Abstract
Osteoporosis (ie, low bone mineral density) is common in ankylosing spondylitis, related to both systemic inflammation and decreased mobility. Vertebral fracture risk is increased; acute back pain in these patients is not always a flare-up of the disease, as it can be related to bone complications. Intervertebral disc fractures in the ankylosed spine are associated with severe neurological complications. As expected from pathophysiology, treatments effective against inflammation have a positive effect on bone, and prospective open studies have shown that tumour-necrosis-factor blockers can improve bone mineral density at the spine and the hip. There is so far no evidence of a decreased risk of fractures with such treatment.
Keywords: Osteoporosis, Ankylosing Spondylitis, Inflammation
Key messages.
Osteoporosis (or low bone mineral density) is common in ankylosing spondylitis, related to both systemic inflammation and decreased mobility.
Patients with ankylosing spondylitis have an increased risk of vertebral fractures.
Effective treatments against inflammation (TNF blockers) have a positive affect on bone mineral density.
There is no evidence of a decreased risk of fractures with the control of inflammation.
Introduction
Osteoporosis is a frequent complication of inflammatory rheumatic disorders and a well-recognised feature of ankylosing spondylitis (AS).1 The disease is characterised by osteoproliferation and spine rigidity. The ankylosed spine is at risk of deformities and fractures. However, low bone mineral density (BMD) has also been observed in early diseases,2 suggesting that decreased mobility is not the single mechanism of bone fragility. Moreover, osteoporosis cannot be related to the underlying characteristics of the patients like in rheumatoid arthritis, as AS is typically a disease of young men, and glucocorticoids are not used in this disease. Systemic inflammation itself can have a deleterious effect on bone remodelling, and this is the rationale for studying the potential positive bone effects of potent anti-inflammatory drugs.
Fractures in AS
Patients with AS have an increased risk of vertebral fractures. A case–control study of 53 108 patients with fractures using the Swedish National Hospital Discharge Register concluded that the risk of fractures was higher in AS than in rheumatoid arthritis, with the largest increase for vertebral fracture (odd ratios (OR) 7.1 and 2.7 for AS and RA, respectively).3 The prevalence of vertebral fractures is highly variable in different studies, up to 30%.4 5 These data are unexpected in a disease affecting a young population, predominantly males. Actually, the definition of a vertebral fracture varies among studies, and three different vertebral complications must be considered.
Spinal fractures in AS
Spinal fractures can occur in patients with an ankylosed spine, even after a minor trauma. They can be transdiscal through the syndesmophytes, or transvertebral, involving the posterior arch.6 They can be located at the cervical spine, which is never involved in typical osteoporotic vertebral fractures.7 Neurological complications of variable degrees, sometimes severe, are usual in these fractures.8 Moreover, the capacity of healing is poor, and pseudoarthrosis with instability can occur, leading to surgery in most of the cases. Unstable cervical fractures are the most frequent, as they are located at the junction between the fused thoracic spine and the mobile head. In such patients, the C7-T1 junction must be analysed carefully. The thoracic hyperkyphosis exposes the patients to a hyperextension trauma of the neck in case of a fall. Patients with a bamboo spine have a high risk of such fractures, because of the calcifications of the spinal longitudinal ligaments and disuse osteoporosis of the vertebral bodies related to immobility. They must be carefully evaluated, as it is sometimes difficult to differentiate between pain from fracture and pain from a flare-up of the inflammatory disease. A retrospective study showed that 60% of cervical spine fractures in AS were undetectable on initial X-rays;9 CT is more sensitive than radiographs. A prospective 22-year cohort study recently showed that the occurrence of spinal fractures in AS, mainly cervical fractures, has an increased occurrence. One potential explanation is that patients with a bamboo spine can have an increasing level of physical activity and thus a greater risk for injuries, because of pain relief related to effective treatment (TNF blockers) of the disease.10
Vertebral deformities in AS
