Abstract
Bioactive peptides and carbohydrates are sourced from a myriad of plant, animal and insects and have huge potential for use as food ingredients and pharmaceuticals. However, downstream processing bottlenecks hinder the potential use of these natural bioactive compounds and add cost to production processes. This review discusses the health benefits and bioactivities associated with peptides and carbohydrates of natural origin and downstream processing methodologies and novel processes which may be used to overcome these.
Keywords: peptides; angiotensin-I-Converting enzyme (ACE-I); renin; platelet activating factor acetylhydrolase (PAF-AH); downstream processing; carbohydrates; chitin, fucan; algaran; ulvans; chitosan; mental health; diabetes; prebiotics
1. Introduction
Carbohydrates play an essential role in human biology and disease development and are a relatively untapped source of bioactive compounds for use as functional foods or pharmaceuticals. In contrast, bioactive peptides or “cryptides” have experienced an explosion of scientific research in recent decades and an impressive array of health attributes have been assigned to peptides generated from food protein sources including dairy, marine, plants and seeds. Bioactive peptides or “cryptides” are sequences of approximately 2–20 amino acids in length that impart a positive health effect to the consumer which goes above and beyond basic human nutrition [1]. They must be bioavailable and capable of exerting this health effect at their target site in the gut, bloodstream or elsewhere [2]. A myriad of positive health beneficial properties are associated with bioactive peptides including antihypertensive, anti-diabetic, anti-obesity, immune-modulatory, relaxing and satiety inducing effects [3]. Furthermore, bioactive peptides can also be generated from meat and underutilized by-products or processing waste/discards produced as a result of food processing [4]. Indeed, bioactive peptides can result from food processing steps including fermentation, high temperature treatment, and pasteurization and cooking [4]. Bioactive peptides derived from natural sources generally act at higher concentrations than their synthetic drug counterparts and functional foods should thus be used for disease prevention rather than treatment. Despite this, bioactive peptides used as functional food ingredients do not accumulate in body tissue [5] and there are only a few reports regarding negative side effects when bioactive peptides are used for preventative healthcare purposes [3]. Bottlenecks in the development of bioactive peptides and their use in food and cosmetic products include costs associated with downstream processing steps, bioavailability of the bioactive peptide and compliance with European Food Safety Authority (EFSA) health claim regulations and other regulatory bodies including the Food Development Authority (FDA) and the Foods of Specific Health Use (FOSHU) system in Japan [6]. Moreover, substantiation of health claims associated with bioactive peptides derived from food sources in the past did not provide enough clinical evidence of the claimed health effect and often the mechanisms of action were not determined [7].
Research efforts concerning the use of bioactive carbohydrates or polysaccharide in functional foods as well as oligosaccharides are still considered under-exploited. However, polysaccharides from natural sources including those isolated from marine and dairy sources have found use in a number of biotechnological and pharmaceutical applications. For example, the polysaccharide chitin which may be generated from prawn and crab shell material and Basidomycete mushrooms, and its de-acetylated form chitosan, have found application as polymers for use in encapsulation technologies [8]. Chitin and chitosan have also been examined for their use as functional food ingredients and have demonstrated anti-obesity and satiety effects in previous studies [8,9]. Furthermore, chitosan is a known coagulant and is used in the manufacture of medical bandages [8,9]. More recently, the prebiotic effects of chitin and chitosan as well as other polysaccharides derived from brown, red and green macroalgae including fucoidan, alginate and ulvans were examined [5,10]. Polysaccharides and oligosaccharides from dairy sources such as yoghurt usually originate from the Generally Recognized as Safe (GRAS) exo-polysaccharide producing bacteria present in the product [11]. Since the dietary fiber intake of many people is below their suggested adequate intake values, strategies to successfully fortify foods with fiber is an important area of food research. In order to provide a source of fiber to the consumer, plant derived polysaccharides may be added to dairy products and sources can include soy and cereals [12]. This paper collates information concerning the varied sources of bioactive carbohydrates and peptides, methods used for purification and downstream processing steps, the bioactivities of these bioactive compounds, their mechanism of action, and bottlenecks concerning their future development.
2. Bioactivities Associated with Peptides and Carbohydrates
Food derived bioactive peptides refer to compounds from animal and plant sources generated by food processing, fermentation, enzymatic or chemical hydrolysis or gastrointestinal digestion and which have regulatory functions in the human system beyond normal and adequate nutrition [13]. Table 1 lists bioactive peptides discovered from a number of sources including soy, wheat, dairy, marine resources including fish processing co-products, meat and others and the commercial products in which they are found. A number of activities have been described for bioactive peptides including antimicrobial, blood-pressure lowering including angiotensin converting enzyme-1 (ACE-I) and renin inhibitory bioactivities, anti-atherosclerotic, antioxidant, antithrombotic, enhancement of mineral absorption, immune-modulatory and opioid activities. Often peptides can have several bioactivities, are multifunctional and exert more than one effect [14].
Table 1.
