Abstract
Objectives
We report the incidence of tuberculosis (TB) across certolizumab pegol (CZP) clinical trials in rheumatoid arthritis (RA), psoriasis, psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), before and after the introduction of stricter TB screening.
Methods
TB incidence rates (IRs) were assessed and stratified according to screening guidelines used at the time of CZP trials. Before 2007 (original trials), purified protein derivative (PPD) tuberculin skin test positivity varied according to local standards (induration ≥5 up to ≥20 mm). Since 2007, all CZP trial protocols have been amended, including trials spanning (intermediate) and initiated after 2007 (current), mandating that any patient with PPD≥5 mm receives treatment for latent TB infection (LTBI). All cases of suspected TB or PPD≥5 mm, in pooled data from 5402 CZP patients across all CZP trials up to 2012, underwent blinded central review by independent experts.
Results
44 TB cases were confirmed in pooled CZP RA trials (IR 0.47/100PY, patient-years) with no cases in Japanese RA trials (J-RAPID, HIKARI). Single TB cases were confirmed in psoriasis and axSpA trials (RAPID-axSpA), and no cases in the PsA trial (RAPID-PsA). IR of TB was 0.51/100PY across original or intermediate RA trials and 0.18/100PY in current trials. The majority of TB cases in RA occurred in Eastern (IR 1.02/100PY) and Central Europe (IR 0.58/100PY). Of 242/370 PPD≥5 mm patients who received 9 months isoniazid (INH) treatment for latent TB infection (LTBI), none developed TB, versus 7.8% of 128 untreated PPD≥5 mm patients.
Conclusions
Implementation of more stringent LTBI screening, plus treatment for LTBI, reduced the IR of TB, even when INH was administered after starting CZP therapy.
Keywords: Tuberculosis, Anti-TNF, Autoimmune Diseases
Key messages.
What is already known on this subject?
Antitumour necrosis factors (anti-TNFs) are associated with an increased risk of opportunistic infections, including active tuberculosis (TB).
What might this study add?
Using a strict purified protein derivative (PPD) cut-off of ≥5 mm induration, as well as a TB questionnaire, may reduce the incidence of TB in patients receiving anti-TNF therapy.
How might this impact on clinical practice?
Antibiotic treatment for latent TB infection (LTBI) at screening, or in patients already on anti-TNF therapy if a history of PPD ≥5 mm is documented, decreases the incidence of TB in patients receiving anti-TNF therapy.
Introduction
Antitumour necrosis factors (anti-TNFs) have provided a major breakthrough in the treatment of chronic inflammatory diseases but are associated with an increased risk of opportunistic infections, including active tuberculosis (TB).1 2 The onset of TB soon after initiation of anti-TNF therapy indicates the probable reactivation of latent TB infection (LTBI), while TB appearing after several months or more of continuous exposure to anti-TNF is likely to arise due to a new infection.2–4 Reports suggest that the incidence of TB in patients treated with biologics has decreased over the past decade thanks to screening of LTBI, as recommended in several countries.5 However, the risk of active TB (particularly non-pulmonary TB) associated with anti-TNFs1 2 4 and underlying inflammatory diseases5–7 remains an important concern. Thus, patients being considered for anti-TNF therapy should undergo initial screening for LTBI, and regular screening for TB infection should be performed during anti-TNF treatment if patients are living in, or have travelled to, TB endemic regions. However, the benefits of repeat screening in TB endemic regions are debatable8 and the perfect approach for screening patients with anti-TNF has not yet been resolved, with different strategies being used to try to identify patients at risk of developing TB.
Prior to 2007, certolizumab pegol (CZP) trials screened for TB by a purified protein derivative (PPD) tuberculin skin test were evaluated according to the respective national guidelines of the recruiting centre. The cut-off value for positivity varied widely (from ≥5 up to ≥20 mm induration), particularly in countries with higher bacillus Calmette-Guérin (BCG) vaccination rates.9 10
In 2007, the WHO introduced stricter practical guidelines to reduce the risk of TB in patients starting anti-TNF treatment and to help identify those at increased risk of developing TB (PPD test results ≥5 mm were considered positive).11 12 Similar screening recommendations have since been introduced by several national rheumatology societies to reduce the risk of TB infection in patients with immune-mediated inflammatory disease (IMID).13–18 In response to an increasing number of TB cases, mainly from high TB incidence countries, and WHO recommendations for more stringent TB screening, CZP trial protocols were amended in 2007;11 12 mandating that any patient with PPD ≥5 mm should begin 9 months isoniazid (INH) treatment for LTBI. This included patients already receiving CZP, even without any signs or symptoms considered to be TB-related. Patient TB questionnaires were also administered, every 6 months, to increase patient/investigator awareness of the symptoms and risk factors for TB (figure 1).
Figure 1.
Evaluation for signs and symptoms of the tuberculosis (TB) questionnaire (sample).
CZP is approved for the treatment of adult patients with moderate-to-severe RA, psoriatic arthritis (PsA), axial spondyloarthritis (axSpA; including ankylosing spondylitis) and Crohn's disease.19 20 This paper reports the incidence rate (IR) of TB during long-term CZP treatment across indications (RA, psoriasis, PsA and axSpA) and examines whether stricter screening rules for LTBI, with subsequent INH treatment when indicated, can reduce the risk of reactivation of TB in CZP-treated patients. The effects of baseline PPD status or different geographic regions on the IR of TB were also evaluated.