Deformities of vertebral bodies are frequent in AS, particularly at the thoracic spine, for a number of reasons: erosions of the anterior corners, squaring, wedging secondary to inflammatory lesions. These deformities are captured by semi-automated methods of morphometry, which use automatic positioning of points on vertebral contours; with such methods, ‘fractures’ are defined as any reduction of the anterior or middle height of the vertebral body larger than 20% as compared to the posterior height, or as compared to the heights of adjacent vertebrae. These methods are very sensitive but need expert adjudication;11 otherwise, they increase the risk of false positives. Short vertebral heights are frequent at the thoracic spine and should not be considered as fractures. Anterior deformities of the thoracic spine, whether they are related to fractures or other causes-related wedging, are responsible for hyperkyphosis, a frequent complication of AS.12
Vertebral fractures in AS
Prevalence of vertebral fractures ranged from 9% to 18% in studies published in the 1990s.4 13 Higher rates have been reported recently in studies using systematic imaging methods of the spine (either X-rays or the vertebral fracture assessment (VFA) method by dual-energy X-ray absorptiometry). In 176 patients (79% males, aged 48.6±13.1 years) with a mean disease duration of 22 years, the prevalence of vertebral fractures was 32.4%; 82% of the fractures were at the thoracic spine, and 65% of them were mild, that is, showed a decrease in at least one vertebral body height of 20–25%. A semiautomated software was used for analysis.5 In 80 patients (84% males, age 38.9±11.8 years) with a mean disease duration of 10 years, the prevalence of moderate or severe fractures (ie, a decrease in vertebral body height of more than 25% and more than 40%, respectively) was 18.8%.14 In early spondyloarthropathies (ie, 7 months of disease duration, but 5.7 years of symptom duration), 15% of the 113 patients (66% males, aged 37.3±9.0 years) had a vertebral fracture; most of them were located at the mid-thoracic spine, half of the fractures were moderate, and none were severe.15 Whether or not the severity of the vertebral fractures is a function of the duration of the disease is unclear.
The use of large databases gives the opportunity to assess the prevalence of VFs in a very large number of patients but with the limits of such method, in particular the absence of the confirmation of the fracture in most of the cases. All the vertebral fractures are those which come to clinical attention, which may represent a minority of them. A nested case control study has been performed in the large General Practice Research Database in the UK; 231 778 patients with fracture and the same number of controls were analysed.16 From medical records, AS was diagnosed in 758 participants. These patients had an increased risk of clinical vertebral fracture: OR=3.26 (1.51–7.02), but no increased risk of non-vertebral fracture, including of the wrist and hip. In a large database in Catalonia, Spain, accounting for 80% of the population, 6474 patients with AS were identified, compared to controls, and followed for a median time of 5 years.17 Among patients with AS, 0.86% and 3.4% sustained a clinical vertebral and a non-vertebral fracture, respectively. This represents a twofold increased risk of clinical vertebral fractures, as compared to controls. Interestingly, an increase in the non-vertebral fracture risk (1.2 fold) was reported in this study, an observation which has never been made previously. Such results have been confirmed in the Danish Health Registries.18 In this case–control study assessing data for the year 2000, the age-matched and gender-matched ORs for patients with AS were 5.4 (2.5–11.7) and 1.4 (1.1–1.7) for vertebral and non-vertebral fractures, respectively. The association between AS and clinical fractures was highest in patients diagnosed for less than 2.5 years, or for more than 12.5 years. However, after adjustment for potential confounders, only clinical spine fracture risk was still significant.18
In one 4-year prospective study conducted in 298 patients, the incidence of vertebral fracture (according to morphometric definition) was 4.7% at 2 years and 13.6% at 4 years. None of the fractures were severe. The risk factors were prevalent vertebral fractures at baseline and increased C reactive protein (CRP) levels at 2 years.19
Risk factors for fractures in AS
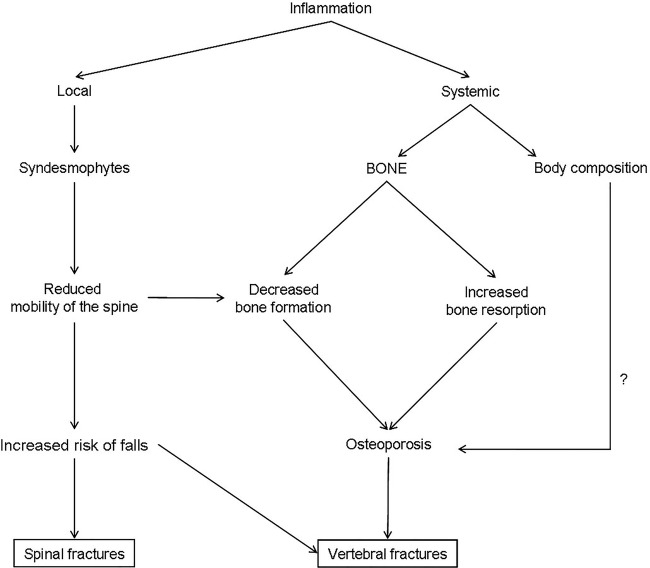
Patients with a bamboo spine, hyperkyphosis and difficulties with peripheral vision have potential impairments in balance and coordination and a high risk of falls.20 Disease duration and wall-occiput distance have been reported as risk factors for vertebral fractures5 (figure 1).
