Abstract
Hyperplasia (i.e., increased adipogenesis) contributes to excess adiposity, the hallmark of obesity that can trigger metabolic complications. As FoxO1 has been implicated in adipogenic regulation, we investigated the kinetics of FoxO1 activation during adipocyte differentiation, and tested the effects of FoxO1 antagonist (AS1842856) on adipogenesis. We found for the first time that the kinetics of FoxO1 activation follows a series of sigmoid curves, and reveals the phases relevant to clonal expansion, cell cycle arrest, and the regulation of PPARγ, adiponectin, and mitochondrial proteins (complexes I and III). In addition, multiple activation-inactivation transitions exist in the stage of terminal differentiation. Importantly, persistent inhibition of FoxO1 with AS1842856 almost completely suppressed adipocyte differentiation, while selective inhibition in specific stages had differential effects on adipogenesis. Our data present a new view of FoxO1 in adipogenic regulation, and suggest AS1842856 can be an anti-obesity agent that warrants further investigation.
Keywords: adipogenesis, AS1842856, FoxO1, mitochondria, obesity
Abbreviations
- AS1842856
5-amino-7-(cyclohexylamino)-1-ethyl-6-fluoro-4-oxo-1, 4-dihydroquinoline-3-carboxylic acid
- BMI
basal media I
- BMII
basal media II
- C1
mitochondrial complex I
- C3
mitochondrial complex III
- DMI
differentiation media I
- DMII
differentiation media II
- FoxO1
forkhead box O1
- G6P
glucose 6-phosphatase
- PEPCK
phosphoenolpyruvate carboxykinase
- PPARγ
peroxisome proliferator-activated receptor gamma
- T2DM
type 2 diabetes mellitus
Introduction
Obesity is a rapidly growing epidemic.1-3 Characterized by excess adiposity, obese individuals show aberrant glucose and lipid metabolism.4 The resultant glucotoxicity and lipoxicity have been found to impair endocrine (e.g., insulin secretion and signaling pathway) and cardiovascular functions, linking obesity to higher risks of type 2 diabetes mellitus (T2DM) and heart diseases.4 Thus, control of adiposity and glycemia is critical to prevent obesity and related metabolic complications.
It has been recognized that hyperplasia — an increase in de novo adipocyte formation (i.e., adipogenesis) contributes to excess white adipose tissue in obese subjects.3 During the development of adipose tissue, pre-adipocytes or precursor/stem cells differentiate into mature adipocytes through complex adipogenic programs that regulate signaling transduction, transcriptional regulation, cell cycle and mitochondrial metabolism.5-10 The transcription factor forkhead box O1 (FoxO1) regulates an array of genes and integrates insulin signaling with metabolic homeostasis, which has been shown to play an important role in adipogenesis.5,11-15 In particular, FoxO1 controls adipogenesis through interacting with peroxisome proliferator-activated receptor gamma (PPARγ) and the cell cycle inhibitor p21 (CDKN1A).15-18 FoxO1 haploinsufficiency protects mice from diet induced insulin resistance and diabetes.15,19 In the liver, activated FoxO1 can regulate lipid metabolism by manipulating mitochondrial function,20,21 and it also increases blood glucose by upregulating glucose 6-phosphatase (G6P) and phosphoenolpyruvate carboxykinase (PEPCK), the gluconeogenic enzymes that promote glucose production.22 These multiple roles in regulating metabolism demonstrate the great potential to target FoxO1 for new therapeutics for obesity and T2DM.5
A selective FoxO1 antagonist, 5-amino-7-(cyclohexylamino)-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (commercially AS1842856), was discovered recently.23 It can potently inhibit the DNA binding of FoxO1 and its transactivation.23-25 In hepatic cells, AS1842856 inhibits glucose production by suppressing G6P and PEPCK mRNA levels, and administration of AS1842856 to diabetic db/db mice significantly reduces fasting blood glucose.23 However, the effect of AS1842856 on adipogenesis has not been examined. In the present study, we targeted FoxO1 with AS1842856 and investigated its effects on adipogenesis. We found that persistent inhibition of FoxO1 by AS1842856 almost completely inhibited adipogenesis, which was accompanied with significant suppression of the adipogenic regulator PPARγ and mitochondrial proteins (complexes I and III). By contrast, selective inhibition at different stages of adipocyte differentiation imposed differential effects on adipogenesis, suggesting that FoxO1 plays stage-dependent roles in adipogenesis. Indeed, we found for the first time that the kinetics of FoxO1 activation and inactivation follows a series of sigmoid curves, which reveals the phases specifically relevant to clonal expansion, cell cycle arrest and PPARγ and mitochondrial regulation. Furthermore, additional sigmoid phases exist in the late stage of kinetics, which warrants future investigation.