Methods
Patient population and study designs
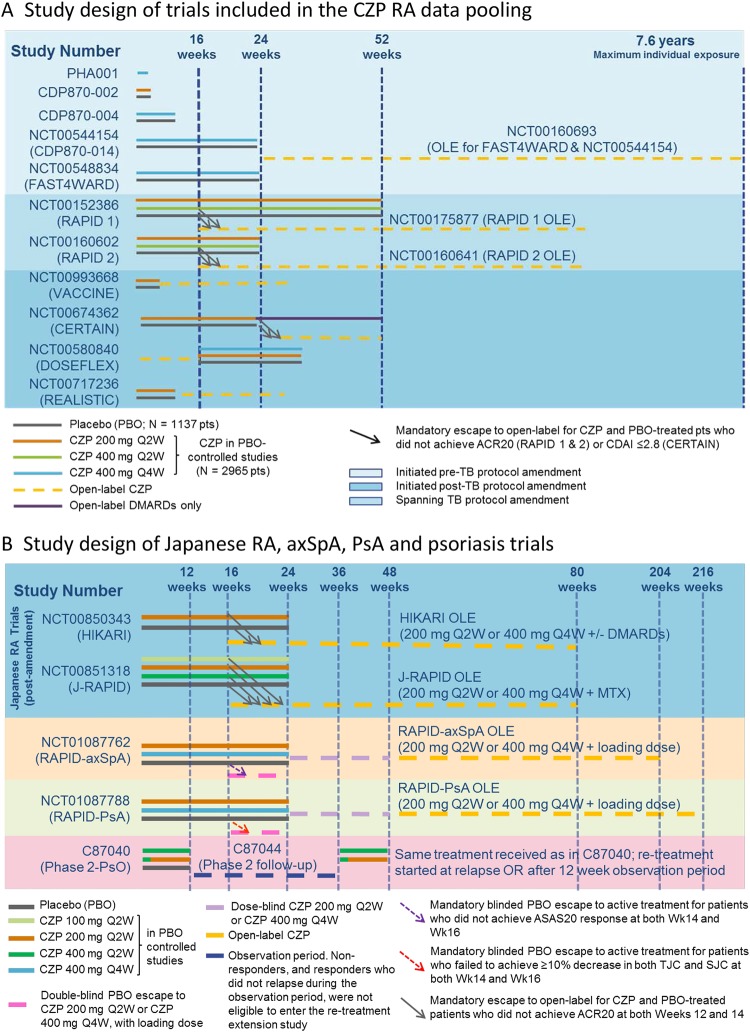
For RA, the pooled CZP safety database included 4049 patients with RA from 14 studies (including randomised controlled trials (RCTs) and open label extensions (OLEs); figure 2A), with maximum CZP exposure of 7.6 years as previously described.21 The first patient was enrolled by the end of 1998 and the safety cut-off date was 30 November 2011. Safety data were also assessed in 528 patients with RA enrolled in two Japanese CZP RCTs and their OLEs (J-RAPID (NCT00791999, NCT00851318), HIKARI (NCT00791921, NCT00850343)) from 19 November 2008 up to 6 June 2013 (figure 2B).
Figure 2.
Study design of CZP trials included in the safety analysis for the incidence of tuberculosis (TB). CZP, certolizumab pegol; OLE, open label extensions; RA, rheumatoid arthritis;
In addition, safety data were assessed for psoriasis phase 2 CZP studies conducted between October 2005 and May 2007 (N=117 patients; C87040 (NCT00245765) and C87044 (NCT00329303)). Safety data were also assessed for the RAPID-PsA15 (N=393 patients; NCT01087788) and RAPID-axSpA18 studies (N=315 patients; NCT01087762) initiated in March 2010, using the new TB screening cut-off criteria of ≥5 mm induration for PPD positivity, up to the cut-off date of 16 November 2012 (figure 2B).
For the majority of RA studies, and for RAPID-PsA and RAPID-axSpA, a loading dose of CZP 400 mg was administered subcutaneously at weeks 0, 2 and 4, followed by CZP 200 mg every 2 weeks (Q2W) or CZP 400 mg every 4 weeks (registered doses). During RAPID1 and RAPID2 RCTs (NCT00152386, NCT00160602), and for ≥6 months during their OLEs (NCT00175877, NCT00160641), CZP was also administered at 400 mg Q2W (twice the registered dose) for all patients. Across trials, patients were exposed to CZP either as a monotherapy or in combination with methotrexate and/or other DMARDs.
TB screening and protocol amendment
All patients were screened for TB at study baseline. The safety database was manually reviewed retrospectively by independent experts (JC, RvV, XM, KW, LC for RA; JC, RvV, XM, KW, LC, JV, JG-R, MW, OL for psoriasis, PsA and axSpA). All cases of suspected TB (PPD positive or exhibiting signs/symptoms compatible with TB) were classified as active TB, LTBI or PPD positive, or as non-evaluable. Active TB cases reported in this paper were confirmed according to the WHO or Centers for Disease Control and Prevention (CDC) criteria for TB.22
In CZP studies before 2007, TB screening was conducted according to respective national guidelines of the recruiting study centre (cut-off for PPD positivity ranged from ≥5 up to ≥20 mm). Since 2007, patients were screened for PPD positivity ≥5 mm at baseline and subsequently received INH treatment for LTBI if they tested PPD positive. This amendment was also enforced retrospectively for all patients already on CZP treatment at the time of protocol amendment. Thus, patients with a baseline PPD of ≥5 mm or no precise information on the wheal size, and no evidence of TB infection, were treated with INH for 9 months, regardless of prior exposure to CZP.11 12 Patient TB questionnaires were also administered every 6 months (figure 1).
Stratification of RA studies
Data from the pooled RA safety database21 (which excluded the Japanese RA trials) were assessed for trials initiated prior to 2007 (2367 patients) in two subgroups: (1) original trials (837 patients), completed prior to TB screening protocol amendment; and (2) intermediate trials (1530 patients from the RAPID1 and RAPID2 trials), which were initiated using PPD positivity cut-off from ≥5 to ≥20 mm but amended retrospectively. Group (3), current trials (1682 patients), were initiated after protocol amendment in 2007 and any patient was subsequently treated for LTBI if they tested PPD positive at study baseline.
Statistical analyses
Safety assessments included all confirmed TB events that occurred after the first dose of CZP or placebo (PBO), and up to 84 days after the last study dose or patient withdrawal. Total exposure to CZP, as used in the IR calculation, was defined as the time from the first dose until the date that the TB event was reported. Exposure-adjusted IRs per 100 patient-years (PY), with 95% CI, are presented in this analysis. In some instances, data are also presented as the percentage of patients with events divided by the total number of patients. All statistics were performed using SAS V.9.1 or a later version.
