Abstract
Overweight and obesity are increasing worldwide and now reach about one-third of the world's population. Obesity also involves patients with inflammatory arthritis. Knowing the impact of obesity on rheumatic diseases (rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis) is thus an important issue. This article first reviews the epidemiological and clinical data available on obesity in inflammatory rheumatic diseases, that is, its impact on incident disease, disease characteristics and the therapeutic response. The second part of this review gives an overview of the factors potentially involved in the specifics of inflammatory arthritis in patients with obesity, such as limitations in the clinical assessment, diet, microbiota and adipokines.
Keywords: Psoriatic Arthritis, Rheumatoid Arthritis, Ankylosing Spondylitis
Key messages.
What is already known about this subject?
Patients with chronic inflammatory rheumatic disease were previously described as cachectic due to hypercatabolism. More recently, obesity has been considered in these patients.
What does this study add?
This review highlights the impact of obesity on incident disease and on disease characteristics with higher DAS28 and higher HAQ but lower radiographic progression in obese patients with RA.
Therapeutic response to conventional disease modifying anti-rheumatic drugs and to tumour necrosis factor inhibitors is also altered in obese patients with chronic inflammatory rheumatic disease.
How might this impact on clinical practice?
Being aware of the difficulties in achieving remission in obese patients who have a good structural prognosis could influence clinicians not to intensify therapy.
Introduction
Overweight and obesity are increasing worldwide and now reach about one-third of the populations in developing countries.1 Although rheumatoid arthritis (RA) was initially described as cachectic due to hypercatabolism, obesity also involves patients with RA and the impact of obesity on RA and rheumatic diseases is thus an important issue.2
In the first part, we review epidemiological and clinical data on obesity in inflammatory rheumatic diseases, namely RA, ankylosing spondylitis (AS) and psoriatic arthritis (PsA). We first present obesity definitions in these diseases, and then summarise the data on obesity and the risk of developing inflammatory rheumatic diseases and the impact of obesity on disease features and the therapeutic response. In the second part, we discuss the factors potentially explaining the specifics of inflammatory rheumatic diseases in the obese population, including assessment difficulties, diet, microbiota and adipokines.
For the first part of this review, we searched MEDLINE via PubMed for articles published up to 31 January 2015 with the following research strategy: (‘body mass index’ OR ‘obese’) AND (‘rheumatoid arthritis’ OR ‘ankylosing spondylitis’ OR psoriasis OR ‘psoriatic arthritis)’. This search led to 827 articles; 65 were excluded because they were not in English and 96 because they concerned animal models. Of the 666 articles retrieved, papers were selected according to their relevance to the topic and after critical discussion between the two authors.
Epidemiological and clinical data on obesity in inflammatory rheumatic diseases
Reliability of body mass index to evaluate fat mass in inflammatory rheumatic diseases
Overweight and obesity are defined by excessive fat mass, which is associated with increased cardiovascular risk. Assessments of overweight or obesity include the calculation of body mass index (BMI, in kg/m2) or more accurate estimations of relative adiposity (body fat (BF) percentage) with several methods (bioelectrical impedance, skinfold thickness, etc.). In the general population, BMIs <25, 25–30 and >30 kg/m2 indicate healthy, overweight and obese people, respectively. However, such cut-offs are questioned in specific populations such as Asians or postmenopausal women.3 4
Patients with RA have an altered body composition, with decreased lean mass and increased fat mass, which could also affect the reliability of BMI cut-offs to define obesity.5–7 Indeed, lower thresholds of BMI were proposed in two studies to define obesity based on bioelectrical impedance and on dual X-ray absorptiometry as a BF assessment reference (obesity if BMI >28 kg/m2; ≥26.1 kg/m2 for women and ≥24.7 kg/m2 for men).8 9 However, these thresholds are not applied in published clinical studies.
Data on body composition in patients with AS and PsA are sparse, controversial and involve few participants. BF percentage (as assessed by X-ray absorptiometry) seems to be higher in patients with PsA than in patients with psoriasis only and controls.10 Lower fat mass assessed by bioelectrical impedance was found in 28 patients with AS compared with 17 controls.11 However, two other studies did not find a difference in the fat mass component (evaluated by dual-energy X-ray absorptiometry and plethysmography) between AS and controls.12
Since BMI is not a reliable tool for assessment of body composition in RA,3 lower thresholds of BMI have been proposed to define overweight and obesity (ie, overweight 23–28 and obesity >28 kg/m2) based on bioelectrical impedance as BF assessment reference.10 Similarly, another study found that the optimal thresholds to define obesity using dual X-ray absorptiometry were ≥26.1 kg/m2 for women and ≥24.7 kg/m2 for men.11 However, these thresholds are not applied in published clinical studies.
Risk of inflammatory rheumatic diseases and obesity
Rheumatoid arthritis
Several recent studies found an increased risk of RA occurrence related to obesity (table 1). In a large American retrospective case–control study, obesity was associated with an increased risk of RA developing (OR 1.24; 95% CI 1.01 to 1.53, adjusted for smoking status).13 In this study, the incidence of RA in Minnesota increased by an increment of 9.2/100 000 among women between 1985 and 2007, with obesity accounting for 52% of this increase. Moreover, two case–control studies (including 515 recent RA with 769 controls and 2748 RA with 3444 controls) found an association between obesity and the probability of developing RA negative for anti-citrullinated protein antibodies (ACPA), with an increased risk of 3.45 (1.73 to 6.87) in the first study and 1.6 (1.2 to 2.2) in women in the second study.14 15 Two cohort studies including large numbers of patients with a long-term follow-up (25 455 participants followed for 14.2 years with 184 incident cases of inflammatory arthritis and more than 4 500 000 person-years with 1181 incident cases of RA) found that obesity is associated with incident seronegative inflammatory polyarthritis (HR 2.75; 95% CI 1.39 to 5.46)16 and seronegative RA (HR 1.34; 95% CI 1.03 to 1.74).17 In the latest study (ie, the Nurses Health Study), the risk for patients with obesity of developing RA with onset before 55 years was further increased in all patients with RA (seropositive and seronegative) (HR 1.65; 95% CI 1.34 to 2.05). Finally, for the 570 patients with undifferentiated arthritis in the Leiden early arthritis cohort, obesity did not affect the probability of developing RA during 1 year follow-up.22
Table 1.