Results and Discussion
Persistent inhibition of FoxO1 by AS1842856 prevented adipocyte differentiation
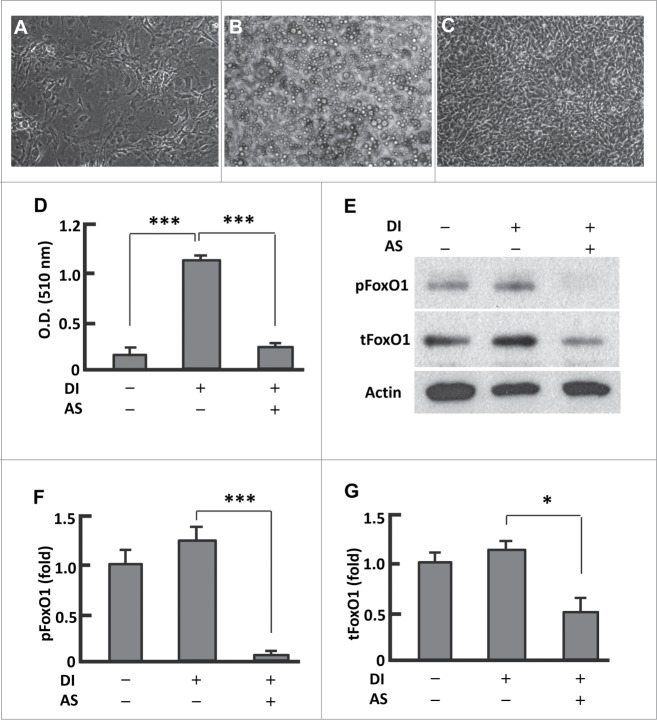
Following an established protocol we cultured 3T3L1 preadipocytes in basal media (days 0-2, BMI), and adipocyte differentiation was induced with differentiation media I (days 3-4, DMI) and differentiation media (days 5-6, DMII), and then the cells were maintained in basal media (days 7-12, BMII) (Fig. S1A).26 BMI and BMII are the same chemically but different in cell differentiation stages. Compared with the preadipocytes, the fully differentiated cells (mature adipocytes) showed significant accumulation of lipid droplets and strong oil red O staining (Fig. 1A and B; Fig. S1B and C). However, persistent treatment of cells with AS1842856 (days 0-12) almost completely prevented the adipogenesis (Fig. 1C and D; Fig. S1D). In line with AS1842856 predominantly blocking dephosphorylated FoxO1 and interfering with its interaction with DNA binding sites to suppress Foxo1-mediated transactivation,23 treatment of the cells with AS1842856 locked FoxO1 in a state of being highly dephosphorylated, and the phosphorylation level was less than 10% of that in untreated adipocytes (P < 0.0001; Figs. 1E and F). In addition, the level of total FoxO1 protein was reduced by 52% (P < 0.05) (Fig. 1E and G). Therefore, AS1842856 induced prevention of adipogenesis was associated with its inhibitory effects on FoxO1.
Figure 1.
Persistent inhibition of FoxO1 by AS1842856 largely suppressed adipogenesis. (A–B) Microscopy images of preadipocytes that were maintained in basal medium (A), and that underwent differentiation induced with the protocol as described in Materials and Methods (B). (C) Microscopy images of cells that were treated with AS1842856 (1.0 μM, days 0–12) and underwent differentiation induction as in (B). All the images were captured on day 12, with the microscope set at 100X. (D) Measurement of extracted Oil red O retained in cells by the absorbance (O.D.) at 510 nm (n = 6). (E) Representative western blot showing that AS1842856 inhibited FoxO1 through binding the de-phosphorylated sites, and to a lesser extent suppressed FoxO1 protein expression. β-actin was probed as the loading control. DI, differentiation induction; AS, AS1842856. (F and G) Densitometric analysis of protein gel blot images with NIH ImageJ software; n = 3−5. * P < 0 .05, and ***, P < 0 .0001.