Results
Incidence of TB in CZP-treated patients across indications
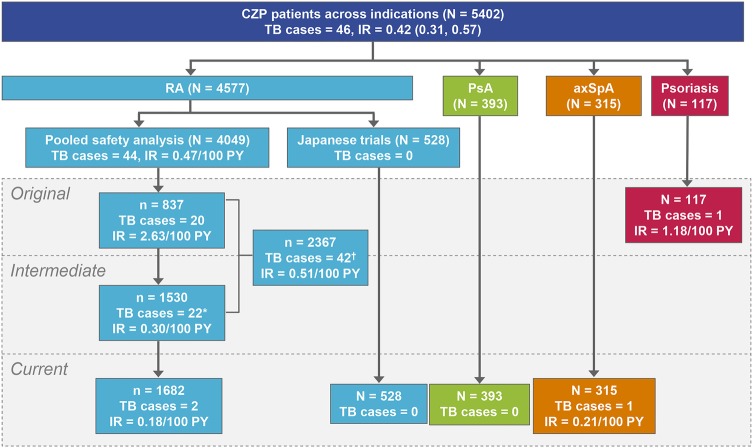
The incidence of TB was assessed in a total of 5402 CZP patients treated for RA, psoriasis, PsA and axSpA, with an overall IR of 0.42/100 PY (95% CI 0.31 to 0.57). For RA, 4049 patients were included in the pooled safety database (9277 PY; mean CZP exposure 782 days, median exposure 267 days).21 Up to 30 November 2011, 44 confirmed TB cases were reported in CZP-treated patients with RA (IR 0.47/100 PY (0.34 to 0.64); also, there were 30 cases of pulmonary TB and 14 cases of non-pulmonary (including isolated lymph node TB) or disseminated TB (occurring in two or more non-continuous organs)). No TB cases were reported in PBO patients during RCTs; however, the total exposure time was relatively short (total exposure 373 PY; mean exposure 110 days), mainly due to mandatory early escape to active CZP treatment for non-responders at weeks 12 and 14 in the RAPID1 and RAPID2 studies, as compared to CZP exposure (total exposure 9277 PY; mean exposure 782 days). Separate analysis of safety data from 528 patients with RA enrolled in Japanese CZP trials (J-RAPID and HIKARI and their OLEs) conducted up to 6 June 2013 confirmed no cases of TB (figure 3).
Figure 3.
Summary of TB cases across indications and trial periods. †A total of 42 TB cases were reported in rheumatoid arthritis (RA) trials initiated or completed prior to protocol amendment; 20 cases in original RA trials (completed prior to protocol amendment) and 22 in intermediate RA trials (initiated before but completed after the introduction of more stringent PPD screening criteria in 2007). *Of the 22 TB cases in patients with RA from intermediate RA trials (RAPID1 and RAPID2), 9 cases were reported after protocol amendment. AxSpA, axial spondyloarthritis; PsA, psoriatic arthritis; IR, incidence rates; PY, patient-years; TB, tuberculosis.
Incidence of TB was also assessed in 117 study patients with psoriasis (85 PY; mean CZP exposure 206 days) up to 9 May 2007 and confirmed one case of disseminated TB after 61 days of CZP exposure (IR 1.18/100 PY (0.03 to 6.58)). The incidence of TB was also assessed in 393 RAPID-PsA patients (612 PY; mean CZP exposure 568 days) and 315 RAPID-axSpA patients (481 PY; mean CZP exposure 558 days). Up to 16 November 2012, a single case of pulmonary TB was confirmed in RAPID-axSpA (IR 0.21/100 PY (0.01 to 1.15)), with no confirmed cases in RAPID-PsA.
Incidence of TB in patients with RA by protocol period and duration of TB exposure
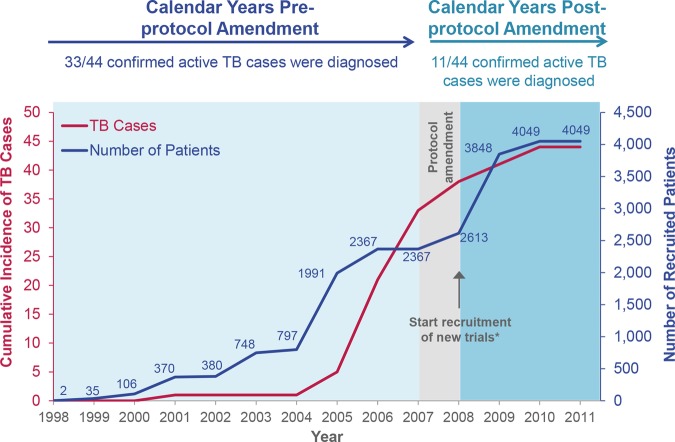
Overall, the IR of TB in CZP-treated patients with RA in trials initiated prior to protocol amendment was 0.51/100 PY (0.37 to 0.69) (42/2367 patients); 2.63/100 PY (1.61 to 4.06) in original trials (20/837 patients) and 0.30/100 PY (0.19 to 0.45) in intermediate trials spanning the period of protocol amendment (22/1530 patients). For current trials initiated with the standardised 5 mm PPD cut-off after protocol amendment was enforced, the IR was 0.18/100 PY (0.02 to 0.66) (2/1682 patients). In total, 11/44 TB cases in RA trials were diagnosed after protocol amendment (9 from intermediate trials [RAPID1 and RAPID2] and 2 from current trials; figures 3 and 4). Of these 11 TB cases, 10 patients were considered PPD negative at baseline (PPD<5 mm) and one patient had an unknown PPD value. All nine patients from the intermediate period had PPD<5 mm at baseline and were from Central or Eastern Europe, with TB onset occurring ≥718 days after the first dose of CZP. Of the confirmed TB cases in patients with RA, 21/44 were reported during the first year of CZP exposure. This included both cases of TB in current RA CZP trials, reported in patients with either negative or unknown baseline PPD test values.
Figure 4.
Cumulative incidence of tuberculosis (TB) in certolizumab pegol (CZP)-treated patients with rheumatoid arthritis (RA) in the pooled RA safety database (N=4049) in relation to study year. Cumulative incidence of TB in RA trials between 1998 and the safety data-cut in November 2011. The cumulative number of recruited patients in each study year are also presented. *Vaccine study, CERTAIN, DOSEFLEX, REALISTIC.