Bioactive peptides derived from food sources and their use as bioactives in commercial products.
| Peptide Sequence | Observed Bioactivity | Product | Producers of Product | Product Type | Co-Product Source | Reference |
|---|---|---|---|---|---|---|
| LKPNM | Antihypertensive | PeptACE™ | Natural Factors Nutritional Products Ltd., British Columbia, Canada | Capsules | Bonito | [32] |
| LKPNM | Antihypertensive | Vasotensin® | Metagenics, USA | Tablet | Bonito | [32] |
| LKPNM | Antihypertensive | Levenorm® | Ocean Nutrition Canada Ltd., Nova Scotia, Canada | N/A | Bonito | [32] |
| LKPNM | Antihypertensive | Peptide ACE 3000 | Nippon Supplement Inc., Osaka, Japan | Capsules | Bonito | [32] |
| LKPNM | Antihypertensive | Peptide Tea | Nippon Supplement Inc., Osaka, Japan | Powder | Bonito | [33] |
| VY | Antihypertensive | Lapis Support | Tokiwa Yakuhin Co., Ltd., Tokyo, Japan | Beverage | Sardine | [33] |
| VY | Antihypertensive | Valtyron® | Senmi Ekisu Co., Ltd., Ohzu-City, Japan | Ingredient | Sardine | [33] |
| FY, VY and IY | Antihypertensive | Wakame Jelly | Riken Vitamin, Tokyo, Japan | Jelly | Undaria pinnatifida (seaweed) | [34] |
| AKYSY | Antihypertensive | Peptide Nori S | Riken Vitamin, Tokyo, Japan | Beverage | Porphyra yezoensis (seaweed) | [35] |
| AKYSY | Antihypertensive | Mainichi Kaisai Nori | Shirako Co., Ltd., Numazu City, Japan | Powder | Porphyra yezoensis (seaweed) | [35] |
| IPP and VPP | Antihypertensive | Ameal S 120 | Calpis Co., Ltd., Tokyo, Japan | Beverage | Milk | [36] |
| IPP and VPP | Antihypertensive | Ameal S | Calpis Co., Ltd., Tokyo, Japan | Tablet | Milk | [36] |
| IPP and VPP | Antihypertensive | Evolus® | Valio Ltd., Helsinki, Finland | Beverage | Milk | [36] |
| VY | Antihypertensive | Sato Marine Super P | Sato Pharmaceutical Co., Ltd., Tokyo, Japan | Tablet | Sardine | [33] |
| FFVAPFPEVFGK | Antihypertensive | Casein DP Peptio Drink | Kracie Pharmaceutical, Tokyo, Japan | Beverage | Milk | [37] |
| FFVAPFPEVFGK | Antihypertensive | C12 Peption | DMV International, Veghel, The Netherlands | Ingredient | Milk | [37] |
| LVY | Antihypertensive | Goma Pepucha | Suntory Beverage & Food Ltd., Tokyo, Japan | Beverage | Sesame | [38] |
| Numerous peptides | Antihypertensive | Bunaharitake | Yakult Health Foods Co., Ltd., Tokyo, Japan | Powder | Mushroom | [39] |
| VY, IY, IVY | Antihypertensive | StayBalance RJ | Api Co., Ltd., Gifu-City, Japan | Beverage | Royal jelly | [40] |
| VVYP | Weight management | Seishou-sabou | Moringa & Co., Ltd., Kanagawa, Japan | Beverage | Blood (bovine and porcine) | none |
| CSPHP | Cholesterol-lowering | Remake CholesterolBlock | Kyowa Hakko, Tokyo, Japan | Beverage | Soy | [41] |
| YLGYLEQLLR | Stress-relief | Lactium® | Ingredia, Arras Cedex, France | Beverage and capsules | Milk | [42] |
| N/A | Stress-relief | Stabilium® 200 | Yalacta, Caen, France | Capsules | Fish | [43] |
| N/A | Stress-relief | AntiStress 24 | Forte Pharma Laboratories, France | Capsules | Fish | [43] |
| N/A | Stress-relief | Protizen® | Copalis Sea Solutions, Boulogne-sur-mer, France | Powder | Fish | [43] |
| N/A | Joint health | CH-Alpha® | Gelita Health Products GmbH, Eberbach, Germany | Beverage | Bovine collagen | |
| N/A | Joint health | Peptan® | Rousselot SAS, Angoulême, France | Powder | Bovine collagen | [44] |
| N/A | Joint health | Collagen HM | Copalis Sea Solutions, Portel France | Powder | Fish collagen | [45] |
| N/A | Joint health | Glycollagen® | Copalis Sea Solutions, Portel, France | Powder | Skate collagen | [45] |
| N/A | Immunomodulatory | PeptiBal™ | InnoVactiv Inc., Rimouski, QC, Canada | Capsules | Shark | [46] |
| N/A | Gastrointestinal health | Seacure® | Proper Nutrition, USA | Capsules | Fish | [47] |
| N/A | Obesity and mental health | Douchi – traditional Chinese soybean product | Traditional Chinese medicine product, Hong Kong, China | N/A | N/A | [48] |
| N/A | Chinese sufu (fermented tofu) | Traditional product | Traditional Chinese medicine product, Hong Kong, China | N/A | N/A | [49] |
| Whey peptides | Blood pressure regulation and cholesterol control | BioZate®3 hydrolysed whey protein | Davisco Foods, Minnesota, MN, USA | Powder product | Whey proteins | [50] |
| Whey peptides | Blood pressure regulation | BioZate (1) hydrolysed whey protein | Davisco Foods, Minnesota, MN, USA | Powder product | Whey proteins | [51] |
| Fish collagen peptides | Skin health | Deyan, China | Deyan, Hubei, China | Powder product | Fish scale collagen peptides | [52] |
| Carnosine and Anserine | Antioxidant and anti-aging | Nivea Q-10 cream, Nivea | Nivea, France | Cream product | Meat muscle protein (beef and chicken) | [53] |
2.1. Heart Health and Coagulation Beneficial Peptides
High blood pressure or hypertension is the major risk factor for myocardial infarction, congestive heart failure, arteriosclerosis, and stroke and end-stage renal disease. The enzymes angiotensin converting enzyme I (ACE-I; EC 3.4.15.1) and renin (EC 3.4.23.15) play an important role in the control and regulation of blood pressure and salt water balance within the renin angiotensin aldosterone system (RAAS) [15]. ACE-I is the main target in treatment of high blood pressure and several synthetic drugs including captopril (Capoten®), lisinopril and enalapril are currently used as pharmaceuticals to treat this problem [15]. However, these drugs have adverse side effects including sleep apnea, dry cough, angioedema and others [16,17]. Food derived bioactive peptides have shown potential for use as mild or moderate ACE-I and renin inhibitory peptides and several of these are documented in the database BIOPEP [15].