AS1842856 reduced PPARγ protein levels
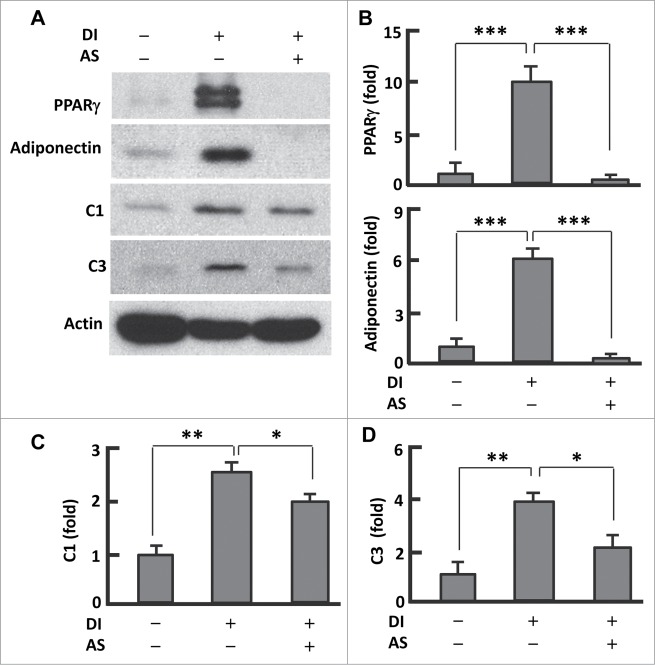
The transcription factor PPARγ plays a key role in adipogenesis,27 and its expression and activity can be regulated by FoxO1.5,16-18 To test whether AS1842856 affects PPARγ, we measured the protein level of PPARγ after induction of differentiation (Fig. 2A and B). In fully differentiated (or mature) adipocytes, the expression of PPARγ was about 10-fold greater (P < 0.0001) than in pre-adipocytes. However, treatment of cells with AS1842856 (days 0-12) significantly suppressed PPARγ (Fig. 2A and B). Loss of PPARγ function may account largely for AS1842856 induced inhibition of adipogenesis (Fig. 1C). Consistently, adiponectin—the adipokine that is secreted predominantly by mature adipocytes6,7—was highly expressed in the fully differentiated cells but barely detected in AS1842856 treated cells (Fig. 2A and C). These data suggest that suppression of adipogenesis by AS1842856 involves interference with the FoxO1-PPARγ axis.
Figure 2.
AS1842856 suppressed PPARγ and mitochondrial protein expression. (A) Western blots showing the effect of AS1842856 on PPARγ, adiponectin, mitochondrial proteins C1 and C3. β-actin was probed as the loading control. DI, differentiation induction; AS, AS1842856. (B–D) Densitometric analysis of western blot images with NIH ImageJ software; n = 3−5. * P < 0 .05; **, P < 0 .01; and ***, P < 0 .0001.
AS1842856 reduced mitochondrial protein levels
Mitochondria underpin adipogenesis and adipocyte function.6,7 As FoxO1 was implicated in mitochondrial regulation,12,20 we asked if AS1842856 interferes with mitochondrial function in adipocytes. Western blot analysis of mitochondrial proteins revealed 2.6-fold (P < 0.01) upregulation of respiration chain complex I (C1) and 3.9-fold (P < 0 .01) elevation of complex III (C3) in matured adipocytes when compared with pre-adipocytes (Fig. 2A, D, and E). However, treatment with AS1842856 substantially reduced the levels of C1 (24%, P < 0.05) and C3 (46%, P < 0.01) when compared with the fully differentiated adipocytes, indicative of an impaired mitochondrial function or efficiency (Fig. 2A, D, and E). Thus, inhibition of FoxO1 by AS1842856 seems to prevent adipogenesis by interfering with mitochondrial function in addition to PPARγ expression.
FoxO1 underwent sigmoid activation during adipocyte differentiation
FoxO1 can repress PPARγ expression and transrepress its transactivation,15-18 implying that silencing FoxO1 may stimulate PPARγ and promotes adipogenesis. However, persistent inhibition of FoxO1 (days 0-12) with AS1842856 markedly suppressed PPARγ and prevented adipogenesis, suggesting FoxO1 activation was indispensable for adipogenesis (Figs. 1-2; Fig. S1). In line with this, silencing FoxO1 in preadipocytes with siRNA severely prevents the cells from differentiation.14 To better understand the physiological role of FoxO1, we studied the kinetics of FoxO1 activation (i.e., dephosphorylation) and inactivation (i.e., phosphorylation) during 3T3L1 cell differentiation.5 As shown in Fig. 3A and B, FoxO1 activation followed a series of sigmoid curves, revealing 3 inactivation peaks (days 1, 5 and 9) and 3 activation peaks (days 3, 7 and 10). FoxO1 was most inactive on day 1 (IP1), when the cells reentered into cell cycle with clonal expansion (Fig. 3B).15 By contrast, FoxO1 reached the first activation peak (AP1) on day 3, when postmitotic growth arrest takes place (Fig. 3B).15 These data strongly support the notion that FoxO1 regulates the cell cycle.5,15
Figure 3.