Risk of inflammatory rheumatic diseases in obese participants: case–control studies and longitudinal studies
| Case–control studies | Longitudinal studies | |
|---|---|---|
| RA | Risk of RA in obese participants: OR=1.24 (1.01 to 1.53); 813 patients with RA and 813 controls matched on age, sex and calendar year13 Risk of ACPA negative RA in obese participants: OR=3.45 (1.73 to 6.87); 515 recent RA with 769 controls matched on age and gender14 Risk of ACPA negative RA in obese women: OR=1.6 (1.2 to 2.2); 2748 RA with 3444 controls15 |
Risk of seronegative inflammatory polyarthritis in obese participants: HR 2.75 (95%CI 1.39 to 5.46) ; 25 455 participants followed for 14.2 years with 184 incident inflammatory arthritis16 Risk of seronegative RA in obese participants: HR=1.34 (1.03 to 1.74); 2 765 195 person-years of follow-up (1976–2008) in NHS and 1 934 518 person-years (1989–2009) in NHSII with 1181 incident cases of RA17 |
| AS | NA | NA |
| PsA | Obesity more frequent in PsA than in RA (45% vs 39%; p=0.007, adjusted for age, sex and race); 294 PsA and 1162 RA18 Obesity more frequent in patients with PsA (37%; n=644) than in patients with psoriasis (29%, n=448), with RA (27%, n=350), or in the general population (18%)19 Risk of PsA in patients with obesity with psoriasis compared to non-obese patients with psoriasis: OR=1.22 (1.02 to 1.47); 75 395 individuals with psoriasis of which 976 developed PsA20 |
Risk of PsA in obese participants: HR=3.12 (1.90 to 5.11); 1 231 693 person-years follow-up with 146 incident PsA21 |
ACPA, anti-citrullinated protein antibodies; AS, ankylosing spondylitis; NA, not available; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
Thus, obesity is associated with an increased risk of RA developing, with more consistent results concerning seronegative RA.
AS and PsA
To the best of our knowledge, no longitudinal study has investigated the effect of BMI on the risk of AS development.
Obesity and weight gain are associated with increased risk of incident psoriasis. The analysis of 892 incident psoriasis cases in 78 626 women included in Nurses Health Study II over a 14-year period showed increased BMI and weight gain associated with a strong risk of incident psoriasis in women. On multivariate analysis, obesity at 18 years was associated with increased risk of psoriasis (OR 1.73, 95% CI 1.24 to 2.41) and weight gain from the age of 18 years was associated with increased risk of incident psoriasis.23 Conversely, another study of more than 16 550 patients with psoriasis and 500 controls found obesity to not be a risk factor for onset of disease but rather a consequence of psoriasis.24 Indeed, psoriasis and PsA promote obesity through social isolation, depression, physical inactivity, high fat diet and alcohol consumption.
Obesity is more frequent in patients with PsA than in those with psoriasis or RA or in healthy participants. Among patients with PsA (n=294) or RA (n=1162) included in the Consortium of Rheumatology Researchers of North America (CORRONA) Registry, obesity was more frequent with PsA than RA (45% vs 39%; p=0.007).18 In a case–control study, obesity was more frequent with PsA (37%; n=644) than psoriasis (29%, n=448) and with RA (27%, n=350) or in the general population (18%) after adjustment for age and gender.19
Concerning obesity and incident PsA, in a case series of 943 patients with psoriasis including 250 patients with PsA, self-reported BMI at age 18 years but not current BMI predicted PsA.25 In the UK general population, 75 395 participants with psoriasis were included between 1995 and 2010; PsA developed in 976.20 The risk of developing PsA was increased with obesity and morbid obesity (BMI ≥35.0 kg/m2) as compared with psoriasis and BMI <25 kg/m2 (OR 1.22, 95% CI 1.02 to 1.47, and 1.48, 1.20 to 1.81, respectively). Similarly, 146 incident PsA cases were identified in Nurses Health Study II during a 1 231 693 person-year follow-up.21 Compared with BMI <25.0 kg/m2, with increasing weight excess, the risk of incident PsA increased (overweight: OR 1.83, 95% CI 1.15 to 2.89; obesity: 3.12, 1.90 to 5.11; morbid obesity: 6.46, 4.11 to 10.16). The risk of PsA was increased with obesity among patients with psoriasis.
Impact of obesity on characteristics, activity and severity of inflammatory rheumatic diseases
Rheumatoid arthritis
In most studies evaluating the impact of BMI on RA activity and/or radiographic joint damage, patients with obesity were less often positive for rheumatoid factor (RF) and/or ACPAs, that is, risk factors of increased subsequent radiographic damages.22 26 Conversely, in a study of 131 patients with established RA, fat areas assessed visceral by abdominal CT were significantly higher in RF-positive patients than RF-negative patients.27
Many different studies and a meta-analysis28 showed that Disease Activity Score in 28 joints (DAS28) is higher among patients with obesity than normal-weight patients with RA. In the QUEST-RA study, including 5161 patients with RA, as compared with normal-weight patients, obesity was associated with increased mean DAS28 (+0.23 points, 95% CI 0.11 to 0.34).29 Additionally, for the 1596 patients with early RA with a mean follow-up of 9.5±3.7 years, compared with normal-weight patients, obesity at inclusion and during follow-up was independently associated with increased disease activity (mean DAS28 3.0±1.2 vs 2.7±1.3, p=0.002), reduced sustained remission rate (20.5% vs 26.6%; p=0.048) and increased mean pain level (32.9±23.9 vs 25.8±23.8; p=0.005).30 This DAS28 increase seems to be mainly explained by an increase in subjective parameters—number of tender joints and patient global assessment26 30 31—and surprisingly not C reactive protein level or erythrocyte sedimentation rate.22 31 In two studies, functional disability assessed by the Health Questionnaire Assessment (HAQ) was also significantly increased in obese as compared with normal-weight patients (0.6±0.7 vs 0.5±0.6; p<0.001, and +0.16 (95% CI 0.03 to 0.30) respectively).26 30
Radiographic joint damage and progression in patients with obesity with RA have been extensively studied. In the Leiden early arthritis cohort including 332 patients with early RA, BMI was inversely correlated with the van der Heijde-Sharp (vdHS) score after 3 years of follow-up.22 The results were replicated in a subgroup of 247 patients participating in the Best study. In both cohorts, BMI was independently and inversely associated with increased vdHS score at 3 years in ACPA-positive, but not ACPA-negative, patients.22 Similarly, another study of 767 patients with early RA showed that normal-weight patients had significantly more joint damage at inclusion and more radiographic progression than patients with obesity evaluated by the Ratingen score.32 This difference in radiographic progression was not observed in RF-negative patients with RA. More recently, the analysis of 1068 participants included in two clinical trials evaluating golimumab (ie, GO-BEFORE and GO-FORWARD) confirmed that obesity was associated with a lower probability of increase in vdHS score at weeks 52 and 104, independent of potential confounders.33 These radiographic results were confirmed by a study of MRI-evidenced progression in erosion score over 2 years.33
Thus, although patients with obesity with RA show increased DAS28 and HAQ score, they have reduced risk of radiographic joint damage and radiographic progression, with consistent results in the literature, especially in RF- and ACPA-positive participants.