Incidence of TB in relation to baseline PPD status and INH treatment for LTBI
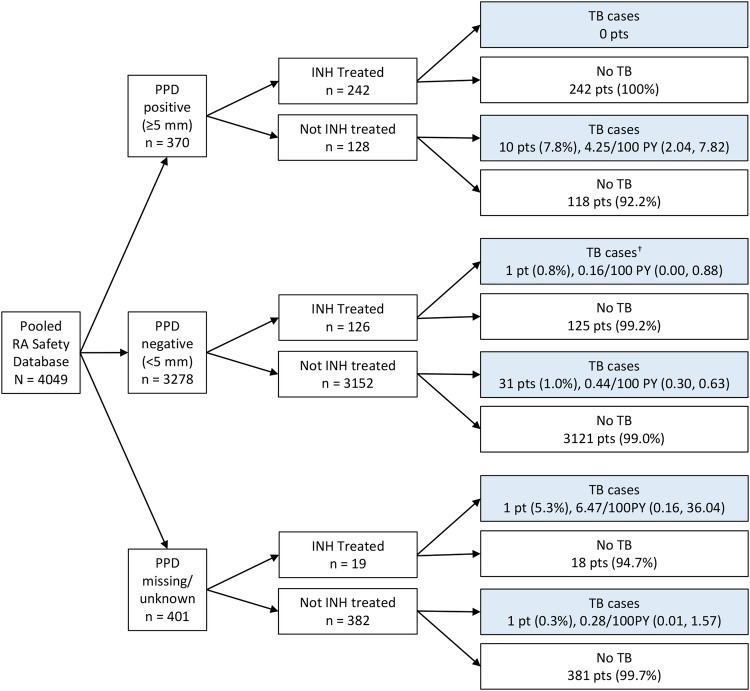
A total of 370/4049 patients in the pooled RA safety database had baseline PPD ≥5 mm. Of these, 242 patients received treatment for LTBI (figure 5); 27 began INH treatment prebaseline, 15 at study baseline, and 200 patients started treatment for LTBI postbaseline following the protocol amendment. No PPD positive patients with RA in receipt of INH treatment for 9 months developed TB (figure 5). One hundred twenty eight patients did not receive INH treatment as they were considered PPD negative according to local guidelines at the time of study enrolment, or had no symptoms of active TB. Of these, 10 patients recruited prior to protocol amendment went on to develop TB. According to the stricter guidelines for PPD positivity cut-off, these patients would have been considered PPD positive at the time of screening. Four had a PPD test result of 5–10 mm and the remaining six patients had baseline PPD >10 mm (table 1). The average time of TB onset after the first dose of CZP was between ∼1 and 1.5 years, and 6/10 cases were isolated pulmonary TB (table 1).
Figure 5.
PPD status of CZP-treated patients with RA in the pooled RA safety database (N=4049) at baseline and TB incidence by INH treatment. †One patient who developed TB started INH treatment 393 days after the first CZP dose was administered. INH was administered from 517 to 251 days before TB was diagnosed. CZP, certolizumab pegol; PPD, purified protein derivative; INH, isoniazid; RA, rheumatoid arthritis; TB, tuberculosis.
Table 1.
Incidence and type of active TB by PPD skin test subgroup for CZP-treated patients with RA in the pooled RA safety database (N=4049)
| TB events | Baseline PPD status |
|||
|---|---|---|---|---|
| <5 mm (n=3278) | 5–10 mm (n=219) | >10 mm (n=151) | PPD missing/unknown (n=401) | |
| Positive TB cases | ||||
| n (%) | 32*(1.0) | 4 (1.8) | 6 (4.0) | 2† (0.5) |
| IR/100 PY (95% CI) | 0.42 (0.29 to 0.59) | 0.49 (0.13 to 1.25) | 1.27 (0.47 to 2.77) | 0.54 (0.07 to 1.95) |
| Time from start of CZP treatment to TB onset (days) | ||||
| Mean (SD) | 552.5 (404.7) | 519.3 (318.8) | 473.7 (404.3) | 171.0 (41.0) |
| Median | 435.5 | 617.5 | 352.0 | 171.0 |
| Q1–Q3 | 204.0–838.5 | 319.0–719.5 | 148.0–806.0 | 142.0–200.0 |
| Minimum–maximum | 50–1473 | 58–784 | 71–1113 | 142–200 |
| Pulmonary TB cases | 22 | 4 | 2 | 2 |
| Non-pulmonary or disseminated TB cases | 10 | 0 | 4 | 0 |
| Positive TB cases/patients treated with INH (%) | 1/126 (0.8) | 0/153 (0.0) | 0/89 (0.0) | 1/19 (5.3) |
| Positive TB cases/patients not treated with INH (%) | 31/3152 (1.0) | 4/66 (6.1) | 6/62 (9.7) | 1/382 (0.3) |
*One patient who developed TB received INH administration. INH treatment started 393 days after the first CZP dose was administered and 517 days before the onset of TB.
†One patient with unknown PPD who developed TB was administered INH treatment before study baseline; INH was administered 141 days prior to the first CZP dose and 340 days before the onset of TB.
%, percentage of patients; CZP, certolizumab pegol; INH, isoniazid; IR, incidence rates; N, number of patients; PPD, purified protein derivative; PY, patient-years; RA, rheumatoid arthritis; TB, tuberculosis.
A total of 3278 patients had a PPD<5 mm and were considered PPD negative; the remaining 401 patients had no PPD test values recorded. Among patients with baseline PPD<5 mm, INH treatment (typically administered postbaseline) had little impact on TB incidence; 1/126 INH-treated patients (0.8%) versus 31/3152 non-INH-treated patients (1.0%) developed TB (figure 5). INH treatment of the PPD negative patient who went on to develop TB was initiated following a positive routine QuantiFERON test, performed 393 days after starting CZP treatment, and TB was reported 517 days later. The average time to TB onset in the 32 patients with PPD<5 mm at baseline was ∼1.5 years after the first dose of CZP, the long latency suggestive of a new TB infection rather than LTBI reactivation. The majority of cases (22/32) were diagnosed as isolated pulmonary TB (table 1). Onset of TB in the two patients with RA with unknown PPD value occurred 142 and 200 days after the first dose of CZP; one patient had a history of positive PPD tests and was treated with INH for 141 days prior to administration of CZP, suggesting reactivation of LTBI.
In the J-RAPID/HIKARI trials, 213/528 patients received INH at study baseline (no cases of TB were reported in these trials). No INH treatment for LTBI was administered to patients enrolled in psoriasis CZP trials (study 040/044), including a patient who developed disseminated TB. In the RAPID-PsA and RAPID-axSpA trials, a total of 26/393 and 25/315 patients, respectively, received INH treatment at study baseline. The single case of pulmonary TB reported in the RAPID-axSpA trial occurred in a PPD negative patient who did not receive treatment for LTBI. Onset of TB in the patient with axSpA, originally randomised to PBO for 24 weeks, was reported after 170 days of CZP exposure.