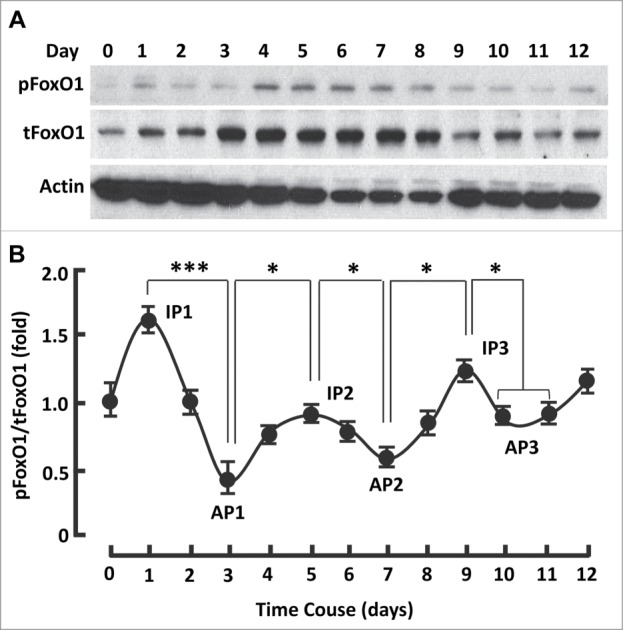
The kinetics of FoxO1 activation followed a series of sigmoid curves during adipogenesis. (A) Western blots showing FoxO1 expression (i.e., tFoxO1), activation (i.e., dephosphorylation) and inactivation (i.e., phosphorylation) during adipogenesis. β-actin was probed as the loading control. (B) Densitometric analysis of protein gel blot images with NIH ImageJ software; n = 3−5. AP, activation peak; IP, inactivation peak. * P < 0 .05; and ***, P < 0 .0001.
The second inactivation phase took place during days 3-5 and peaked on day 5 (IP2), which was accompanied with gradual upregulation of PPARγ and adiponectin (Fig. 3B; Fig. 4A and B). Concomitantly, the expression of mitochondrial C1 and C3 increased (Fig. 4A and C). Interestingly, the expression of PPARγ and mitochondrial proteins was minimally changed in phase IP1 (days 0-2; Fig. 4A-C). Thus, phase IP2 seems to be relevant primarily to PPARγ and mitochondrial regulation, while phase IP1 to clonal expansion.
Figure 4.
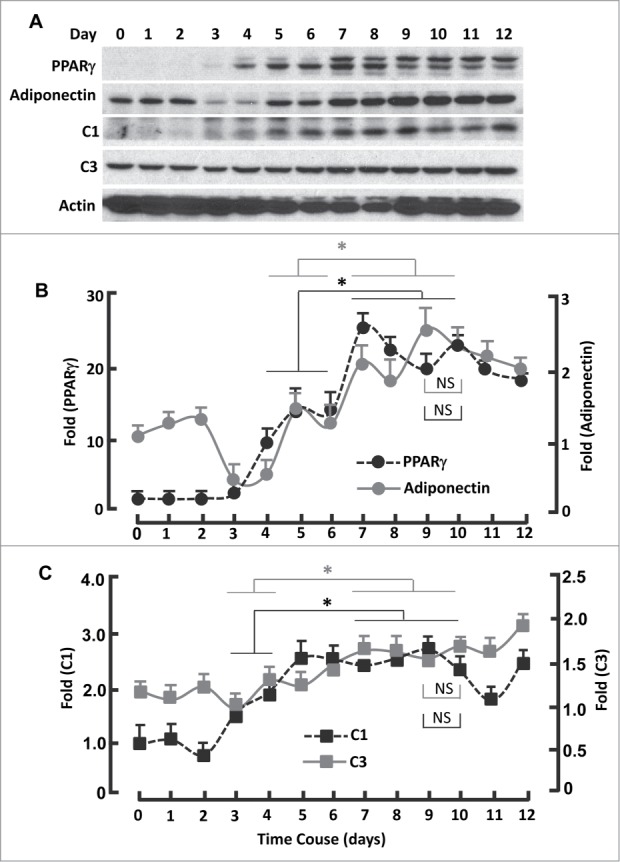
Kinetics of FoxO1-regulated protein expression during adipogenesis. (A) Western blots showing the expression of PPARγ, adiponectin, mitochondrial proteins C1 and C3. β-actin was probed as the loading control. (B) Densitometric analysis of western blot images for PPARγ and adiponectin with NIH ImageJ software. (C) Densitometric analysis of protein gel blot images for C1 and C3 with NIH ImageJ software. n = 3−5. * P < 0 .05; NS, not significant.