2.1.1. Sources and Structure of ACE-I Inhibitory Peptides
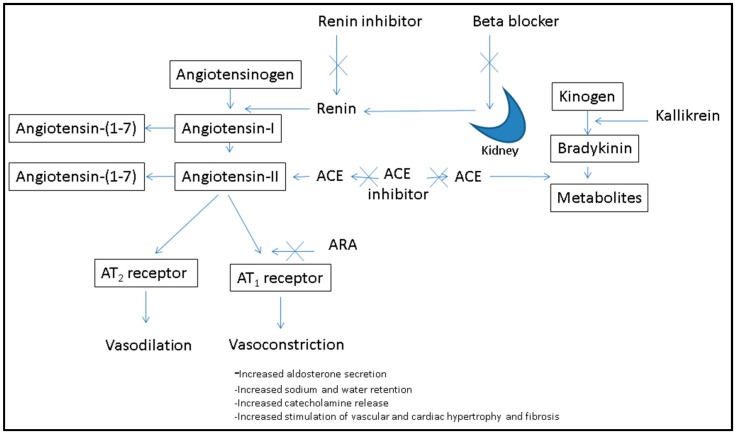
ACE-I inhibitory peptides were first identified by the British scientist Sir John Vane who observed the vasodilatory effect of snake venom [18]. ACE-I catalyzes the conversion of the vasodilatory, decapeptide angiotensin I to the vasoconstrictor angiotensin II within the RAAS (Figure 1). ACE-I also catalyzes the degradation of the vasodilatory compound bradykinin, which results in increased blood pressure [18]. ACE-I inhibitory peptides have been isolated from numerous sources including dairy products such as fermented yoghurts and cheese [19,20], marine co-product proteins [21], in particular collagen from fish skins [22], meat by-products [23], soy [24], hemp seed [25], Chinese and Iranian traditional medicines [26], vegetables including cruciferous vegetables such as broccoli [27], cereals [28] and micro and macroalgae [29,30]. ACE-I inhibitory peptides act on sub-sites of the active site of ACE-I via the C-terminal tri-peptide sequence at the end of a peptide. Many authors have highlighted the importance of the affinity of ACE-I competitive inhibitors to ACE-I of hydrophobic, aromatic or bulky branched side chain amino acid residues. The presence of C-terminal amino acids with a positive charge on the ε-amino group can also contribute to the potency of ACE-I inhibition [31]. Molecular weight is also an important attribute to consider when designing ACE-I inhibitory peptides. In general, ACE-I inhibitory peptides are short sequences of hydrophobic amino acids, and have low molecular weights. In order to determine if ACE-I inhibitory peptides are competitive or non-competitive, it is necessary to determine the minimum quantity of the peptide that inhibits the enzyme by 50% (the IC50 value of the peptide) and to assess the rate of inhibition using the Michaelis Menton equation and Lineweaver-Burk plots [32].
Figure 1.
The Renin-Angiotensin-Aldosterone System (RAAS) can be inhibited by ACE-I inhibitors, angiotensin II type 1 receptor antagonists (ARA), renin inhibitors and beta blockers. ACE-I also plays a role in bradykinin metabolism and metabolism of angiotensin-(1–7).