Overall, of the 46 cases of confirmed TB across CZP trials (44 in RA (20 in original, 22 in intermediate and 2 in current trials), 1 in psoriasis, 1 in axSpA), 34 were observed prior to the implementation of stricter screening rules (33 in RA, 1 in psoriasis) and predominantly occurred in patients who did not receive INH treatment.
Incidence of TB in relation to geographic location
The geographic distribution of CZP-treated patients in the pooled RA safety database was 19.8% from Western Europe, 20.0% from Central Europe, 12.4% from Eastern Europe, 41.3% from North America and 6.5% from the Rest of the World. United Nations statistics for the lower bound incidence of TB in the general population report a higher risk in Eastern and Central Europe (table 2). Consistent with this, the majority of TB cases observed with CZP originated in Eastern Europe (21 cases; IR 1.02/100 PY (0.63 to 1.56)) and Central Europe (18 cases; IR 0.58/100 PY (0.34 to 0.92)). 29/32 TB cases in patients with RA with PPD<5 mm at baseline were reported in patients from Eastern or Central Europe. Nevertheless, the rate of TB in patients with RA was far below the expected rate observed in the general population in each respective country (table 2). Three TB cases were confirmed in Western Europe (2 in Germany, 1 in the UK) along with single cases in North America (US) and the Rest of the World (Argentina) (table 2). The single cases of TB in the psoriasis and axSpA trials were in France and Argentina, respectively.
Table 2.
TB cases in CZP-treated patients with RA in the pooled RA safety database (N=4049) by region and country (risk vs incidence)
| Baseline PPD status (n) |
Incidence of TB within CZP treatment groups |
|||||||
|---|---|---|---|---|---|---|---|---|
| Geographic region | TB incidence rate in general population (per 105)* | Patients (n) | <5 mm | ≥5 mm | missing/ unknown | IR/100 PY (95% CI) | Number of events (in patients with PPD<5 mm) | INH-treated, no active TB (no TB/INH treated) |
| Overall | 4049 | 3278 | 370 | 401 | 0.47 (0.34 to 0.64) | 44 (32) | 385/387 | |
| Western Europe† | 7.9 | 800 | 464 | 42 | 294 | 0.23 (0.05 to 0.68) | 3 (2) | 19/19 |
| Austria | 6.9 | 125 | 40 | 1 | 84 | – | 0 (−) | 0/0 |
| Belgium | 8.5 | 19 | 18 | 1 | 0 | – | 0 (−) | 0/0 |
| Finland | 4.9 | 8 | 8 | 0 | 0 | – | 0 (−) | 0/0 |
| France | 7.7 | 69 | 24 | 2 | 43 | – | 0 (−) | 0/0 |
| Germany | 4.9 | 351 | 294 | 19 | 38 | 0.34 (0.04 to 1.22) | 2 (2) | 6/6 |
| Ireland | 7.5 | 8 | 4 | 4 | 0 | – | 0 (−) | 2/2 |
| Italy | 5.8 | 20 | 18 | 2 | 0 | – | 0 (−) | 2/2 |
| The Netherlands | 5.5 | 4 | 4 | 0 | 0 | – | 0 (−) | 0/0 |
| Spain | 12.0 | 32 | 26 | 5 | 1 | – | 0 (−) | 5/5 |
| Sweden | 6.3 | 32 | 0 | 0 | 32 | – | 0 (−) | 0/0 |
| UK | 14.0 | 132 | 28 | 8 | 96 | 0.35 (0.01 to 1.94) | 1 (0) | 4/4 |
| Central Europe | 25.1 | 810 | 633 | 177 | 0 | 0.58 (0.34 to 0.92) | 18 (13) | 150/151 |
| Bulgaria | 28.0 | 52 | 45 | 7 | 0 | 0.50 (0.01 to 2.79) | 1 (1) | 0/0 |
| Czech Republic | 4.7 | 314 | 220 | 94 | 0 | 0.36 (0.12 to 0.84) | 5 (2) | 76/77 |
| Estonia | 20.0 | 25 | 18 | 7 | 0 | 2.10 (0.25 to 7.59) | 2 (1) | 6/6 |
| Hungary | 16.0 | 72 | 53 | 19 | 0 | – | 0 (−) | 10/10 |
| Latvia | 49.0 | 44 | 44 | 0 | 0 | 2.24 (0.46 to 6.54) | 3 (3) | 0/0 |
| Lithuania | 58.0 | 92 | 83 | 9 | 0 | 1.30 (0.42 to 3.04) | 5 (5) | 7/7 |
| Poland | 19.0 | 132 | 118 | 14 | 0 | 0.55 (0.07 to 2.00) | 2 (1) | 20/20 |
| Slovakia | 5.9 | 79 | 52 | 27 | 0 | – | 0 (−) | 31/31 |
| Eastern Europe | 46.8 | 503 | 413 | 90 | 0 | 1.02 (0.63 to 1.56) | 21 (16) | 135/135 |
| Croatia | 13.0 | 14 | 11 | 3 | 0 | 1.94 (0.05 to 10.82) | 1 (0) | 2/2 |
| Russia | 77.0 | 226 | 171 | 55 | 0 | 1.00 (0.48 to 1.83) | 10 (7) | 79/79 |
| Serbia | 20.0 | 104 | 92 | 12 | 0 | 0.99 (0.27 to 2.54) | 4 (4) | 10/10 |
| Ukraine | 77.0 | 159 | 139 | 20 | 0 | 1.00 (0.37 to 2.17) | 6 (5) | 44/44 |
| North America‡ | 3.6 | 1673 | 1552 | 45 | 76 | 0.05 (0.00 to 0.30) | 1 (0) | 55/56 |
| Canada | 4.0 | 157 | 111 | 9 | 37 | – | 0 (−) | 10/10 |
| USA | 3.2 | 1516 | 1441 | 36 | 39 | 0.06 (0.00 to 0.33) | 1 (0) | 45/46 |
| Rest of the World§ | 16.7 | 263 | 216 | 16 | 31 | 0.11 (0.00 to 0.60) | 1 (1) | 26/26 |
| Argentina | 21.0 | 132 | 131 | 1 | 0 | 0.19 (0.00 to 1.06) | 1 (1) | 10/10 |
| Australia | 5.7 | 43 | 14 | 2 | 27 | – | 0 (−) | 2/2 |
| Chile | 14.0 | 12 | 12 | 0 | 0 | – | 0 (−) | 2/2 |
| Israel | 6.7 | 45 | 37 | 8 | 0 | – | 0 (−) | 6/6 |
| Mexico | 19.0 | 7 | 5 | 2 | 0 | – | 0 (−) | 4/4 |
| New Zealand | 6.6 | 20 | 17 | 3 | 0 | – | 0 (−) | 2/2 |
| Singapore | 44.0 | 4 | 0 | 0 | 4 | – | 0 (−) | 0/0 |
*Based on UN statistics for the lower bound of incidence in 2012 per 100 000 population in individual countries within each geographic region.28
†PPD test results missing for 294 patients, including the patient in the UK who developed TB.