A third inactivation peak (IP3) appeared on day 9, before which FoxO1 activation reached the second maximum (AP2) on day 7 and after which was AP3 on day 10 (Fig. 3B). Interestingly, the expression of PPARγ and adiponectin continuously increased in the phase transition of IP2 → AP2 (days 5-7; Fig. 4A and B), so did mitochondrial proteins C1 and C3 (Fig. 4A and C). The observation of additional transitions (IP2 →AP2, AP2→ IP3 and IP3→AP3) is novel, which fall into the same stage of “terminal differentiation” (days 4-12),15 and warrants further study to advance our understanding of FoxO1 in adipogenic regulation.
AS1842856 had stage-dependent effects on adipogenesis
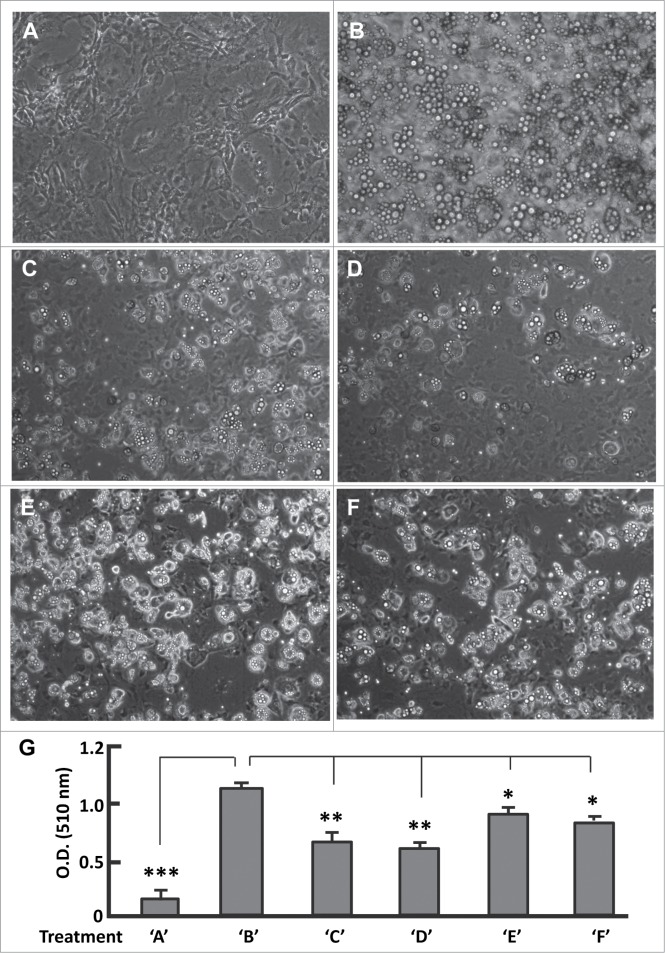
To test the importance of the additional transitions of IP2→AP3 for adipogenesis, we added AS1842856 (0.1 μM) to BMII at the end of day 6, locking FoxO1 in an inhibited state during days 7-12 (Fig. S2A). As controls, mature adipocytes showed significant accumulation of lipid droplets and strong Oil red O staining, which were not observed in the pre-adipocytes (Fig. 5A and B; Fig. S3A and B). By contrast, treatment of the cells with AS1842856 from day 7 to day 12 potently prevented adipogenesis regardless of differentiation induction (Fig. 5C; Fig. S3C). Thus, FoxO1 re-activation and the progression to IP2→AP3 are critical for adipogenesis. We then moved the treatments to earlier stages (Fig. S2B-D). Addition of AS1842856 (0.1 μM) to DMI led to a similar phenotype than to BMII (Fig. 5D; Fig. S3D). However, when it was supplemented in BMI and DMII, AS1842856 suppressed adipogenesis to a lesser extent than in DMI, suggestive of a milder disturbance of FoxO1 action in adipogenesis (Fig. 5E and F; Fig. S3E and F).
Figure 5.
(See previous page). Effects of phase-specific FoxO1 inhibition on adipogenesis. (A and B) Images of cells that were maintained in basal medium (A), and that underwent differentiation induced with the protocol as described in Materials and Methods (B). (C) Images of cells that were treated with AS1842856 during stage BMII (days 7–12) and underwent differentiation induction as in (B). (D) Images of cells that were treated with AS1842856 during stage DMI (days 3–4) and underwent differentiation induction as in (B). (E) Images of cells that were treated with AS1842856 during stage BMI (days 1–2) and underwent differentiation induction as in (B). (F) Images of cells that were treated with AS1842856 during stage DMII (days 5–6) and underwent differentiation induction as in (B). The final concentration of AS1842856 was 0.1 μM. All the images were captured on day 12, and the microscope was set at 100X. (G) Measurement of extracted Oil red O retained in cells by the absorbance (O.D.) at 510 nm (n = 6). Treatments of ‘A’–‘F’ refer to the individual treatments as described in panels A-F. * P < 0 .05; **, P < 0 .01; and ***, P < 0 .0001.