2.1.2. Sources and Structure of Renin Inhibitory Peptides
The enzyme renin (also known as angiotensinogenase) was first reported by Tigerstedt and Bergman [52] in 1898 when they observed that an extract from rabbit kidney increased blood pressure in rabbits. Renin is a member of the aspartic protease family, which also includes the enzymes pepsin, cathepsin, and chymosin. It is a monospecific enzyme that displays specificity for its only known substrate, angiotensinogen [53]. It is found primarily in the granular cells of the juxtaglomerular apparatus situated in the macula densa mechanism of the kidneys and is produced in response to three main stimuli: (i) Decrease in arterial blood pressure; (ii) Decrease in sodium chloride (NaCl) levels in the ultrafiltrate of the nephron in the kidneys; and (iii) sympathetic nervous system activities which also control blood pressure levels. Renin is produced through the activation of Pro-renin, the enzymatic precursor of Renin. Pro-renin is inactive due to a 43 amino acid N-terminal pro-peptide that covers the active site and blocks access of the active site to angiotensinogen. It is activated either through proteolytic cleavage of the pro-peptide chain or by non-proteolytic activation in the juxtaglomerular cells by the unfolding of the proteolytic pro-peptide, which is how the majority of circulating renin is produced. Renin inhibition is the rate limiting step within the RAAS. However, compared to ACE-I inhibitors, few renin inhibitory peptides have been discovered from food or natural products. Peptides generated following enzymatic hydrolysis of flaxseed fractions were found to inhibit both human recombinant renin and ACE [54]. Li and Aluko identified the renin inhibitory peptide with the amino acid sequence IR from fractions of pea protein isolates with IC50 values <25 mM [55]. More recently, Fitzgerald and colleagues identified the renin inhibitory tridecapeptide IRLIIVLMPILMA from the red seaweed Palmaria palmata [56]. Renin inhibitory peptides were also isolated from buckwheat protein, cereals and rice [57] and hemp seed previously.
In contrast to ACE-I inhibitors, renin inhibitors cause an increase in renin release (Figure 1). This is because they interrupt the negative feedback-mediated regulation of renin release. The combination of an ACE-I inhibitor, angiotensin receptor antagonist or renin inhibitor with another drug from these groups, or with a diuretic, markedly amplifies the increase in renin concentrations. Increases in the concentration of renin concentrations may be 100-fold, which then offsets the inhibition of the renin-angiotensin system by these antihypertensive drugs [58,59]. This may attenuate any reduction in blood pressure.
2.1.3. Sources of Platelet Activating Factor Acetylhydrolase (PAF-AH) Inhibitory Peptides
Platelet activating factor acetylhydrolase (PAF-AH) plays a role in atherosclerosis and inflammation and this is thought to be due to the formation of lysophosphatidyl choline and oxidized non-esterified fatty acids. This enzyme is considered a promising therapeutic target for the prevention of atherosclerosis [60]. Recently, our group isolated the PAF-AH inhibitory tetrapeptide NIGK from the red seaweed Palmaria palmata. The dried powdered alga was hydrolyzed using the food grade enzyme papain, and the resultant peptide containing fraction generated using RP-HPLC. The peptide NIGK had an IC50 value of 2.32 mM [61].
2.1.4. Sources of Dipeptidyl Dipeptidase IV (DPP-IV) Inhibitory Peptides
Inhibition of the enzyme dipeptidyl peptidase-IV (DPP-IV; EC 3.4.14.5) has potential in the prevention of diseases related to the development of metabolic syndrome including type-2 diabetes, heart health and obesity. DPP-IV degrades and inactivates glucagon-like peptide-1 (GLP-1) and gastric-inhibitory peptide (GIP), two incretin hormones which contribute to the enhancement of glucose-induced insulin secretion [62]. Recently, gelatin hydrolysis of fish skins were suggested as a good source of natural inhibitors of dipeptidyl peptidase-IV and could potentially be used in the management of type 2-diabetes and/or neuropathological disorders [63]. GLP-1 is released from intestinal L-cells in response to food entering the GI tract [62]. Collated data suggests that specific macronutrient constituents found in dairy foods act as secretagogues for GLP-1. Tryptophan is considered an important amino acid in the sequence of peptides with potential to inhibit DPP-IV [63]. In a recent study, twenty seven tryptophan containing dipeptides were evaluated for their ability to inhibit dipeptidyl peptidase IV (DPP-IV). Novel DPP-IV inhibitors were identified comprising of three potent dipeptides (Trp-Arg, Trp-Lys and Trp-Leu) with half maximum inhibitory concentration (IC50 values) <45 μM. With the exception of Leu-Trp which was approximately 20 times less potent than Trp-Leu, their reverse peptide did not inhibit DPP-IV. Trp-Asp was the only peptide studied herein with an N-terminal Trp residue which was not a DPP-IV inhibitor [64].
3. Peptides in Mental Health and Prevention of Diabetes PEP/POP Enzyme
3.1. Sources and Mechanisms of Action of Prolyl oligopeptidase (POP) or Prolyl endopeptidase (PEP) (EC 3.4.21.26) Inhibitory Peptides
POP or PEP is a proline-specific endopeptidase that is expressed in the brain and is known to cleave neuroactive peptides implicated in memory, learning and also in neurodegeneration. It is highly conserved and cleaves peptide bonds at the carboxyl side of Proline residues in proteins with a relatively small molecular weight (30 amino acids in size) containing the recognition sequence X-Pro-Y, where X is a peptide or protected amino acid and Y is either an amide, a peptide, an amino acid, an aromatic amine or an alcohol [65]. Furthermore, it is thought that POP may be involved in thalamocortical neurotransmission, memory and learning functions of hippocampal formation and GABAergic regulation of voluntary and in the development of Multiple Sclerosis (MS). Welches and colleagues found that POP is also a major component of the enzymatic pathways that participate in angiotensin metabolism in canine hypothalamus. Several POP inhibitors have been isolated from microbes, medical plants and foods or have been chemically synthesised in recent times and their anti-amnesic effects have been studied in rat models. Sørensen and colleagues [66] found that peptide fractions generated from cod, salmon and trout hydrolysates, autolysates, and water-soluble extracts of cheeses inhibited POP in hydrolysing Z-Gly-Pro-amidomethylcoumarin [66]. Natural POP inhibitors have also been isolated from wine [67], caseins [68], unsaturated fatty acid [69] and plant phenolics [70]. More recently, a protease treated sample of “Barquillo” was found to inhibit POP in vitro. “Barquillo” is a by-product obtained from cocoa processing by pressing and rolling cocoa butter. It is of high biological value due to its high protein content of between 20%–27% [71].