‡PPD test results missing for 76 patients, including the patient who developed TB.
§PPD test results missing for 31 patients.
CZP, certolizumab pegol; INH, isoniazid; IR, incidence rates; PPD, purified protein derivative; PY, patient-years; RA, rheumatoid arthritis; TB, tuberculosis.
Of note, for current RA trials, the number of patients recruited from Central Europe and Eastern Europe was 0 and 40 patients, respectively, and may have contributed to the reduced rates of TB observed in patients with RA after protocol amendment. The majority of patients with RA in current trials were recruited from North America (1319/1682 patients).
Discussion
Since the introduction of stricter screening rules for LTBI in CZP clinical trials (cut-off for PPD positivity of ≥5 mm and a TB questionnaire including 13 questions relating to symptoms and risk factors for TB, in addition to chest X-rays), and mandating INH monotherapy for 9 months in patients at risk, the IR of TB infection in CZP-treated patients with RA has decreased from 2.63/100 PY (original trials) to 0.18/100 PY (current trials). This rate is comparable to that reported for other monoclonal anti-TNF antibodies (IR ∼0.3/100 PY)23–25 and suggests beneficial effects of stricter LTBI screening prior to administering anti-TNFs in patients with IMID. A similar IR of 0.21/100 PY was reported for CZP-treated RAPID-axSpA patients (1 case), with no TB cases in RAPID-PsA patients to date.
Previous reports suggest that the risk of TB is elevated ∼2− to 4-fold in patients with RA compared to the general population6 7 and that it further increased 1.6–25.1 times following anti-TNF therapy. 1 3 6 26 Over the past decade, there are signs of a global decrease in TB incidence among patients with anti-TNF,5 but the IRs in some areas (eg, large cities) remain higher than the national rates.27 Furthermore, the overall risk of TB may depend on many factors including the population exposed, clinical setting, comorbidities, concomitant medications and anti-TNF administered. Studies suggest that the majority of TB cases which develop soon after initiation of anti-TNF treatment are due to reactivation of LTBI.2 3 For CZP trials: 21/44 TB cases in patients with RA and two confirmed cases in patients with psoriasis and axSpA were reported during the first year of CZP treatment; implying that the cases were most likely due to reactivation of LTBI despite PPD negativity (<5 mm) at baseline, whereas in patients with onset of TB >1 year after the start of CZP therapy, yet testing PPD negative at baseline, it is likely to be a new TB infection.2 17 No new cases of TB were reported in those who received INH as a result of a positive tuberculin skin test (PPD≥5 mm), emphasising the benefits of more stringent screening of patients for LTBI and administration of LTBI therapy, regardless of vaccination status. Only two patients received INH treatment prior to TB diagnosis. The lower IR of TB in intermediate trials compared with original trials (0.30/100 PY vs 2.63/100 PY) suggests beneficial effects of treatment for LTBI in patients on long-term CZP treatment, even when administered sometime after the start of anti-TNF therapy. In current trials, recruitment of fewer patients from high TB incidence countries, changes in study design, introduction of a 6-monthly TB questionnaire to increase patient/physician awareness (and facilitate appropriate management of TB) and recruitment of fewer biologically naïve patients5 are likely to have contributed to reduced TB rates. During this study, only INH monotherapy was used for treatment of LTBI. However, no specific compliance check for patient adherence to 9 months of INH treatment was performed. The shorter regimen of INH and Rifampicin dual therapy (typically 3 months) was not used, even though this approach is associated with better treatment compliance.
The majority of TB cases in RA were reported from Central and Eastern Europe, consistent with the higher notification rates and risk of TB in these regions.27 28 A high proportion of these patients were PPD negative, in line with lower rates of PPD positivity than TB rates in TB endemic countries and high anergy rates for TB screening in patients with severe inflammatory diseases.29 Glucocorticoid treatment may also lead to false negative PPD skin test results,28 potentially resulting in inadequate LTBI treatment of at-risk patients. In contrast, prior BCG vaccination, sensitisation resulting from infection with environmental mycobacteria or an increase in non-TB mycobacteria may generate false positive test results.1 30 Nevertheless, in most cases, routine BCG vaccination in newborns in TB endemic countries is unlikely to account for positive PPD skin tests during adulthood as reactivity declines during early childhood.31 32 Consequently, frequent monitoring of patients in TB endemic countries may be required. Repeated PPD tuberculin skin tests are one approach, but potential booster effects could limit the interpretation of results.
In vitro interferon-γ release assays (IGRAs; ie, QuantiFERON Gold or ELISPOT TB) data may provide an alternative LTBI screening method with improved specificity in BCG-vaccinated patients, reducing unnecessary administration of INH treatment for LTBI and its associated risks.33–35 Although IGRAs can provide improved specificity,33 34 they can also produce false positives.34 Thus, reliance on a single screening test is not without problems and, in high TB endemic areas, the use of algorithms that combine the results of dual testing (PPD skin test and IGRAs) may provide greater benefits.35 36 The lack of a gold standard for identifying BK infections does not allow an optimal evaluation of any TB screening method. In countries with low TB incidence or where BCG vaccination is generalised, using both tests may lead to an overuse of treatment for LTBI and use of IGRAs alone should be encouraged.18 33 One limitation of the present paper is the lack of concomitant IGRAs data, not available at the start of the CZP programme.