Aligning stages BMI, DMI, DMII and BMII with FoxO1 activation trajectory (Fig. S2E), one can see the same primary mark in stages BMI and DMII, i.e., to inactivate FoxO1 at IP1 and IP2, respectively. Evidently, addition of AS1842856 to media BMI and DMII promotes FoxO1 inactivation in stages BMI and DMII, in concert with the physiological role of FoxO1 thus favorable for adipogenesis. However, AS1842856 prevents FoxO1 re-activation in the late stages of BMI (day 2) and DMII (day 6), which counteracts the physiological role of FoxO1 and may account at least in part for the overtly mild suppression of adipogenesis (Fig. 5E and F; Fig. S3E and F). By contrast, the primary mark in stages DMI and BMII is FoxO1 activation at AP1 and AP2 (Fig. S2E). Supplement of AS1842856 in media DMI and BMII prevents FoxO1 from reactivation, thereby largely inhibiting adipogenesis (Fig. 5C and D; Fig. S3C and D). Thus, a finely tuned activity of FoxO1 is critical for its physiological role in adipogenesis.
Conclusion
Our data suggest targeting FoxO1 with the selective antagonist AS1842856 dramatically suppresses adipogenesis. Antagonizing FoxO1 with AS1842856 also reduces the expression of mitochondrial proteins and adiponectin, supporting the notion that mitochondrial function is critical for adipocyte differentiation.6,7 Since excess adipogenesis contributes to obesity development, AS1842856 may be an anti-obesity candidate that warrants further investigation. It should be noted that the expression and activation of FoxO1 during adipocyte differentiation is fairly dynamic other than steady-state, which may explain the controversy arising from stable overexpression of FoxO1.13-15 As FoxO1 activation and inactivation must follow sigmoid curves during the differentiation process, however, it is reasonable to observe that adipocytes overexpressing of constitutively active FoxO1 fail to differentiate.15 The presence of multiple activation-inactivation transitions in the stage of “terminal differentiation” (days 4-12) suggests a complex regulation of FoxO1, and molecular events involved in these transitions remain largely unknown and warrant future study.
Materials and Methods
Materials
3T3-L1 preadipocytes (ATCC® CL-173™) were purchased from ATCC (Manassas, VA). Dulbecco's modified Eagle's (DMEM) medium was from Corning Inc. (Manassas, VA). Fetal bovine serum (FBS) was from GeneMate (Kaysville, UT). Dexamethasone, 3-isobutyl-1-methylxanthine (IBMX) and rosiglitazone were purchased from Cayman chemical (Ann Arbor, MI). Penicillin/streptomycin (P/S) was from GE Healthcare Life Sciences HyClone Laboratories (Logan, UT). Insulin was from Sigma-Aldrich (St. Louis, MO). FoxO1 inhibitor AS1842856 was from EMD Millipore (San Diego, CA).
Cell culture and differentiation induction
3T3-L1 preadipocytes were cultured in basal media (i.e., DMEM media supplemented with 10% FBS, 100 units/ml penicillin and 100 μg/ml streptomycin (1 × P/S)), at 37°C in a humidified atmosphere of 5% CO2. The media were replaced every 2 d Differentiation of 3T3-L1 cells was induced as described previously with slight modification (Fig. 1s).26 Briefly, 3T3-L1 cells were grown to confluence (day 0), and maintained in fresh basal media (BMI) for 2 d (days 1-2). At the end of day 2, BMI medium was changed to differentiation medium I (DMI): DMEM supplemented with 10% FBS, P/S (1 ×), IBMX (0.5 mM), dexamethasone (1 μM), insulin (1 μg/ml) and rosiglitazone (2 μM). At the end of day 4, DMI medium was changed to differentiation medium II (DMII): DMEM supplemented with 10% FBS, P/S (1 ×), and insulin (1 μg/ml). At the end of day 6, DMII medium was changed to basal media (BMII), and the cells were maintained in BMII (replaced with fresh basal medium every 2 days) until fully differentiated (day 12). As a control, preadipocytes were maintained in BMI till day 12, and the medium was replaced fresh one every 2 d Images of the cells were captured on day 12 with a Nikon ECLIPSE TS100 microscope.