3.2. Sources of DPP-IV Inhibitory Peptides
Diets rich in specific bio-functional ingredients, including food protein derived peptides, have emerged as a potential strategy for the prevention and management of type-2-diabetes (T2D). It is thought that the number of persons diagnosed with diabetes will increase from 285 to 439 million by 2030 [3]. Blood vessel damage in the brain of patients with diabetes and high cholesterol can lead to symptoms of Alzheimer’s disease (AD) and prevention of diabetes and high cholesterol can help to reduce the risk of developing AD [72]. Glucagon-like peptide 1 (GLP-1) and glucose dependent insulinotropic polypeptide (GIP) both control blood glucose levels in the body. These are degraded by DPP-IV and several research groups are looking at the development of DPP-IV inhibitory agents to control glucose and prevent T2D [72,73,74,75]. Pharmaceutical DPP-IV inhibitory drugs available today include Saxagliptin (Onglyza™), and vildagliptin (Galvus®). These drugs do have some side effects including urinary and upper tract infections. Food derived bioactive peptides that inhibit DPP-IV provide an alternative for the potential prevention and treatment of both T2D and AD. Recently, Amylin, a pancreatic peptide, 37 amino acids in length which passes through the blood brain barrier easily provided the template for the amylin analog pramlintide which serves as an effective drug in the clinical treatment of T2D [72]. Furthermore, when injected, this peptide reduced behavioural impairment and brain amyloid pathology in murine models of Alzheimer’s disease.
Peptides derived from dairy, salmon [74], tuna [76], rice [77], amaranth and lysozyme proteins were found previously to inhibit DPP-IV in vitro. Kannan identified a pentapeptide from a bran rice hydrolysate which showed enhanced anti-Alzheimer’s activity. Few in vivo studies with DPP-IV inhibitors have been carried out to date in relation to their possible role in the prevention of AD. Several recent reports however have identified that dipeptidyl DPP-IV inhibitors have suppressive effects on atherosclerosis in apolipoprotein E-null (Apoe−/−) mice. Furthermore, the protective effects of the DPP-IV inhibitor sitagliptin in the blood-retinal barrier in a T2D animal model were shown previously [77].
3.3. Acetylcholinesterase Inhibitory Peptides (AChE Inhibitory Peptides)
The enzyme AChE (EC 3.1.1.7), present in the Central Nervous System (CNS) catalyzes the hydrolysis of Acetylcholine (Ach) to choline. ACh is released in the synaptic cleft, where it activates both postsynaptic and presynaptic cholinergic receptors, which results in cognition improvement [78,79]. AChE—a cholinesterase enzyme-terminates this neural-stimulating activity Protein hydrolysates are also a source of AchE inhibitory peptides. Tuna liver is a fish by-product and is normally discarded and/or used as fish and animal feed due to poor functionality. In a study carried out by Ahn and colleagues, tuna fractionated hydrolysates produced by the commercial enzymes alcalase, neutrase and protamex following flavourzyme hydrolysis showed excellent antioxidant activities against DPPH [76]. Furthermore, all fractionated hydrolysates inhibited acetylcholinesterase activity and the high MW fractions showed greater AChE inhibitory activities than LMW fractions [76]. The AChE inhibitory activity of Douchi—a traditional Chinese salt-fermented soybean food was examined and observed inhibition was attributed bioactive peptides generated from soybean protein following fermentation [80]. Similarly, the AChE inhibitory activity of Chinese sufu (fermented tofu) was observed by Chen and colleagues [81]. In a further study which examined the anti-obesity and anti-Alzheimer’s effect of rice bran, bioactive peptides <5 kDa in size were identified [71].
4. Bioactive Carbohydrates
Bioactive carbohydrates include prebiotics and immune cell regulators. Sources include polysaccharides from marine and terrestrial plants and animals and include fish mucus coating, Phaeophyceae and dairy products. Marine macroalgae or seaweeds are a rich source of commercially available polysaccharides which are used as functional ingredients in food manufacture [82]. Seaweeds, are classified according to their pigmentation into brown (Phaeophyceae), red (Rhodophyta), and green (Chlorophyta). The species of seaweeds that are industrially used belong to the divisions Rhodophyta and Phaeophyta. Bioactivities associated with seaweed polysaccharides include anti-thrombotic, anti-viral, antimicrobial and antioxidant properties.
These bioactivities are thought to be due to sulphated polysaccharides, which are not found in terrestrial plants and which may have specific functions in ionic regulation. Seaweed dietary fibers are particularly rich in soluble fractions, which in red seaweeds are mostly composed of sulphated galactans, such as agar or carrageenans. Soluble dietary fiber polysaccharides derived from brown seaweeds include the alginates, fucans, and laminaran [83]. These polymeric carbohydrates, or polysaccharides are non-toxic, have unique properties, such as their ability to form gels at low concentrations and have technofunctional applications [84].