AS and PsA
Few data are available for spondyloarthritis, but the findings are similar to those for RA. In a cross-sectional study, increased BMI in 46 patients with AS was associated with increased functional impairment (ie, Bath Ankylosing Spondylitis Functional Index (BASFI) and HAQ score) as well as increased disease activity (Bath Ankylosing Spondylitis Disease activity Index (BASDAI)).34 In another study of 26 patients with AS, a significant correlation was found between the visceral adipose tissue amount and BASDAI.35
In 270 patients with PsA, disease characteristics of obese and non-obese patients with PsA did not differ in terms of axial, peripheral or mutilant presentation, Psoriasis Area Severity Index (PASI), HAQ score, number of tender or swollen joints or CRP level.36 Such results corroborated those from Eder et al37, who found a higher consumption of non-steroidal anti-inflammatory drugs in patients with obesity than in normal-weight patients but no significant difference in the HAQ score, CRP level, swollen and tender joint count and joint damage.
Thus, obesity might be associated with increased activity in spondyloarthritis, but no clear impact was observed for PsA.
Impact of obesity on therapeutic response
Rheumatoid arthritis
A Swedish study of 495 early patients with RA recently reported that, as compared with normal-weight patients, patients receiving synthetic disease-modifying anti-rheumatic drugs (DMARDs), including 86% methotrexate, with a BMI ≥25 kg/m2 had a 51% lower likelihood of achieving DAS28 low disease activity (OR 0.49, 95% CI 0.31 to 0.78) and a 42% lower odds of remission (OR 0.58, 0.37 to 0.92) at 6 months (table 2).38
Table 2.
Impact of obesity on therapeutic response
| Disease | Drug studied | Patients (n) | Results |
|---|---|---|---|
| RA | cDMARD | 495 | Likelihood in overweight patients compared to normal-weight patients of achieving at 6 months LDAS: OR=0.49 (95% CI 0.31 to 0.78) and remission: OR=0.58 (0.37 to 0.92)38 |
| RA | cDMARD and infliximab | 508 | Likelihood in overweight patients compared to normal-weight patients of not achieving at 1 year DAS <2.4: RR=1.20 (1.05 to 1.37)31 |
| RA | Infliximab | 89 | Percentage of responders (DAS28 ≥1.2): 50% for patients with obesity, 75% for those with BMI 20–30 kg/m2 and 84% for those with BMI <20 kg/m2 (p=0.04)39 |
| RA | TNFi | 641 | DAS28 remission at 12 months less frequent in patients with obesity: 15.2% in patients with obesity, 30.4% in overweight patients and 32.9% in patients with a BMI <25 kg/m2, the difference being only significant in IFX treated patients (not in ADA or ETN)40 |
| RA | Tocilizumab | 222 | Likelihood in patients with obesity compared to normal-weight patients of achieving at 6 months EULAR response: OR 1.19, 95% CI 0.31 to 4.48, p=0.7841 |
| AS | Infliximab | 155 | Lower BASDAI50 therapeutic response at 6 months in patients with obesity (26.5%) than in normal-weight (77.6%) individuals42 |
| AS | TNFi | 170 | Likelihood of achieving BASDAI50 at 12 months in patients with obesity compared to normal-weight participants: OR=3.57 (1.15 to 11.11), the difference being only significant in IFX treated patients (not in ADA or ETN)43 |
| PsA | TNFi | 270 | Likelihood of not achieving MDA in patients with obesity: HR=4.90 (3.04 to 7.87; p<0.001), and in morbidly patients with obesity: HR=5.40 (3.09 to 9.43, p<0.001), with no difference between the 3 TNFi36 |
| PsA | cDMARD and TNFi | 557 | Likelihood of achieving MDA in patients with obesity: OR 0.53; p<0.000137 |
ADA, adalimumab; AS, ankylosing spondylitis; BASDAI, Bath ankylosing spondylitis disease activity index; BMI, body mass index; cDMARD, conventional disease-modifying antirheumatic drug; DAS, disease activity score; ETN, etanercept; EULAR, European League Against Rheumatism; IFX, infliximab; LDAS, low disease activity score; MDA, minimal disease activity; PsA, psoriatic arthritis; RA, rheumatoid arthritis; TNFi: tumour necrosis factor inhibitor.
Several studies have shown decreased efficacy of antitumour necrosis factor (anti-TNF) therapy with obesity in RA. In the 508 patients with recent-onset RA in the Best trial initially receiving combination therapy with prednisone and initial and delayed infliximab treatment, BMI ≥25 kg/m2 was independently associated with failure to achieve remission.31 For 89 patients with RA receiving infliximab for established RA, BMI and DAS28 decrease were found to be negatively associated after 16 weeks after multiple adjustment.39 These results were confirmed in an Italian study including 641 patients receiving TNF inhibitors (adalimumab, etanercept or infliximab). DAS28 remission at 12 months was more frequently reached in lean participants: 15.2% in obese participants, 30.4% in overweight participants and 32.9% in patients with BMI <25 kg/m2. The difference in remission between obese and non-obese patients was significant for only patients receiving infliximab, but not those receiving adalimumab and etanercept, without any clear explanation to date.40
Conversely, results for the interleukin 6 (IL-6) blocking agent tocilizumab were not similar to those with anti-TNF therapy: in a retrospective study of 222 patients with RA, BMI and EULAR responses to tocilizumab at 6 months were not associated after multiple adjustments.41
Thus, in RA, obesity seems to be associated with reduced probability of achieving remission with synthetic DMARDs and TNF inhibitors. Further studies are required and should include other biological agents.
