubMed] [Google Scholar]