Figure 1.
Bone fragility in ankylosing spondylitis.
A low BMD is common in patients with AS with progressive disease. However, increased BMD can be due to artefacts related to the presence of syndesmophytes or other structural lesions as ankylosed posterior arch and periosteal bone formation. Active or past hip arthritis (coxitis) can impair the internal rotation of the lower limb, which is mandatory for an accurate hip BMD measurement.
The definition of ‘low BMD’ varies among studies. In theory, T scores are used only for postmenopausal women, and Z scores should be used in young males; an abnormal BMD could then be defined as Z ≤−2. Recognising these different definitions, the prevalence of low BMD measured by dual energy X-ray absorptiometry is 14–27% and 4–14% at the spine and hip, respectively, in patients with long-lasting disease.16 21–24 Prevalence is higher in studies using quantitative CT at the spine, as this technique allows measurement of central areas of vertebral bodies, avoiding the cortex and bone constructions. The prevalence of osteoporosis (based on dual energy X-ray absorptiometry measurements) in patients within the first decade after diagnosis is 16% and 13% at the lumbar spine and femoral neck, respectively.25 In 267 patients with symptoms suggestive of axial SpA, we showed recently that patients with a confirmed diagnosis (N=93) had lower BMD than patients with an unconfirmed SpA (N=74). The positive likelihood ratio of low BMD for an axial SpA diagnosis was 2.60 and 3.12 at the spine and hip, respectively.26 In 80 patients aged 39 years, the prevalence of osteoporosis (T ≤−2.5 at any site) was 25%, which is higher than expected in such a young population. Low weight and low body mass index (BMI), long disease duration, male gender and markers of disease activity were associated with osteoporosis.14 In 204 patients (57% men, mean age 50±13 years), the prevalence of osteoporosis (T≤−2.5) was 21% in participants aged ≥50 years. Low BMD was associated with age, BMI, disease duration and inflammatory parameters.23 Patients with vertebral fractures had lower BMD than patients without, and femoral neck was the best discriminant site in this population where the mean modified Stoke Ankylosing spondylitis spine score (MSASSS) was 14.2 (median 5.5).24 There is a debate about low BMD as a risk factor for vertebral fracture in AS. Such a relationship has not been found in patients with early disease.25 27 In a study of 113 patients with a disease duration of 7 months, aged 37 years on average, the majority of patients with vertebral fractures do not fulfil the densitometry-based criteria for osteoporosis.25 The proportion of patients with low BMD varies with time. In 130 patients (66% males) with an early disease (time since diagnosis 6.6 months, and disease duration 6.3 years), 9% of these patients aged 38±9 years had osteoporosis (ie, T score ≤−2.5 at the spine and or hip).28 In patients within 5 years of onset of AS, the prevalence of osteoporosis was 11% and 15% at the hip and spine, respectively; these rates were 30% and 4%, respectively, in patients with a disease duration higher than 10 years.28 There is an association between the presence of syndesmophytes and a low hip BMD,29 suggesting the role of both the severity of the disease and the reduced mobility of the patients.