Spondyloarthritis
In a cohort of 155 patients with AS starting infliximab, Ottaviani et al42 reported a significantly lower therapeutic response at 6 months (BASDAI50) in patients with obesity (26.5%) than in normal-weight (77.6%) participants. In another study of 170 patients with AS receiving a first-line TNF inhibitor, the proportion of patients achieving BASDAI50 at 12 months was 72.8% for normal-weight patients, as compared with 54.5% for overweight patients and 30.4% for obese participants, with an increased risk of therapeutic failure in patients with obesity as compared with normal-weight participants (OR 3.57, 95% CI 1.15 to 11.11).43 This difference was mainly explained by patients receiving infliximab, with a BASDAI50 response of 79.0% for normal-weight participants and 16.7% for patients with obesity (p<0.001). As in RA, the therapeutic response did not differ between normal-weight patients and patients with obesity with AS receiving adalimumab or etanercept.
In PsA, achieving minimal disease activity (MDA) with TNF inhibitors (80 receiving adalimumab, 111 etanercept and 79 infliximab) was compared between 135 patients with obesity and 135 normal-weight patients. After adjustment, obesity was associated with increased risk of not achieving MDA (HR 4.90, 95% CI 3.04 to 7.87; p<0.001), especially in morbidly patients with obesity (HR 5.40; 95% CI 3.09 to 9.43, p<0.001).36 However, MDA achievement did not differ between the three TNF inhibitors. In another Canadian cohort of 557 patients with PsA mainly receiving synthetic DMARDs (10–17% of patients receiving only TNF inhibitors), achieving sustained MDA was lower for patients in the higher BMI categories than in those with BMI <25 kg/m2 after multiple adjustments. 37
Thus, obesity is associated with poor response to infliximab in AS patients and with decreased probability of achieving and maintaining MDA in PsA patients receiving synthetic DMARDs and TNF inhibitors.
Impact of weight loss on inflammatory arthritis
Rheumatoid arthritis
Since obese status has an impact on disease activity and RA structural progression and may modulate response to some therapeutic agents, weight loss represents an important issue, although it is poorly studied. In a recent study of 53 patients with RA who underwent bariatric surgery, disease activity was significantly decreased 6 months after the surgery, with 68% of patients achieving remission.44
Spondyloarthritis
The effect of weight loss on TNF inhibitor response was assessed in a study with 126 patients with PsA, following a hypocaloric diet or free managed diet.45 MDA was more often achieved with the hypocaloric than the free managed diet (HR 1.85, 95% CI 1.02 to 3.35). Regardless of the type of diet, after 6 months’ treatment, MDA achievement was significantly higher for participants with 5–10% weight loss (OR 3.75, 95% CI 1.36 to 10.36) and >10% weight loss (OR 6.67, 2.41 to 18.41) as compared with those with <5% weight loss. Weight loss may be more important than type of diet. Psoriasis also improves with weight loss.46
Obesity and mortality in RA, AS, and PsA
Patients with RA, AS and PsA exhibit increased risk of cardiovascular events and mortality.47 48 Patients with obesity with RA are at increased risk of cardiovascular risk factors such as hypertension, low high-density lipoprotein, insulin resistance and metabolic syndrome.49 However, the effect of BMI on mortality is surprising. Survival in a cohort of 779 patients with RA adjusting for comorbidity, RA disease severity and other potential confounders was inversely correlated with BMI. Patients with obesity with the lowest mortality (1.7 deaths per 100 person-years (95% CI 1.1 to 2.5) vs 3.3 (2.3 to 4.9) for normal-weight patients).50 This result was confirmed in another cohort of 24 535 patients with RA over 12.3 years. For overweight and obese patients, the risk for all-cause and cardiovascular mortality was lower as compared with normal-weight patients (relative risk (RR) 0.8, 95% CI 0.8 to 0.9 and 0.8, 0.7 to 0.8, respectively), with and without comorbidity adjustment, although the number of comorbidities was higher in these BMI groups.51 No such studies are available in spondyloarthritis.
Thus, obese RA patients are at increased risk of cardiovascular risk factors and events but seem to have a decreased mortality.
Potential mechanisms explaining the specifics of inflammatory rheumatic diseases in patients with obesity
Much evidence shows that obesity affects the occurrence and outcomes of inflammatory arthritis. What are the potential underlying mechanisms?
Misdiagnosis and assessment difficulties
Obesity mainly predisposes to seronegative RA, and patients with obesity with RA have less structural damage, with lower radiographic progression, than non-obese patients with RA, although the former have higher disease activity and poorer therapeutic response. The poorer therapeutic response may be explained by a misdiagnosis of RA in patients with obesity that could have another cause, such as in hand osteoarthritis (OA), because the risk of hand OA is increased by two points in overweight and patients with obesity.52 Obesity is associated with increased pain and reduced quality of life52 and could be responsible for increased DAS28 and HAQ scores as compared with non-obesity. Likewise, the high disease activity could lead to treatment intensification, but this needs to be proven.