4.1. Prebiotics
Prebiotics are non-digestible food ingredients that selectively stimulate the growth and/or activity of one or a limited number of beneficial bacteria (probiotics) in the colon [85]. Prebiotic carbohydrates currently available include fructooligosaccharides, lactulose, inulin and galactooligosaccharides from lactose (GOS-La) [86]. Currently, there is considerable interest in the discovery of new carbohydrates with potential prebiotic properties including galactooligosaccharides from lactulose (GOS-Lu). In order to be defined as prebiotic carbohydrates the carbohydrate must fulfill certain criteria [67,87]. The carbohydrate must firstly be resistant to gastric acidity, hydrolysis by mammalian enzymes, gastrointestinal absorption and fermentation by intestinal microflora [87]; Secondly, the carbohydrate in question must have selective stimulation on the growth and/or activity of intestinal probiotic bacteria [87]. As is the case with all functional foods, the final demonstration of a prebiotic effect must be carried out in vivo using the appropriate species. Recently O’Sullivan and colleagues examined the potential of seaweed polysaccharides as prebiotics [10]. Potential prebiotic carbohydrates from plant sources include soybean oligosaccharides, glucooligosaccharides, cyclodextrins, gentiooligosaccharide, germinated barley, oligodextrans, glucuronic acid, pectic oligosaccharide and whole grains [88].
4.1.1. Prebiotics Carbohydrates in Brown Seaweeds
Sulfated fucans derived from brown seaweeds such as Ascophyllum nodosum and Fucus species are frequently referred to as fucans, fucosan, fucansulphate or fucoidans and were first isolated by Killing in 1913 [89]. These polysaccharides consist mainly of sulphated l-fucose and are easily extracted from the cell wall of brown algae with hot water or acid solutions. Fucans can make up greater than 40% of the dry weight of isolated cell walls. There are different types of sulphated fucans which include ascophyllan or xylofucoglycuronan. This polysaccharide is based on a backbone of uronic acid (mannuronic acid) with fucose containing branches (3-O-d-xylosyl-l-fucose-4-sulfate). Sargassans or glycuronofucoglycan are also sulphated fucans, based on linear chains of d-galactose with branches of l-fucose-3-sulfate or occasionally uronic acid. The International Union of Pure and Applied Chemistry (IUPAC) recommend defining sulphated fucan as a polysaccharide based mainly on sulphated l-fucose, with less than 10% of other monosaccharides [82]. The polysaccharides known as alginates are used in the food industry as thickeners, stabilizers and gelling agents and in recent decades, alginates (E400–E404) have become widely adopted as dietetic food hydrocolloids. Ascophyllum nodosum is the brown seaweed that is most exploited for alginate content worldwide. Studies regarding the dietary effects of alginates on faecal microbial fauna in male volunteers have shown that daily administration of 10 g of Sodium alginate for 2 weeks significantly increases the content of Bifidobacteria, while the number of Enterobacteriacea and the frequency of the occurrence of lectinase-negative Clostridia tend to decrease [90].
4.1.2. Prebiotic Carbohydrates in Green Seaweeds
In the green algae, most work has focused on polysaccharides known as ulvans as they display several biological activities of potential interest for therapeutic, nutraceutical and personal care applications. Ulvans are poorly or not degraded by faecal bacteria and therefore could serve as stabilizers and promoters for the binding of growth factors to the high affinity receptors of the cells in the intestinal membrane [91]. A polysaccharide from the green marine algae Ulva lactuca was isolated and was studied for antiviral activity in vitro against a number of human and avian influenza viruses and considerable inhibition detected [92].
4.1.3. Prebiotic Carbohydrates in Red Seaweeds
Sulphated galactans are found in the thallus of red seaweeds. Carrageenans are sulphated galactans extracted from seaweeds of the order Gigartinales, and from some genera of Dumontiales, Halymeniales and Rhodymeniales [82]. Agarans are sulphated galactans in which the α-galactose or 3,6-anhydro-α-galactose units belong to the l-series. Agarose which is biosynthesized by members of the orders Gelidiales and Gracilariales is an agaran polysaccharide. Many reports exist regarding the anticoagulant activity of carrageenan [82]. The species Chondrus crispus is the primary source of λ-carrageenan, whereas Eucheuma cottoni and E. spinosum are the sources of κ- and ι-carrageenans, respectively. The principal basis of the anticoagulant activity of carrageenan appeared to be an anti-thrombotic property [83]. λ-carrageenan showed greater anti-thrombotic activity than κ-carrageenan probably due to its higher sulphate content, whereas the activity of the un-fractionated material remained between the two. Λ-carrageenan consistently prolonged the clotting time and was more toxic than κ-carrageenan [84]. The toxicity of carrageenan depends on the molecular weight. The effect of carrageenan on human platelets was also examined previously. Ex vivo, carrageenan had an anticoagulant effect and inhibited platelet aggregation. The mechanism of anticoagulant activity of λ-carrageenan is exhibited via thrombin inhibition [84].