Attention has been paid recently to bone microarchitecture changes in patients with AS30 measured by high-resolution peripheral quantitative CT of the ultradistal radius and tibia. Patients with AS have lower cortical BMD at peripheral sites, a result which is in accordance with the role of systemic inflammation and thus a systemic bone effect in this disease. In contrast, the presence of syndesmophytes was not associated with any sign of hyperostosis in the peripheral microarchitecture, suggesting that osteoproliferation is not a systemic process.30
Physiopathology of bone fragility in AS
Osteoporosis can occur in AS because of reduced physical activity, and decreased functional capacity related to pain, stiffness and ankylosis.28 However, low BMD is found in patients with early disease, before any structural changes.26 28 31
Vitamin D receptor gene may contribute to BMD differences in patients with AS, and some polymorphisms are also linked to inflammation.32–34
The HLA B27 transgenic rat, a validated model of spondyloarthritis, having colitis, skin lesions, peripheral arthritis and spondylitis, exhibits a decreased bone strength, without any defect in the mineralisation process; histomorphometric indices indicate a decrease in bone volume, trabecular number and trabecular thickness, that is, an osteoporosis.35
Two prospective studies have shown that spine and hip BMD decrease predominantly in patients with active disease.36 37 In 332 (52% males) patients with early inflammatory back pain suggestive of spondyloarthropathies (disease duration of symptoms 1.6 years), we found that male gender, either increased erythrocyte sedimentation rateor CRP, and presence of bone marrow oedema on MRI were associated with a low BMD. Interestingly, bone inflammatory lesions on MRI were one of the determinants of low spine BMD, and the single determinant of low hip BMD, suggesting the systemic effect of inflammation.31 In a 1-year prospective study in 30 patients with inflammatory back pain, there was no change in hip and spine BMD; however, in a post hoc analysis, hip bone loss (not of the spine) was found to be associated with raised baseline CRP and sacroiliitis diagnosed by MRI. 38
All these data support the role of inflammation in bone loss in SpA. Advances in pathogenesis have been provided by a new mouse model that highlights the role of IL23 in entheseal inflammation. Gut-derived IL23 (even in subclinical gut involvement) can act on a previously unidentified subpopulation of entheseal resident T cells, which, in reaction, produce cytokines such as IL22 and IL17, involved in osteoproliferation and bone loss, respectively.39
Patients with active and long-lasting AS are at risk of muscle loss because of reduced physical activity and inflammation; tumour-necrosis-factor (TNF) increases resting energy expenditure, stimulates muscle protein breakdown and downregulates the systemic and local expression of anabolic hormones and growth factors.40 Adipokines, produced by adipocytes from fat tissue, can have immune regulatory function and affect bone metabolism; some of them (resistin, visfatin) may be involved in radiographic damage in patients with AS.41
Thus, inflammation plays a key role in bone loss in AS, and a beneficial effect of anti-inflammatory drugs on bone is expected, not only through the increased mobility related to pain relief, but also through a direct effect on bone.
Effect of pharmacological treatments on osteoporosis
NSAIDs
In a primary care-based nested case control study, the risk of any clinical fracture was decreased in patients with AS taking non-steroidal anti-inflammatory drugs (NSAIDs) (OR: 0.65 (0.50, 0.84)), after adjustments.16 In a population-based cohort study, the increased risk of fractures in patients with AS was apparent only in those not on regular NSAIDs treatment.17 However, this result was not confirmed by a nationwide case–control study: after stratifying by NSAID use, the excess risk of any clinical fracture in patients with AS is higher in NSAID users, which may be related to a higher utilisation of NSAIDs in patients with a more severe disease.18 All these data should thus be interpreted with caution.