Adipose tissue and adipokines
The impact of obesity on RA risk, disease activity and structural protection may be explained by the intervention of adipose tissue products, called adipokines.53 This term includes all the soluble mediators predominantly synthetised by adipocytes and/or infiltrating immune cells. Adipokines are involved in metabolism (ie, insulin resistance, appetite) and in inflammation and immunity. Likewise, in obese participants, adipokines are responsible for the low-grade inflammatory state and participate in metabolic disturbances such as insulin resistance and cardiovascular complications. Since leptin, adiponectin and visfatin, the most studied adipokines, are also involved in RA immunopathology, they could represent the cornerstone of RA and obesity.54
Leptin is considered a pro-inflammatory adipokine in immune cells and joint cells, although its role in animal models of arthritis remains unclear, with contrasting results (ie, aggravation or limitation of arthritis).55 Visfatin, also called nicotinamide phosphoribosyltransferase (Nampt), is a proinflammatory adipokine in immune and joint cells, and its blockade reduces arthritis severity similar to that of anti-TNF drugs. Adiponectin has several isoforms and acts as an anti-inflammatory adipokine at a systemic level on endothelial cells and immune cells, which explains a low blood adiponectin level in patients with obesity, who are thus not protected against cardiovascular diseases. However, in vitro, all adiponectin isoforms may stimulate fibroblastic synoviocytes to induce proinflammatory mediators and matrix proteolytic enzyme release.56
Many studies have shown that the blood level of adiponectin and leptin may reflect disease activity.54 Some reports have also shown that serum adiponectin and possibly leptin are independently associated with radiographic damages or may predict radiographic progression in RA.57 Therefore, the paradoxical lower radiographic joint damage among patients with obesity with RA could be mechanistically explained by adiponectin: a low level of serum adiponectin usually found in patients with obesity could be responsible for less proinflammatory action on joint cells and thus explain why patients with obesity with RA have less radiographic progression. In 197 patients with RA, an inverse association between visceral fat area assessed by total-body dual X-ray absorptiometry and radiographic damage was attenuated when adiponectin was modelled as a mediator.58
However, adipokines are synthetised not only by adipose tissue but also by haematopoietic cells and joint cells (ie, synoviocytes, chondrocytes, osteoblasts) and thus may also be involved in RA progression, independent of obesity, similar to other ‘classical’ proinflammatory mediators such as TNFα or IL-6. This hypothesis is emphasised by the persistent association between serum adipokine levels and RA damage or progression on multivariate analyses that adjusted for BMI. Likewise, adipokines may explain in part the link between obesity and RA, but other factors may play a role. Moreover, dysfunction of regulatory B cells in adipose tissue could contribute to adipose tissue progression and obesity59 as well as RA development.60 Studies investigating serum adipokine levels in association with AS or PsA and obesity remain scarce, with elevated serum level of resistin and increased level of leptin in AS.61 62 Interestingly, a recent study reported that elevated baseline visfatin levels can predict subsequent progression of radiographic damage in patients with AS.63
Metabolic syndrome and dyslipidaemia associated with obesity may also play a role in the increased prevalence of inflammatory rheumatic diseases in obese participants. Indeed, a recent work from the Nurse Health Study II showed that hypercholesterolaemia is associated with incident psoriasis and PsA after adjusting for potential confounders.64 Hypercholesterolaemia affects regulatory and effector T cells,65 66 which could promote diseases such as psoriasis and PsA. However, further work is needed to investigate the mechanisms behind these associations.
Diet and microbiota
Diet and microbiota could also explain the link between obesity and inflammatory arthritis. The ‘obesogenic’ diet is characterised by increased consumption of energy-dense processed foods and reduced consumption of nutrient-rich foods such as fruits and vegetables, corresponding to low fibre and high fat contents. Long-term intake of fish and other sources of long-chain n-3 polyunsaturated fatty acids (n-3 PUFAs) was found to be protective for RA development.67 Conversely, consumption of ≥1 sugar-sweetened soda per day was associated with a 63% increased risk of developing seropositive RA as compared with no consumption of sugar-sweetened soda or <1 soda per month.68
Processed food is enriched in salt. Two recent publications postulate that salt-rich diets could also affect the immune system and explain the increasing prevalence of autoimmunity.69 70 Increased salt concentrations found locally under physiological conditions in vivo greatly boost the induction of murine and human Th17 cells. Moreover, mice fed a high-salt diet show a more severe form of experimental autoimmune encephalomyelitis, an animal model for multiple sclerosis, in line with increased Th17 cells.69 Such results agree with a recent study showing that an increase in salt concentration promotes IL-23R expression and enhances Th17 cell differentiation in vitro and in vivo.70 A low salt diet may also prevent the development of collagen-induced arthritis,71 whereas a high-salt diet enhanced inflammation and joint destruction in the same model.72
Despite few studies, RA microbiota composition seems altered73 74 and antibiotic use exacerbates arthritis in mice.75 The impact of microbiota on RA development may be direct or indirect. First, a genetic background such as shared epitopes, which predispose to RA, affects the composition of microbiota, which could in turn be involved in arthritis development.76 Moreover, since obesity can be transmitted to lean mice via faecal microbiota transplantation from obese mice,77 microbiota could influence obese status and thus indirectly modulate RA risk and severity.78 Fibre can only be broken down by commensal bacteria. Short chain fatty acids (SCFAs) are the metabolites of this fibre digestion and the concentrations are thus influenced by composition of the beneficial commensal flora.79 SCFAs promote regulatory T cell (Treg) development in the gut and have well-established anti-inflammatory effects.79 The implication of SCFAs in RA is supported by the fact that mice deficient in SCFA receptors (Gpr43−/−) showed exacerbated inflammation in models of arthritis.79 Pathogenic bacteria produce endotoxins such as lipopolysaccharides that activate the nuclear factor-kB pathway and promote the development of inflammatory arthritis.80 However, from a therapeutic point of view, the potential therapeutic effect of probiotics such as Lactobacillus casei in inflammatory arthritis has never been shown.81
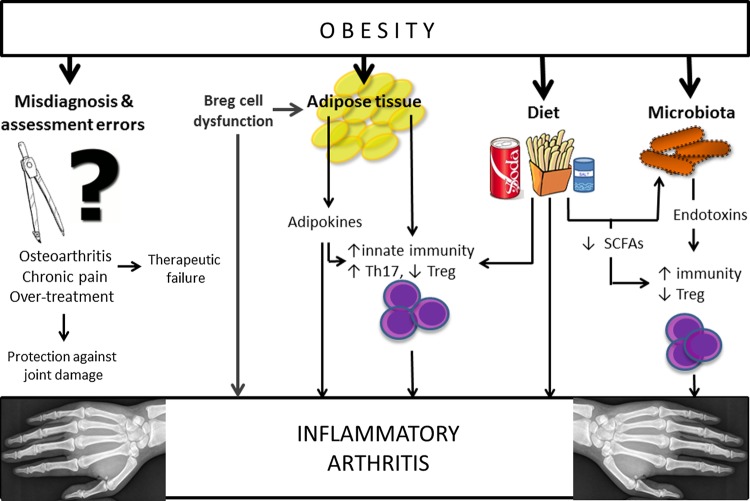
Thus, microbiota in patients with obesity could enhance autoimmune diseases such as RA directly via bacterial endotoxin-induced inflammation and Treg deficiency or indirectly in that microbiota disturbances are tightly associated with diet and obesity, which may in turn increase the risk of RA (figure 1).