AS1842856 treatments
AS1842856 has an IC50 of 0.033 μM to inhibit FoxO1 and can potently block FoxO1 at a final concentration of 0.05-1 μM without showing cytotoxicity.23-25 We tested 0.1 μM and 1 μM, and found AS1842856 severely suppressed adipogenesis at both concentrations, thus choosing to use 0.1 μM for the treatments if not specified elsewhere. AS1842856 or the vehicle 0.1% DMSO as a treatment control was supplemented through the whole procedure of BMI→DMI→DMII→BMII (Fig. 1s), or in the individual stage of BMI, DMI, DMII, BMII (Fig. 2s). Other than the treatment stages, the cells were maintained in media identical to the protocol as described above. Images of the cells were captured on day 12 with a Nikon ECLIPSE TS100 microscope.
Oil red O staining
The Oil Red O working solution was freshly prepared by mixing 0.35% stock solution with dH2O (6:4) and filtered, and the staining was conducted as described.26 At the end of differentiation procedure (day 12), the media were removed and the cells were washed once with phosphate buffered saline (PBS), and fixed in 4% formaldehyde at room temperature for 10 min and then at 4°C overnight. Subsequently, the cells were washed once with dH2O, once with 60% isopropanol, and air dried. Oil Red O working solution was added and the staining at room temperature lasted for 1 h. Afterwards, the stained cells were washed with dH2O for 4 times, and the images were captured with a Nikon ECLIPSE 80i microscope. Oil Red O retained in the cells was extracted with isopropanol, and quantified by the absorbance (O.D.) at 510 nm.28
Western blot
For cell lysates, the cells were washed with ice-cold PBS (Caisson Labs) and lysed with a Bullet Blender® (Next Advance, Inc..), in PLC lysis buffer:20,29 (30 mM Hepes, pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 10 mM NaPPi, 100 mM NaF, 1 mM Na3VO4) supplemented with protease inhibitor cocktail (Roche), 1 mM PMSF, 10 μM TSA (Trichostatin A, Selleckchem) and 5 mM Nicotinamide (Alfa Aesar). Total protein concentrations of the cell lysates were determined using the DC protein assay (Bio-Rad). Western blot and image analysis were conducted as described previously.20 Antibody catalog numbers and vendors are as follows: C1 (A31857) and C3 (A21362) from Invitrogen; PPARγ (MA5-14889) and β-actin (MA5-15739) from Pierce; Adiponectin (2789s), phospho-FoxO1 (9464) and FoxO1 (9454) from Cell Signaling Technology.
Statistical analyses
All results are expressed as means ± s.d. and are analyzed by analysis of variance (ANOVA) to determine p values; p < 0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Funding for this work was provided in part, by the Virginia Agricultural Experiment Station and the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture (Z.C.).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Cheng Z, Almeida FA. Mitochondrial alteration in type 2 diabetes and obesity: an epigenetic link. Cell Cycle 2014; 13:890-7; PMID:24552811; http://dx.doi.org/ 10.4161/cc.28189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng Z, Schmelz EM, Liu D, Hulver MW. Targeting mitochondrial alterations to prevent type 2 diabetes-Evidence from studies of dietary redox-active compounds. Mol Nutr Food Res 2014; 58(8):1739-49; PMID:24668725; http://dx.doi.org/ 10.1002/mnfr.201300747 [DOI] [PubMed] [Google Scholar]
- 3. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19:1338-44; PMID:23995282; http://dx.doi.org/ 10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niswender K. Diabetes and obesity: therapeutic targeting and risk reduction - a complex interplay. Diabetes Obes Metab 2010; 12:267-87; PMID:20380648; http://dx.doi.org/ 10.1111/j.1463-1326.2009.01175.x [DOI] [PubMed] [Google Scholar]
- 5. Cheng Z, White MF. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxidants & Redox Signaling 2011; 14:649-61; PMID:20615072; http://dx.doi.org/ 10.1089/ars.2010.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Pauw A, Tejerina S, Raes M, Keijer J, Arnould T. Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am J Pathol 2009; 175:927-39; PMID:19700756; http://dx.doi.org/ 10.2353/ajpath.2009.081155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kusminski CM, Scherer PE. Mitochondrial dysfunction in white adipose tissue. Trend endocrinol Metab: TEM 2012; 23:435-43; http://dx.doi.org/ 10.1016/j.tem.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell 2007; 131:242-56; PMID:17956727; http://dx.doi.org/ 10.1016/j.cell.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 9. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab 2006; 4:263-73; PMID:17011499; http://dx.doi.org/ 10.1016/j.cmet.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014; 156:20-44; PMID:24439368; http://dx.doi.org/ 10.1016/j.cell.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng Z, White MF. The AKTion in non-canonical insulin signaling. Nat Med 2012; 18:351-3; PMID:22395698; http://dx.doi.org/ 10.1038/nm.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng Z, Tseng Y, White MF. Insulin signaling meets mitochondria in metabolism. Trend Endocrinol Metab: TEM 2010; 21:589-98; http://dx.doi.org/ 10.1016/j.tem.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim H, Hiraishi A, Tsuchiya K, Sakamoto K. (-) Epigallocatechin gallate suppresses the differentiation of 3T3-L1 preadipocytes through transcription factors FoxO1 and SREBP1c. Cytotechnology 2010; 62:245-55; PMID:20596890; http://dx.doi.org/ 10.1007/s10616-010-9285-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Munekata K, Sakamoto K. Forkhead transcription factor Foxo1 is essential for adipocyte differentiation. In Vitro Cell Dev Biol An 2009; 45:642-51; http://dx.doi.org/ 10.1007/s11626-009-9230-5 [DOI] [PubMed] [Google Scholar]
- 15. Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell 2003; 4:119-29; PMID:12530968; http://dx.doi.org/ 10.1016/S1534-5807(02)00401-X [DOI] [PubMed] [Google Scholar]
- 16. Dowell P, Otto TC, Adi S, Lane MD. Convergence of peroxisome proliferator-activated receptor gamma and Foxo1 signaling pathways. J Biol Chem 2003; 278:45485-91; PMID:12966085; http://dx.doi.org/ 10.1074/jbc.M309069200 [DOI] [PubMed] [Google Scholar]
- 17. Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, Karnieli E. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem 2006; 281:19881-91; PMID:16670091; http://dx.doi.org/ 10.1074/jbc.M600320200 [DOI] [PubMed] [Google Scholar]
- 18. Fan W, Imamura T, Sonoda N, Sears DD, Patsouris D, Kim JJ, Olefsky JM. FOXO1 transrepresses peroxisome proliferator-activated receptor gamma transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J Biol Chem 2009; 284:12188-97; PMID:19246449; http://dx.doi.org/ 10.1074/jbc.M808915200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim JJ, Li P, Huntley J, Chang JP, Arden KC, Olefsky JM. FoxO1 haploinsufficiency protects against high-fat diet-induced insulin resistance with enhanced peroxisome proliferator-activated receptor gamma activation in adipose tissue. Diabetes 2009; 58:1275-82; PMID:19289458; http://dx.doi.org/ 10.2337/db08-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med 2009; 15:1307-11; PMID:19838201; http://dx.doi.org/ 10.1038/nm.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng Z, White MF. Foxo1 in hepatic lipid metabolism. Cell Cycle 2010; 9:219-20; PMID:20023377; http://dx.doi.org/ 10.4161/cc.9.2.10567 [DOI] [PubMed] [Google Scholar]
- 22. Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab 2008; 8:65-76; PMID:18590693; http://dx.doi.org/ 10.1016/j.cmet.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagashima T, Shigematsu N, Maruki R, Urano Y, Tanaka H, Shimaya A, Shimokawa T, Shibasaki M. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Mol Pharmacol 2010; 78:961-70; PMID:20736318; http://dx.doi.org/ 10.1124/mol.110.065714 [DOI] [PubMed] [Google Scholar]
- 24. Diep CH, Charles NJ, Gilks CB, Kalloger SE, Argenta PA, Lange CA. Progesterone receptors induce FOXO1-dependent senescence in ovarian cancer cells. Cell Cycle 2013; 12:1433-49; PMID:23574718; http://dx.doi.org/ 10.4161/cc.24550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PloS one 2013; 8:e54514; PMID:23342163; http://dx.doi.org/ 10.1371/journal.pone.0054514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zebisch K, Voigt V, Wabitsch M, Brandsch M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem 2012; 425:88-90; PMID:22425542; http://dx.doi.org/ 10.1016/j.ab.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 27. Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 1998; 47:507-14; PMID:9568680; http://dx.doi.org/ 10.2337/diabetes.47.4.507 [DOI] [PubMed] [Google Scholar]
- 28. Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochem 1992; 97:493-7; http://dx.doi.org/ 10.1007/BF00316069 [DOI] [PubMed] [Google Scholar]
- 29. Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, Rossetti L, Sajan M, Farese RV, White MF. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol 2009; 29:5070-83; PMID:19596788; http://dx.doi.org/ 10.1128/MCB.00138-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.