TNF blockers
Prospective open studies in patients with AS receiving TNF blockers show a positive effect on BMD.42 In a 2-year follow-up study of 106 patients, we observed 5.8% and 2.3% increases in the lumbar spine and hip BMD, respectively. 40 Over 6 years of continuous administration of such treatment in 59 patients, the increase in BMD was 11.8 and 3.6% at these two sites, even after exclusion of patients with prevalent and/or incident syndesmophytes.43 A systematic review of eight studies (including 1 randomised control trial) with a total of 568 patients with AS showed an average of 8.6% and 2.5% increases in BMD at the lumbar spine and hip, respectively.44 There is a strong biological rationale beyond these results, as TNFα plays a key role in bone resorption and formation. Osteoclastogenesis and osteoclast activity are enhanced by TNFα, which also inhibits osteoclast apoptosis.45 46 On the other hand, excess TNFα inhibits the bone formation process, and sclerostin is over expressed in TNFα transgenic mice.47 As expected, changes in bone remodelling markers in patients treated with TNFα blockers are those expected with a treatment having an antiresorptive activity.40 48 49 An increase in body weight is observed in patients receiving TNF blockers, mostly due to a gain in fat mass,40 with an early increase in abdominal (both visceral and subcutaneous) fat.50
As a consequence, the bone effect of anti-TNF treatment should be taken into account before introducing antiosteoporotic treatment in patients with AS with osteoporosis. However, there is so far no evidence of an anti-fracture effect of TNF blockers in AS.51 In a prospective study conducted in 298 patients, markers of inflammation were associated with incident vertebral fracture; among 26 patients with new vertebral fracture, 6 were on TNF inhibitors and the study was not powered to identify a protective effect of this treatment.19 There are no guidelines for treatment of osteoporosis in AS. In patients with an indication for TNF blocker treatment, without prevalent non-traumatic fracture, it seems logical to assess first the benefit of this treatment. However, in patients with severe osteoporosis and prevalent fractures, available guidelines in osteoporotic participants and male osteoporosis must be applied.
Rehabilitation treatments have been reported to improve management of patients with AS receiving TNF blockers, but which exercise protocols should be recommended is not yet defined.52
Conclusion
Ankylosing spondylitis raises a paradox: patients have both osteoporosis and an excess of bone formation. Local changes and systemic bone loss are underlined by different mechanisms. AS is an appropriate model for studies of bone effect of inflammation.
Footnotes
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Roux C. Osteoporosis in inflammatory joint diseases. Osteoporos Int 2011;22:421–33. 10.1007/s00198-010-1319-x [DOI] [PubMed] [Google Scholar]
- 2.Will R, Palmer R, Bhalla AK et al. . Osteoporosis in early ankylosing spondylitis. A primary pathological event? Lancet 1989;2:1483–5. 10.1016/S0140-6736(89)92932-2 [DOI] [PubMed] [Google Scholar]
- 3.Weiss RJ, Wick MC, Ackermann PW et al. . Incresed fracture risk in patients with rheumatic disorders and other inflammatory diseases. A case-control study with 53,108 patients with fracture. J Rheumatol 2010;37:2247–50. 10.3899/jrheum.100363 [DOI] [PubMed] [Google Scholar]
- 4.Cooper C, Carvone L, Michet CJ et al. . Fracture risk in patients with ankylosing spondylitis: a population based study. J Rheumatol 1994;21:1877–82. [PubMed] [Google Scholar]
- 5.Montala N, Juanola X, Collantes E et al. . Prevalence of vertebral fractures by semiautomatedmortphometry in patients with ankylosing spondylitis. J Rheumatol 2011;38:893–7. 10.3899/jrheum.100851 [DOI] [PubMed] [Google Scholar]