5. Bioactive Carbohydrates from Plant and Food Processing Co-Products
5.1. Chitin
Chitin is a naturally occurring biopolymer and derivatives include chitosan and chitooligosaccharides as well as glucosamine [8,9]. It is the major structural component of mushroom stalks (in particular Basidomycete mushrooms) and shell waste such as crab and prawn [8,9]. Chitin is a high molecular weight, linear polymer of N-acetyl-d-glucosamine units and can be easily processed into many other bioactive derivatives. Chitosan (CTS) results from the removal of considerable amounts of acetyl groups from chitin. CTS is water soluble and is a positively charged, heteropolymer of d-glucosamine. Both chitin and CTS are user friendly, non-toxic, biocompatible and biodegradable in nature. In the United States CTS was approved by the FDA for certain food applications including as an edible film to protect foods [85]. CTS produced by the Norwegian company Primex® has Generally Recognized as Safe (GRAS) approval and is recognized as a functional food while chitosan-glucan isolated from Aspergillus niger obtained a novel food health claim from EFSA in 2011 [86]. CTS is known to lower density lipoprotein-cholesterol [87]. The positive charge nature of CTS and COS along with its polyelectrolyte properties, gel-forming abilities and the presence of reactive functional groups govern most of their biological activities. Other bioactivities associated with chitin and chitosan include ACE-I inhibition, antimicrobial and anti-obesity bioactivities [8,9].
5.2. Plant Sourced Prebiotic Oligoscaccharides, Obesity and Hypertension Prevention
The oligosaccharides known as raffinose which are isolated from the lupin seeds (Lupinus albus var. Multolupa) are known bifidogenic prebiotics and have been used during the manufacture of probiotic fermented milk [88]. Studies have shown that selection of B. lactis Bb-12 and Lactobacillus acidophilus in a mixed culture at a 1:1 ratio and addition of raffinose oligosaccharides to produce a fermented milk product would have the advantages of rapid growth and acidification rate and would likely increase the probiotic effect of the final functional product [88].
Soluble, plant derived prebiotics such as pectin, konjac mannan and modified starches are soluble in solutions and have viscous effects. These physicochemical properties have been found to affect physiological responses such as the lowering of blood cholesterol and increasing satiety due to delayed gastric emptying [89]. Fiber is associated with a reduction of obesity following consumption. Decreasing obesity by consumption of prebiotics could help to prevent the incidence of hypertension [90]. Various other mechanisms have been postulated to explain the ability of prebiotics to reduce the risk of high blood pressure. The lipid and cholesterol lowering effects of prebiotics could be attributed to the production of short chain fatty acids [91]. Prebiotics resist digestion in the small intestine and in the colon are fermented by microflora to produce short chain fatty acids (SCFAs). SCFAs produced in the bowel are absorbed in the portal vein and subsequently affect various metabolic processes. Propionate for example, could hinder fatty acid and cholesterol synthesis and lactate produced in the bowel cold play a role in lowering the synthesis of triaclyglycerol fatty acids [91].
6. Dairy Prebiotic Oligosaccharides
The dairy prebiotics market directly benefits from the growth in probiotics. Prebiotics from dairy sources include lactulose and galactooligosaccharides.
6.1. Lactulose
Lactulose is produced from lactose through alkali isomerization of the glucose moiety of lactose to fructose, thereby making it a combination of fructose and galactose and it cannot be degraded by mammals [92]. Lactulose is known to have prebiotic effects and may be used in that capacity or as a low-calorie sweetener. It is also used as a pharmaceutical agent to prevent constipation and in patients with hepatic encephalopathy to reduce blood ammonia concentration. Lactulose can selectively stimulate Bifidobacteria and Lactobacilli populations and is considered a prebiotic.
6.2. Galactooligosaccharides (GOS)
GOS is produced from lactose. It is heat and acid stable during storage unlike other prebiotics such as fructooligosaccharide (FOS). GOS are defined as a mixture of those substances produced from lactose, comprising between 2 and 8 saccharide units, with one of these units being a terminal glucose and the remaining saccharide units being galactose and disaccharides comprising 2 units of galactose. Three health claims on GOS have passed EFSA’s pre-screening, namely: “maintains a healthy normal digestive system”, “prebiotic/bifidogenic”, and “calcium absorption” [93].
7. Downstream Processing
Downstream processing of bioactive peptides and carbohydrates involves the recovery and purification of bioactive peptides and carbohydrates. Traditionally bioactive compounds have been extracted using methods which require use of solvent, thermal and/or physical methods. These methods are generally time and energy intensive. In addition, many bioactive compounds are sensitive to high temperature heat and the use of solvents. Recent research has focused on the development of novel extraction techniques that are more efficient in terms of yield, time, and costs and in addition are environmentally friendly and can better preserve the activity of target compounds. Extraction technologies which use ultrasound, microwaves, enzymes, supercritical fluid and pressurized liquid have been researched for extraction of bioactive compounds for food and pharmaceutical applications. Extraction of bioactive carbohydrates and peptides generally involves grinding, precipitation in an acid or basic medium followed by filtration and drying. Application of both classical and novel technologies for the extraction of bioactive compounds from various sources has been reviewed extensively [94,95]. Novel processing technologies has been employed for cell structure disintegration, thus facilitating the extraction of bioactive compounds from complex matrix whereas in some cases novel techniques when used in combination with enzymes enhance digestion reaction rates. Pre-treatment of samples using novel techniques followed by conventional extraction have been demonstrated to enhance extraction yields of bioactive carbohydrates and recovery of proteins for the production of bioactive peptides. Following section outlines application of novel technologies for enhancing reaction rates and cell disruption are discussed.