Figure 1.
Potential mechanisms explaining the link between obesity and inflammatory arthritis. Breg, regulatory B cells; Th17, T helper 17; SCFAs, short chain fatty acids; Treg, regulatory T cells.
In conclusion, obesity is associated with increased risk of RA and PsA incidence, increased disease activity and reduced radiographic joint damage and progression in RA and reduced therapeutic response in RA and spondyloarthritis, especially for infliximab use (see table 1 for summary). These findings may be explained by assessment drawback due to obesity, OA and a more painful state. Adipokines are probably involved in such links, especially with RA and obesity. The impact of diet and microbiota affecting the immune system may have an additional role. A better knowledge of the arthritis–obesity interaction will help us improve the personalised management of inflammatory rheumatism in this specific setting with potential molecular targets that could improve both diseases.
Footnotes
Competing interests: CID: consulting fees, congresses financial support and/or grant researches: Pfizer, Roche, Chugai, MSD, BMS, UCB, Abbvie. JS: consulting fees and/or grant researches: Pfizer, Roche, Chugai, MSD, BMS, UCB, Servier, Laboratoire Expanscience, Abbvie.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol 2006;35:93–9. 10.1093/ije/dyi272 [DOI] [PubMed] [Google Scholar]
- 2.Roubenoff R, Roubenoff RA, Ward LM et al. Rheumatoid cachexia: depletion of lean body mass in rheumatoid arthritis. Possible association with tumor necrosis factor. J Rheumatol 1992;19:1505–10. [PubMed] [Google Scholar]
- 3.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 4.Blew RM, Sardinha LB, Milliken LA et al. Assessing the validity of body mass index standards in early postmenopausal women. Obes Res 2002;10:799–808. 10.1038/oby.2002.108 [DOI] [PubMed] [Google Scholar]
- 5.Elkan AC, Engvall IL, Cederholm T et al. Rheumatoid cachexia, central obesity and malnutrition in patients with low-active rheumatoid arthritis: feasibility of anthropometry, Mini Nutritional Assessment and body composition techniques. Eur J Nutr 2009;48:315–22. 10.1007/s00394-009-0017-y [DOI] [PubMed] [Google Scholar]
- 6.Giles JT, Ling SM, Ferrucci L et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum 2008;59:807–15. 10.1002/art.23719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binymin K, Herrick A, Carlson G et al. The effect of disease activity on body composition and resting energy expenditure in patients with rheumatoid arthritis. J Inflamm Res 2011;4:61–6. 10.2147/JIR.S16508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y et al. Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis 2007;66:1316–21. 10.1136/ard.2006.060319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz PP, Yazdany J, Trupin L et al. Sex differences in assessment of obesity in rheumatoid arthritis. Arthritis Care Res 2013;65:62–70. 10.1002/acr.21810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedreira PG, Pinheiro MM, Szejnfeld VL. Bone mineral density and body composition in postmenopausal women with psoriasis and psoriatic arthritis. Arthritis Res Ther 2011;13:R16 10.1186/ar3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sari I, Demir T, Kozaci LD et al. Body composition, insulin, and leptin levels in patients with ankylosing spondylitis. Clin Rheumatol 2007;26:1427–32. 10.1007/s10067-006-0509-6 [DOI] [PubMed] [Google Scholar]
- 12.Plasqui G, Boonen A, Geusens P et al. Physical activity and body composition in patients with ankylosing spondylitis. Arthritis Care Res 2012;64:101–7. 10.1002/acr.20566 [DOI] [PubMed] [Google Scholar]
- 13.Crowson CS, Matteson EL, Davis JM et al. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res 2013;65:71–7. 10.1002/acr.21660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen M, Jacobsen S, Klarlund M et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther 2006;8:R133 10.1186/ar2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wesley A, Bengtsson C, Elkan AC et al. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: results from a population-based case-control study. Arthritis Care Res 2013;65:107–12. 10.1002/acr.21749 [DOI] [PubMed] [Google Scholar]
- 16.Lahiri M, Luben RN, Morgan C et al. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register—the EPIC-2-NOAR Study). Ann Rheum Dis 2014;73:219–26. 10.1136/annrheumdis-2012-202481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu B, Hiraki LT, Sparks JA et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis 2014;73:1914–22. 10.1136/annrheumdis-2014-205459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labitigan M, Bahče-Altuntas A, Kremer JM et al. Higher rates and clustering of abnormal lipids, obesity, and diabetes mellitus in psoriatic arthritis compared with rheumatoid arthritis. Arthritis Care Res 2014;66:600–7. 10.1002/acr.22185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhole VM, Choi HK, Burns LC et al. Differences in body mass index among individuals with PsA, psoriasis, RA and the general population. Rheumatol Oxf Engl 2012;51:552–6. 10.1093/rheumatology/ker349 [DOI] [PubMed] [Google Scholar]
- 20.Love TJ, Zhu Y, Zhang Y et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis 2012;71:1273–7. 10.1136/annrheumdis-2012-201299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Han J, Qureshi AA. Obesity and risk of incident psoriatic arthritis in US women. Ann Rheum Dis 2012;71:1267–72. 10.1136/annrheumdis-2011-201273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van der Helm-van Mil AH, van der Kooij SM, Allaart CF et al. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis 2008;67:769–74. 10.1136/ard.2007.078832 [DOI] [PubMed] [Google Scholar]
- 23.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Intern Med 2007;167:1670–5. 10.1001/archinte.167.15.1670 [DOI] [PubMed] [Google Scholar]
- 24.Herron MD, Hinckley M, Hoffman MS et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol 2005;141:1527–34. 10.1001/archderm.141.12.1527 [DOI] [PubMed] [Google Scholar]
- 25.Soltani-Arabshahi R, Wong B, Feng BJ et al. Obesity in early adulthood as a risk factor for psoriatic arthritis. Arch Dermatol 2010;146:721–6. 10.1001/archdermatol.2010.141 [DOI] [PubMed] [Google Scholar]