- 6.Campagna R, Pessis E, Feydy A et al. . Fractures of the ankylosedspine: MDCT and MRI with emphasis of individual anatomic spinal structures. Am J Roentgenol 2009;192:987–95. 10.2214/AJR.08.1616 [DOI] [PubMed] [Google Scholar]
- 7.Stenhouse G, Ulbricht C, Khanna M. Spinal injury in ankylosing spondylitis. BMJ 2014;348:g3849 10.1136/bmj.g3849 [DOI] [PubMed] [Google Scholar]
- 8.Vosse D, Feldtkeller E, Erlendsson J et al. . Clinical vertebral fractures in patients with ankylosing spondylitis. J Rheumatol 2004;31:1981–5. [PubMed] [Google Scholar]
- 9.Anwar F, Al-Khayer A, Joseph G et al. . Delayed presentation and diagnosis of cervical spine injuries in long-standing ankylosing spondylitis. Eur Spine J 2011;20:403–7. 10.1007/s00586-010-1628-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson Y, Sanden B, Olerud C. Increased occurrence of spinal fractures related to ankylosing spondylitis: a prospective 22-year cohort study in 17,764 patients from a national registry in Sweden. Patient Saf Surg 2013;7:2 10.1186/1754-9493-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vosse D, Heijckmann C, Landewé R et al. . Comparing morphometric X-ray absorptiometry and radiography in defining vertebral wedge fractures in patients with ankylosing spondylitis. Rheumatology 2007;46:1667–71. 10.1093/rheumatology/kem135 [DOI] [PubMed] [Google Scholar]
- 12.Geusens P, Vosse D, Van der Heijde DM et al. . High prevalence of thoracic vertebral deformities and discal wedging in ankylosing spondylitis patients with hyperkyphosis. J Rheumatol 2001;28:1856–61. [PubMed] [Google Scholar]
- 13.Raltson SH, Urquhart GDK, Bzeski M et al. . Prevalence of vertebral compression fractures due to osteoporosis in ankylosing spondylitis. Br Med J 1990;300:563–5. 10.1136/bmj.300.6724.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghozlani I, Ghazi M, Nouijai A et al. . Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone 2009;44:772–6. 10.1016/j.bone.2008.12.028 [DOI] [PubMed] [Google Scholar]
- 15.Van der Weijden MAC, Van der Horst-Bruinsma IE, van Denderen JC et al. . High frequency of vertebral fractures in early spondylarthropathies. Osteoporos Int 2012;23:1683–90. 10.1007/s00198-011-1766-z [DOI] [PubMed] [Google Scholar]
- 16.Vosse D, Landewé R, van der Heijde D et al. . Ankylosing spondylitis and the risk of fracture: results from a large primary care-based nested case control study. Ann Rheum Dis 2009;68:1839–42. 10.1136/ard.2008.100503 [DOI] [PubMed] [Google Scholar]
- 17.Munoz-Ortego J, Vestergaard P, Rubio JB et al. . Ankylosing spondylitis is associated with an increased risk of vertebral and non-vertebral clinical fractures: a populations based cohort study. J Bone Miner Res 2014;29:1770–6. 10.1002/jbmr.2217 [DOI] [PubMed] [Google Scholar]
- 18.Prieto-Alhambra D, Munoz-Ortego J, De Vries F et al. . Ankylosing spondylitis confers substantially increased risk of clinical spine fractures: a nationwide case-control study. Osteoporos Int 2015;26:85–91. 10.1007/s00198-014-2939-3 [DOI] [PubMed] [Google Scholar]
- 19.Kang KY, Kim IJ, Jung SM et al. . Incidence and predictors of morphometric vertebral fractures in patients with ankylosing spondylitis. Arthr Res Therap 2014;16:R124 10.1186/ar4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatemi G, Gensler LS, Learch TJ et al. . Spine fractures in ankylosing spondylitis: a case report and review of imaging as well as predisposing factors to falls and fractures. Semin Arthritis Rheum 2014;44:20–4. 10.1016/j.semarthrit.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jun JAE, Joo KB, Her MY et al. . Femoral bone mineral denstity is associated with vertebral fractures in patients with ankylosing spondylitis: a cross-sectional study. J Rheumatol 2006;33:1637–41. [PubMed] [Google Scholar]