A comparison of TB incidence in CZP trials with findings from other anti-TNF trials is limited by different inclusion/exclusion criteria, trial locations and other risk factors. Moreover, prior to the recent introduction of a specific Medical Dictionary for Regulatory Activities (MedDRA) preferred term for TB,37 different sponsors may have reported positive PPD test results (LTBI), suspected TB or confirmed TB as a TB event.23 38 39 A recent meta-analysis assessing the risk of TB in patients with IMID treated with biologicals or tofacitinib reported elevated TB rates across trials, with higher rates for CZP.40 However, inclusion of suspected or LTBI cases from CZP trials and the recruitment of patients from high TB incidence countries is likely to have contributed to this finding. In general, data on the IR of TB from biological clinical trials that enrol patients from TB endemic countries are limited.40 In CZP trials, non-TB mycobacteria may also be a confounding factor, but specific diagnostic tools allowing systematic screening are missing and no non-TB mycobacteria cases were identified by the investigators. For the soluble TNF-receptor agent, reports evidenced a lower TB incidence,1 2 consistent with less stable inhibition of membrane-bound TNF and reduced effectiveness in the treatment of granulomatous inflammatory conditions such as Crohn's disease.
In summary, methods of screening for LTBI and decisions on whether to administer INH therapy can influence the incidence of TB in anti-TNF patients. In CZP trials, several cases of TB occurred in patients with negative PPD (<5 mm) at screening who did not receive INH treatment. However, the likelihood of developing TB was greatest in those with PPD ≥5 mm who did not receive treatment for LTBI. Physicians should be aware of the importance of adequate TB screening for biologicals patients, particularly in TB endemic countries and in discrete high TB incidence regions of countries with relatively low national TB rates. Increasing the stringency of TB assessments (eg, PPD positivity cut-off of ≥5 mm induration irrespective of vaccination status, alongside repeated/alternative screening tests) may reduce TB incidence in patients treated with anti-TNF and enable those receiving treatment for LTBI to continue biological therapy for their chronic autoimmune disease.
Acknowledgments
The authors would like to thank John J Cush (Dallas), Kevin Winthrop (Portland) and Leonard Calabrese (Cleveland) for their contribution to the external medical review of suspected TB cases. The authors acknowledge ‘Matladi N. Ndlovu, PhD, UCB Pharma, Brussels, Belgium, for publication coordination and Costello Medical Consulting, UK, for writing and editorial assistance, which was funded by UCB.
Correction notice: This article has been corrected since it was published Online First. The Key messages text box has been included.
Contributors: All authors were involved in planning for the analysis of data, reviewed the data, as well as approved each draft of the manuscript including approval of the final version. XM, JV, OL, JG-R, MW and RvV manually reviewed the suspected TB cases.
Funding: This study was funded by UCB Pharma.
Competing interests: XM has received grant/research support from: Pfizer, Roche; and consultancy fees from: BMS, GSK, Pfizer, Roche, UCB Pharma. JV has received consultancy fees from: Abbott, Pfizer, MSD, UCB Pharma, Samsung Bioepics; and speaker's bureau from: UCB Pharma, Pfizer, Abbott, MSD. OL has received grant/research support from: MSD; consultancy fees from: MSD, Astellas, Gilead, Pfizer; and speaker's bureau from: Astellas, Gilead, Merck/Schering, Pfizer. JG-R has received grant/research support from: MSD, Roche, UCB Pharma; consultancy fees from: BMS, GSK, MSD, Pfizer, Roche, UCB Pharma; and speaker's bureau from: BMS, GSK, MSD, Pfizer, UCB Pharma. MdL is an employee of UCB Pharma. PR has received consultancy fees from UCB Pharma. MW has received grant support from: BMS, Crescendo Bioscience, UCB Pharma; and consultancy fees from: Amgen, Abbvie, BMS, Crescendo Bioscience, UCB Pharma, Roche. RvV has received research support from: Abbvie, BMS, GSK, Pfizer, Roche, UCB Pharma; and consultancy fees/honoraria from: Abbvie, Biotest, BMS, GSK, Janseen, Lilly, Merck, Roche, UCB Pharma, Vertex.
Ethics approval: Independent ethics committee or institutional review board at participating sites.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Solovic I, Sester M, Gomez-Reino JJ et al. . The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J 2010;36:1185–206. 10.1183/09031936.00028510 [DOI] [PubMed] [Google Scholar]
- 2.Tubach F, Salmon D, Ravaud P et al. . Risk of tuberculosis is higher with anti–tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French Research Axed on Tolerance of Biotherapies Registry. Arthritis Rheum 2009;60:1884–94. 10.1002/art.24632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe F, Michaud K, Anderson J et al. . Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum 2004;50:372–9. 10.1002/art.20009 [DOI] [PubMed] [Google Scholar]
- 4.Baronnet L, Barnetche T, Kahn V et al. . Incidence of tuberculosis in patients with rheumatoid arthritis. A systematic literature review. Joint Bone Spine 2011;78:279–84. 10.1016/j.jbspin.2010.12.004 [DOI] [PubMed] [Google Scholar]
- 5.Arkema EV, Jonsson J, Baecklund E, et al. Are patients with rheumatoid arthritis still at an increased risk of tuberculosis and what is the role of biological treatments? Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204960. Published Online First: 7 March 2014. doi: 10.1136/annrheumdis-2013–204960. [DOI] [PubMed] [Google Scholar]
- 6.Askling J, Fored CM, Brandt L et al. . Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum 2005;52:1986–92. 10.1002/art.21137 [DOI] [PubMed] [Google Scholar]
- 7.Carmona L, Hernandez-Garcia C, Vadillo C et al. . Increased risk of tuberculosis in patients with rheumatoid arthritis. J Rheumatol 2003;30:1436–9. [PubMed] [Google Scholar]
- 8.Bonfiglioli KR, Ribeiro ACM, Moraes JCB et al. . LTBI screening in rheumatoid arthritis patients prior to anti-TNF treatment in an endemic area. Int J Tuberc Lung Dis 2014;18:905–11. [DOI] [PubMed] [Google Scholar]
- 9.Shashidhara AN, Chaudhuri K. The tuberculin skin test. National Tuberculosis Institute News Letter, 1990;26. [Google Scholar]
- 10.[No authors listed] Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep 2000;49:1–51. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention: Division of Tuberculosis Elimination. Fact Sheet: TB Elimination—Tuberculin Skin Testing 2011. http://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf (accessed 14 Jan 2013).