- 26.Baker JF, George M, Baker DG et al. Associations between body mass, radiographic joint damage, adipokines and risk factors for bone loss in rheumatoid arthritis. Rheumatol Oxf Engl 2011;50:2100–7. 10.1093/rheumatology/ker294 [DOI] [PubMed] [Google Scholar]
- 27.Giles JT, Allison M, Blumenthal RS et al. Abdominal adiposity in rheumatoid arthritis: association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum 2010;62:3173–82. 10.1002/art.27629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal C, Barnetche T, Morel J et al. Influence of body mass index on disease activity and radiographic joint damage in rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Rheumatol 2014;66(11 Suppl):S167. [DOI] [PubMed] [Google Scholar]
- 29.Jawaheer D, Olsen J, Lahiff M et al. Gender, body mass index and rheumatoid arthritis disease activity: results from the QUEST-RA Study. Clin Exp Rheumatol 2010;28:454–61. [PMC free article] [PubMed] [Google Scholar]
- 30.Ajeganova S, Andersson ML, Hafström I et al. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long-term followup from disease onset. Arthritis Care Res 2013;65:78–87. 10.1002/acr.21710 [DOI] [PubMed] [Google Scholar]
- 31.Heimans L, van den Broek M, le Cessie S et al. Association of high body mass index with decreased treatment response to combination therapy in recent-onset rheumatoid arthritis patients. Arthritis Care Res 2013;65:1235–42. 10.1002/acr.21978 [DOI] [PubMed] [Google Scholar]
- 32.Westhoff G, Rau R, Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum 2007;56:3575–82. 10.1002/art.23033 [DOI] [PubMed] [Google Scholar]
- 33.Baker JF, Ostergaard M, George M et al. Greater body mass independently predicts less radiographic progression on X-ray and MRI over 1–2years. Ann Rheum Dis 2014;73:1923–8. 10.1136/annrheumdis-2014-205544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durcan L, Wilson F, Conway R et al. Increased body mass index in ankylosing spondylitis is associated with greater burden of symptoms and poor perceptions of the benefits of exercise. J Rheumatol 2012;39:2310–14. 10.3899/jrheum.120595 [DOI] [PubMed] [Google Scholar]
- 35.Aydin M, Aydin F, Yuksel M et al. Visceral fat reflects disease activity in patients with ankylosing spondylitis. Clin Investig Med Médecine Clin Exp 2014;37:E186. [DOI] [PubMed] [Google Scholar]
- 36.Di Minno MN, Peluso R, Iervolino S et al. Obesity and the prediction of minimal disease activity: a prospective study in psoriatic arthritis. Arthritis Care Res 2013;65:141–7. 10.1002/acr.21711 [DOI] [PubMed] [Google Scholar]
- 37.Eder L, Thavaneswaran A, Chandran V et al. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann Rheum Dis 2015;74:813–7. 10.1136/annrheumdis-2013-204448 [DOI] [PubMed] [Google Scholar]
- 38.Sandberg ME, Bengtsson C, Källberg H et al. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann Rheum Dis 2014;73:2029–33. 10.1136/annrheumdis-2013-205094 [DOI] [PubMed] [Google Scholar]
- 39.Klaasen R, Wijbrandts CA, Gerlag DM et al. Body mass index and clinical response to infliximab in rheumatoid arthritis. Arthritis Rheum 2011;63:359–64. 10.1002/art.30136 [DOI] [PubMed] [Google Scholar]
- 40.Gremese E, Carletto A, Padovan M et al. Obesity and reduction of the response rate to anti-tumor necrosis factor α in rheumatoid arthritis: an approach to a personalized medicine. Arthritis Care Res 2013;65:94–100. 10.1002/acr.21768 [DOI] [PubMed] [Google Scholar]
- 41.Pers YM, Godfrin-Valnet M, Lambert J et al. Response to tocilizumab in rheumatoid arthritis is not influenced by the body mass index of the patient. J Rheumatol 2015;42:580–4. 10.3899/jrheum.140673 [DOI] [PubMed] [Google Scholar]
- 42.Ottaviani S, Allanore Y, Tubach F et al. Body mass index influences the response to infliximab in ankylosing spondylitis. Arthritis Res Ther 2012;14:R115 10.1186/ar3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gremese E, Bernardi S, Bonazza S et al. Body weight, gender and response to TNF-α blockers in axial spondyloarthritis. Rheumatol Oxf Engl 2014;53:875–81. 10.1093/rheumatology/ket433 [DOI] [PubMed] [Google Scholar]
- 44.Sparks C, Moots R, Psarelli E et al. Impact of obesity on 1 year outcomes: results from the meteor foundation international rheumatoid arthritis cohort. Arthritis Rheumatol 2014;66:(11Suppl):S468. [Google Scholar]
- 45.Di Minno MN, Peluso R, Iervolino S et al. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Ann Rheum Dis 2014;73:1157–62. 10.1136/annrheumdis-2012-202812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Mutairi N, Nour T. The effect of weight reduction on treatment outcomes in obese patients with psoriasis on biologic therapy: a randomized controlled prospective trial. Expert Opin Biol Ther 2014;14:749–56. 10.1517/14712598.2014.900541 [DOI] [PubMed] [Google Scholar]
- 47.Avina-Zubieta JA, Thomas J, Sadatsafavi M et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–9. 10.1136/annrheumdis-2011-200726 [DOI] [PubMed] [Google Scholar]
- 48.Mathieu S, Gossec L, Dougados M et al. Cardiovascular profile in ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Care Res 2011;63:557–63. 10.1002/acr.20364 [DOI] [PubMed] [Google Scholar]
- 49.Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF et al. Associations of obesity with modifiable risk factors for the development of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis 2009;68:242–5. 10.1136/ard.2008.095596 [DOI] [PubMed] [Google Scholar]
- 50.Escalante A, Haas RW, del Rincón I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med 2005;165:1624–9. 10.1001/archinte.165.14.1624 [DOI] [PubMed] [Google Scholar]
- 51.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res 2012;64:1471–9. 10.1002/acr.21627 [DOI] [PubMed] [Google Scholar]
- 52.Taylor R, Pergolizzi JV, Raffa RB et al. Pain and obesity in the older adult. Curr Pharm Des 2014;20:6037–41. 10.2174/1381612820666140316131431 [DOI] [PubMed] [Google Scholar]
- 53.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006;6:772–83. 10.1038/nri1937 [DOI] [PubMed] [Google Scholar]