- 22.El Maghraoui A, Borderie D, Edouard R et al. . Osteoporosis, body composition and bone turnover in ankylosing spondylitis. J Rheumatol 1999;26:2205–9. [PubMed] [Google Scholar]
- 23.Klingberg E, Geijer M, Göthlin J et al. . Vertebral fractures in ankylosing spondylitis are associated with lower bone mineral density in both central and peripheral skeleton. J Rheumatol 2012;39:1987–95. 10.3899/jrheum.120316 [DOI] [PubMed] [Google Scholar]
- 24.Klingberg E, Lorentzon M, Mellström D et al. . Osteoporosis in ankylosing spondylitis: prevalence, risk factors and methods of assessment. Arthr Res Therap 2012;14:R108 10.1186/ar3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Weijden MAC, Claushuis TAM, Nazari T et al. . High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis: a systematic review. Clin Rheumatol 2012;31:1529–35. 10.1007/s10067-012-2018-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forien M, Molto A, Etcheto A et al. . Bone mineral density in patients with symptoms suggestive of spondyloarthritis. Osteoporos Int 2015;26:1647–53. 10.1007/s00198-015-3044-y [DOI] [PubMed] [Google Scholar]
- 27.Mitra D, Elvins DM, Speden DJ et al. . The prevalence of vertebral fractures in mild ankylosing spondylitis and their relationship to bone mineral density. Rheumatology (Oxford) 2000;39:85–9. 10.1093/rheumatology/39.1.85 [DOI] [PubMed] [Google Scholar]
- 28.Van Der Weijden MAC, Van Denderen JC, Lems WF et al. . Low bone mineral density is related to male gender and decreased functional capacity in early spondylarthopathies. Clin Rheumatol 2011;30:497–503. 10.1007/s10067-010-1538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karberg K, Zochling J, Sieper J et al. . Bone loss is detected more frequently in patients with ankylosing spondylitis with syndesmophytes. J Rheumatol 2005;32:1290–8. [PubMed] [Google Scholar]
- 30.Klingberg E, Lorentzon M, Göthlin J et al. . Bone microarchitecture in ankylosing spondylitis and the association with bone mineral density, fractures, and syndesmophytes. Arthr Therap 2013;15:R179 10.1186/ar4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briot K, Durnez A, Paternotte S et al. . Bone oedema on MRI is highly associated with low bone mineral density in patients with early inflammatory back pain: results from the DESIR cohort. Ann Rheum Dis 2013;72:1914–19. 10.1136/annrheumdis-2012-201845 [DOI] [PubMed] [Google Scholar]
- 32.Lange U, Teichmann J, Strunk J et al. . Association of 1.25 vitamin D3 deficiency, disease activity and low bone mass in ankylosing spondylitis. Osteoporos Int 2005;16:1999–2004. 10.1007/s00198-005-1990-5 [DOI] [PubMed] [Google Scholar]
- 33.Obermayer-Pietsch BM, Lange U, Tauber G et al. . Vitamin D receptor initiation codon polymorphism, bone density and inflammatory activity of patients with ankylosing spondylitis. Osteoporos Int 2003;14:995–1000. 10.1007/s00198-003-1501-5 [DOI] [PubMed] [Google Scholar]
- 34.Arends S, Spoorenberg A, Bruyn GAW et al. . The relation between bone mineral density, bone turnover markers, and vitamin D status in ankylosing spondylitis patients with active disease: a cross-sectional analysis. Osteoporos Int 2011;22:1431–9. 10.1007/s00198-010-1338-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauner M, Stupphann D, Haas M et al. . The HLA-B27 transgenic rat, a model of spondyloarthritis, has decreased bone mineral density and increased RANK L to osteoproteogerin in RNA ratio. J Rheumatol 2009;36:120–6. 10.3899/jrheum.080475 [DOI] [PubMed] [Google Scholar]
- 36.Gratacos J, Collado A, Pons F et al. . Significant loss of bone mass in patients with early, active ankylosing spondylitis. A follow-up study. Arthritis Rheum 1999;42:2319–24. [DOI] [PubMed] [Google Scholar]
- 37.Maillefert JF, Aho S, El Maghraoui A et al. . Changes in bone density in patients with ankylosing spondylitis: a 2 year follow-up study. Osteoporos Int 2001;12:605–9. 10.1007/s001980170084 [DOI] [PubMed] [Google Scholar]