- 12.W.H.O. Global Tuberculosis Control 2010.
- 13.Singh JA, Furst DE, Bharat A et al. . 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. 10.1002/acr.21641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding T, Ledingham J, Luqmani R et al. . BSR and BHPR rheumatoid arthritis guidelines on safety of anti-TNF therapies. Rheumatology (Oxford) 2010;49:2217–19. 10.1093/rheumatology/keq249a [DOI] [PubMed] [Google Scholar]
- 15.Mease PJ, Fleischmann R, Deodhar A et al. . Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24 week results of a phase 3 double blind randomized placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. 10.1136/annrheumdis-2013-203696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reich K, Ortonne JP, Gottlieb AB et al. . Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol 2012;167:180–90. 10.1111/j.1365-2133.2012.10941.x [DOI] [PubMed] [Google Scholar]
- 17.Schreiber S. Certolizumab pegol for the treatment of Crohn's disease. Therap Adv Gastroenterol 2011;4:375–89. 10.1177/1756283X11413315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landewé R, Braun J, Deodhar A et al. . Efficacy of certolizumab pegol on signs and symptoms of axila spondyloarthritis including ankylosing spondylitis: 24 week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014;73:39–47. 10.1136/annrheumdis-2013-204231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EMA. Annex 1: Summary of Product Characteristics (Cimzia), 2014. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001037/human_med_001294.jsp&mid=WC0b01ac058001d124. (accessed 12 Dec 2014).
- 20.UCB. CIMZIA Prescribing Information 2014. http://www.ucb.com/our-products/product-list/immunology-inflammation/ (accessed 12 Dec 2014).
- 21.Bykerk VP, Cush J, Winthrop K et al. . Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015;74:96–103. 10.1136/annrheumdis-2013-203660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prevention CfDCa. Tuberculosis (TB) (Mycobacterium tuberculosis). National Notifiable Diseases Surveillance System (NNDSS), 2009. [Google Scholar]
- 23.Burmester GR, Mease P, Dijkmans BAC et al. . Adalimumab safety and mortality rates from global clinicial trials of six immune-mediated inflammatory diseases. Ann Rheum Dis 2009;68:1863–9. 10.1136/ard.2008.102103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burmester GR, Panaccione R, Gordon KB et al. . Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013;72:517–24. 10.1136/annrheumdis-2011-201244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kay J, Fleischmann R, Keystone E et al. . Golimumab 3-year safety update: an analysis of pooled data from the long-term extensions of randomised, double-blind, placebo-controlled trials conducted in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. Ann Rheum Dis 2015;74:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez-Reino JJ, Carmona L, Valverde VR et al. . Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 2003;48:2122–7. 10.1002/art.11137 [DOI] [PubMed] [Google Scholar]
- 27.de Vries G, Aldridge RW, Cayla JA et al. . Epidemiology of tuberculosis in big cities of the European Union and European Economic Area countries. Eurosurveillance 2014;19:5 10.2807/1560-7917.ES2014.19.9.20726 [DOI] [PubMed] [Google Scholar]
- 28.Tuberculosis incidence rate per 100,000 population (lower bound). UN Statistics. [Updated 07 July 2014]. http://mdgs.un.org/unsd/mdg/SeriesDetail.aspx?srid=789 (accessed 2 Oct 2014).
- 29.Ponce de Leon D, Acevedo-Vasquez E, Sanchez-Torres A et al. . Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis 2005;64:1360–1. 10.1136/ard.2004.029041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrejak C, Nielsen R, Thomsen VO et al. . Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax 2013;68:256–62. 10.1136/thoraxjnl-2012-201772 [DOI] [PubMed] [Google Scholar]
- 31.Chan P-C, Chang L-Y, Wu Y-C et al. . Age-specific cut-offs for the tuberculin skin test to detect latent tuberculosis in BCG-vaccinated children. Int J Tuber Lung Dis 2008;12:1401–6. [PubMed] [Google Scholar]
- 32.Hizel K, Maral I, Karakus R et al. . The influence of BCG immunisation on tuberculin reactivity and booster effect in adults in a country with a high prevalence of tuberculosis. Clin Microbiol Infect 2004;10:980–3. 10.1111/j.1469-0691.2004.00970.x [DOI] [PubMed] [Google Scholar]
- 33.Mazurek GH, Jereb J, Vernon A et al. . Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 2010;59:1–25. [PubMed] [Google Scholar]
- 34.Mariette X, Baron G, Tubach F et al. . Influence of replacing tuberculin skin test with ex vivo interferon gamma release assays on decision to administer prophylactic antituberculosis antibiotics before anti-TNF therapy. Ann Rheum Dis 2012;71:1783–90. 10.1136/annrheumdis-2011-200408 [DOI] [PubMed] [Google Scholar]
- 35.Klein M, Jarosova K, Forejtova S et al. . Quantiferon TB Gold and tuberculin skin tests for the detection of latent tuberculosis infection in patients treated with tumour necrosis factor alpha blocking agents. Clin Exp Rheumatol 2013;31:111–17. [PubMed] [Google Scholar]
- 36.Winthrop KL, Weinblatt ME, Daley CL. You can't always get what you want, but if you try sometimes (with two tests -TST and IGRA—for tuberculosis) you get what you need. Ann Rheum Dis 2012;71:1757–60. 10.1136/annrheumdis-2012-201979 [DOI] [PubMed] [Google Scholar]
- 37.Term. M. Latent TB 2013. http://bioportal.bioontology.org/ontologies/MEDDRA?p=classes&conceptid=http%3A%2F%2Fpurl.bioontology.org%2Fontology%2FMEDDRA%2F10065048
- 38.Dixon WG, Hyrich KL, Watson KD et al. . Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis 2010;69:522–8. 10.1136/ard.2009.118935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Vollenhoven RF, Emery P, Bingham CO III et al. . Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis 2013;72:1496–502. 10.1136/annrheumdis-2012-201956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Souto A, Maneiro JR, Salgado E et al. . Risk of tuberculosis in patients with chronic immune-mediated inflammatory diseases treated with biologics and tofacitinib: a systematic review and meta-analysis of randomized controlled trials and long-term extension studies. Rheumatology (Oxford) 2014;53:1872–5. 10.1093/rheumatology/keu172 [DOI] [PubMed] [Google Scholar]