- 54.Gómez R, Conde J, Scotece M et al. What's new in our understanding of the role of adipokines in rheumatic diseases? Nat Rev Rheumatol 2011;7:528–36. 10.1038/nrrheum.2011.107 [DOI] [PubMed] [Google Scholar]
- 55.Busso N, So A, Chobaz-Péclat V et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol 2002;168:875–82. 10.4049/jimmunol.168.2.875 [DOI] [PubMed] [Google Scholar]
- 56.Frommer KW, Schäffler A, Büchler C et al. Adiponectin isoforms: a potential therapeutic target in rheumatoid arthritis? Ann Rheum Dis 2012;71:1724–32. 10.1136/annrheumdis-2011-200924 [DOI] [PubMed] [Google Scholar]
- 57.Meyer M, Sellam J, Fellahi S et al. Serum level of adiponectin is a surrogate independent biomarker of radiographic disease progression in early rheumatoid arthritis: results from the ESPOIR cohort. Arthritis Res Ther 2013;15:R210 10.1186/ar4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giles JT, Allison M, Bingham CO et al. Adiponectin is a mediator of the inverse association of adiposity with radiographic damage in rheumatoid arthritis. Arthritis Rheum 2009;61:1248–56. 10.1002/art.24789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishimura S, Manabe I, Takaki S et al. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell Metab 2013;S1550-4131:386–0. [DOI] [PubMed] [Google Scholar]
- 60.Daien CI, Gailhac S, Mura T et al. Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheumatol 2014;66:2037–46. 10.1002/art.38666 [DOI] [PubMed] [Google Scholar]
- 61.Toussirot E, Streit G, Nguyen NU et al. Adipose tissue, serum adipokines, and ghrelin in patients with ankylosing spondylitis. Metabolism 2007;56:1383–9. 10.1016/j.metabol.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 62.Kocabas H, Kocabas V, Buyukbas S et al. The serum levels of resistin in ankylosing spondylitis patients: a pilot study. Rheumatol Int 2012;32:699–702. 10.1007/s00296-010-1651-7 [DOI] [PubMed] [Google Scholar]
- 63.Syrbe U, Callhoff J, Conrad K et al. Adipokine serum levels in patients with ankylosing spondylitis and their relation to clinical parameters and radiographic spinal progression. Arthritis Rheumatol 2015;67:678–85. [DOI] [PubMed] [Google Scholar]
- 64.Wu S, Li WQ, Han J et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol 2014;66:304–10. 10.1002/art.38227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maganto-García E, Tarrio ML, Grabie N et al. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation 2011;124:185–95. 10.1161/CIRCULATIONAHA.110.006411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robertson AK, Zhou X, Strandvik B et al. Severe hypercholesterolaemia leads to strong Th2 responses to an exogenous antigen. Scand J Immunol 2004;59:285–93. 10.1111/j.0300-9475.2004.01403.x [DOI] [PubMed] [Google Scholar]
- 67.Di Giuseppe D, Wallin A, Bottai M et al. Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women. Ann Rheum Dis 2014;73:1949–53. 10.1136/annrheumdis-2013-203338 [DOI] [PubMed] [Google Scholar]
- 68.Hu Y, Costenbader KH, Gao X et al. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am J Clin Nutr 2014;100:959–67. 10.3945/ajcn.114.086918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kleinewietfeld M, Manzel A, Titze J et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013;496:518–22. 10.1038/nature11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu C, Yosef N, Thalhamer T et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013;496:513–7. 10.1038/nature11984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sehnert B, Pohle S, Schröder A et al. A low salt diet ameliorates clinical manifestations in collagen- induced arthritis. Arthritis Rheumatol 2014;66:(11Suppl):S145 10.1002/art.38530 [DOI] [Google Scholar]
- 72.Jung SM, Min HK, Koh JH et al. Salt Aggravates Arthritis By Th17 Polarization. Arthritis Rheumatol 2014;66:(11Suppl):S145 10.1002/art.38530 [DOI] [Google Scholar]
- 73.Vaahtovuo J, Munukka E, Korkeamäki M et al. Fecal microbiota in early rheumatoid arthritis. J Rheumatol 2008;35:1500–5. [PubMed] [Google Scholar]
- 74.Scher JU, Sczesnak A, Longman RS et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013;2:e01202 10.7554/eLife.01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dorożyńska I, Majewska-Szczepanik M, Marcińska K et al. Partial depletion of natural gut flora by antibiotic aggravates collagen induced arthritis (CIA) in mice. Pharmacol Rep PR 2014;66:250–5. 10.1016/j.pharep.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 76.Gomez A, Luckey D, Yeoman CJ et al. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS ONE 2012;7:e36095 10.1371/journal.pone.0036095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ridaura VK, Faith JJ, Rey FE et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013;341:1241214 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simões CD, Maukonen J, Kaprio J et al. Habitual dietary intake is associated with stool microbiota composition in monozygotic twins. J Nutr 2013;143:417–23. 10.3945/jn.112.166322 [DOI] [PubMed] [Google Scholar]
- 79.Tan J, McKenzie C, Potamitis M et al. The role of short-chain fatty acids in health and disease. Adv Immunol 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 80.Lorenz W, Buhrmann C, Mobasheri A et al. Bacterial lipopolysaccharides form procollagen-endotoxin complexes that trigger cartilage inflammation and degeneration: implications for the development of rheumatoid arthritis. Arthritis Res Ther 2013;15:R111 10.1186/ar4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alipour B, Homayouni-Rad A, Vaghef-Mehrabany E et al. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int J Rheum Dis 2014;17:519–27. [DOI] [PubMed] [Google Scholar]
- 82.Horreau C, Pouplard C, Brenaut E et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol 2013;27(Suppl 3):12–29. 10.1111/jdv.12163 [DOI] [PubMed] [Google Scholar]
- 83.Yusuf E, Nelissen RG, Ioan-Facsinay A et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis 2010;69:761–5. 10.1136/ard.2008.106930 [DOI] [PubMed] [Google Scholar]
- 84.Choe JY, Bae J, Lee H et al. Lack association of body mass index with disease activity composites of rheumatoid arthritis in Korean population: cross-sectional observation. Clin Rheumatol 2014;33:485–92. 10.1007/s10067-013-2427-8 [DOI] [PubMed] [Google Scholar]