- 38.Haugeberg G, Bennett AN, McGonagle D et al. . Bone loss in very early inflammatory back pain in undifferentiated spondyloarthropathy: a 1-year observational study. Ann Rheum Dis 2010;69:1364–6. 10.1136/ard.2009.124982 [DOI] [PubMed] [Google Scholar]
- 39.Sherlock JP, Joyce-Shaikh B, Turner SP et al. . IL-23 induces spondyloarthropathy by acting on ROR-γt+CD3+CD4−CD8−entheseal resident T cells. Nat Med 2012;18:1069–76. 10.1038/nm.2817 [DOI] [PubMed] [Google Scholar]
- 40.Briot K, Gossec L, Kolta S et al. . Prospective assessment of body weight, body composition, and bone density changes in patients with spondyloarthropathy receiving anti-tumor necrosis factor-alpha treatment. J Rheumatol 2008;35:855–61. [PubMed] [Google Scholar]
- 41.Syrbe U, Callhoff J, Conrad K et al. . Serum adipokine levels in patients with ankylosing spondylitis and their relationship to clinical parameters and radiographic spinal progression. Arthritis Rheumatol 2015;67:678–85. 10.1002/art.38968 [DOI] [PubMed] [Google Scholar]
- 42.Allali F, Breban M, Porcher R et al. . Increase in bone mineral density of patients with spondyloarthropathy treated with anti-tumour necrosis factor alpha. Ann Rheum Dis 2003;62:347–9. 10.1136/ard.62.4.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durnez A, Paternotte S, Fechtenbaum J et al. . Increase in bone density in patients with spondyloarthritis during anti-tumor necrosis factor therapy: 6-year follow-up study. J Rheumatol 2013;40:1712–18. 10.3899/jrheum.121417 [DOI] [PubMed] [Google Scholar]
- 44.Haroon NN, Sriganthan J, Al Ghanim N et al. . Effect of TNF-alpha inhibitor treatment on bone mineral density in patients with ankylosing spondylitis: a systematic review and meta-analysis. Semin Arthritis Rheum 2014;44:155–61. 10.1016/j.semarthrit.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 45.Lam J, Takeshita S, Barker JE et al. . TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000;143:1108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glantsching H, Fisher JE, Wesolowski G et al. . M-CSF, TNF-alpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ 2003;10:1165–77. 10.1038/sj.cdd.4401285 [DOI] [PubMed] [Google Scholar]
- 47.Heiland GR, Zwerina K, Baum W et al. . Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann Rheum Dis 2010;69:2152–9. 10.1136/ard.2010.132852 [DOI] [PubMed] [Google Scholar]
- 48.Gengenbacher M, Sebald HJ, Villiger PM et al. . Infliximab inhibits bone resorption by circulating osteoclast precursor cells in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis 2008;67:620–4. 10.1136/ard.2007.076711 [DOI] [PubMed] [Google Scholar]
- 49.Visvanathan S, van der Heijde D, Deodhar A et al. . Effects of infliximab on markers of inflammation and bone turnover and associations with bone mineral density in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:175–82. 10.1136/ard.2007.084426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hmamouchi I, Roux C, Paternotte S et al. . Early increase of abdominal adiposity in patients with spondyloarthritis receiving anti-tumor necrosis factor-α treatment. J Rheumatol 2014;41:1112–17. 10.3899/jrheum.131150 [DOI] [PubMed] [Google Scholar]
- 51.Kawai VK, Grijalva CG, Arbogast PG et al. . Initiation of tumor necrosis factor α antagonists and risk of fractures in patients with selected rheumatic and autoimmune diseases. Arthritis Care Res 2013;65:1085–94. 10.1002/acr.21937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masiero S, Bonaldo L, Pigatto M et al. . Rehabilitation treatment in patients with ankylosing spondylitis stabilized with tumor necrosis factor inhibitor therapy. A randomized controlled trial. Arthritis Care Res 2012;64:101–7. 10.1002/acr.20566 [DOI] [PubMed] [Google Scholar]