7.1. High Pressure Processing
High pressure processing (HPP) is a novel technique which employs pressure in the range of 100–800 MPa or even up to 1000 MPa. HPP is applied to enhance solvent permeability and facilitates extraction of bioactives from various matrices. Increase in cell permeability occurs due to the large differential pressure between cell internal and exterior of cell membranes. Increase in cell permeability allows solvent penetration, dissolution rates and improves mass transfer rates. Application of HPP followed by enzymatic hydrolysis or in the presence of enzyme has been investigated to enhance proteolytic hydrolysis of various protein sources. Hydrolysis of food proteins under HPP and enzymes including chymotrypsin, trypsin, and pepsin has been reported to enhance proteolytic reaction and changes the proteolytic pattern [96]. HPP is also reported to allow rapid removal of intact proteins leading to a reduction in binding properties as observed in the case β-lactoglobulins [97] and Ovalbumin [98]. The effect of HPP on whole egg white was examined using in vitro pepsin digestion and proteomic methods. Pepsin incubations conducted with an enzyme to protein ratio of 3:1 following high pressure treatment (400–800 MPa and 9 °C) resulted in increased hydrolysis of egg white proteins. HPP treatment of egg white at 800 MPa resulted in greater susceptibility to pepsin hydrolysis compared to thermal treatment at 95 °C. HPP treatment is also reported to improve antioxidant properties of enzymatic pea protein hydrolysates compared to heat treatment alone [99]. In a study, Garcia-Mora et al. [100] studied the effect of high pressure on hydrolytic efficiency of commercial protein enzymes and they observed that the proteolysis at 300 MPa led to a complete degradation of lentil proteins and increased peptides (<3 kDa). HPP causes protein conformational changes leading to a partial or full unfolding of polypeptides resulting in exposing peptides responsible for antioxidant activity. Unfolding of proteins and opening of protein structure also allows improved hydrolysis of proteins using proteolytic enyzymes.
7.2. Ultrasound Processing
Application of ultrasound (above 20 kHz) to enhance enzymatic reaction and/or extraction yield has been reported extensively. There are two main types of ultrasound equipment which can be employed, namely an ultrasonic water bath and an ultrasonic probe system fitted with horn transducers [100]. Ultrasonic water baths are relatively inexpensive and are commonly used to sonicate laboratory samples. Ultrasonic probe system with horn transducers introduces vibrations directly into the sample and may be used in batch or continuous mode [101]. The main driving force for the extraction effects of ultrasound is acoustic cavitation. Phenomenon of the creation, expansion, and implosive collapse of microbubbles in ultrasonically irradiated liquids is known as “acoustic cavitation”. The formation and collapse of cavitation bubbles generates macro-turbulence, high-velocity inter-particle collisions, and agitation in micro-porous particles of the biomass [102] allows disintegration of matrix. Impingement by these micro-jets results in surface peeling, erosion, and particle break down facilitating release of bioactive compounds from the biological matrix thus increasing extraction efficiency by improving mass transfer [103,104]. Ultrasound in combination with other conventional extraction techniques has been demonstrated as a potential technique for extraction of bioactive compounds [105]. Ultrasound can be carried out at low temperature, which facilitates the extraction of thermo-labile compounds with minimal damage, preserving bioactivity. Ultrasound can be employed with a wide range of solvents including aqueous extraction of bioactive compounds, i.e., for water-soluble components [106]. Application of ultrasound with enzymes has been demonstrated to improve extraction yields of bioactives by facilitating an increase in collisions between enzyme and substrate. Enhanced enzymatic activity of amylases, glucose oxidase, cellulases, dextranase has been reported [107,108]. Studies show that the low intensity ultrasound enhances reaction rates by enhancing both the catalytic and specificity constants, altering composition and modifications of α-helix and β-sheet fractions [107]. Application of ultrasound along with enzymes has a potential to enhance extraction yields of polysachararides from various plant matrices including wheat bran [108,109].
7.3. Microwave Assisted Extraction
Microwave assisted extraction involves the use of electromagnetic radiation in a frequency ranging from 300 MHz to 300 GHz to heat solvents in contact with a sample to separate compounds of interest from the sample matrix. Microwave possess both electric and magnetic field and allow heating of solvent and the sample via dipolar rotation and ionic conduction. The use of microwave energy in extraction process allows disruption of weak hydrogen due to dipole rotation of the molecules and improves solvent penetration into the matrix and thus facilitates the solvation [110]. Microwave assisted extraction has been reported to enhance the extraction yield of bioactives from various matrices compared to traditional solid liquid extraction [111]. The use of microwave assisted extraction may induce degradation of bioactive carbohydrates due to localised high temperature [112]. However, improved antioxidant capacity of bioactive polysaccharides from Auricularia auricular (AAP) using microwave assisted extraction has been reported [113]. Microwave assisted extraction has also been applied for extraction of bioactive sulfated polysaccharides from seaweed including Fucus vesiculosus [114] and Ascophyllum nodosum [115]. Microwave assisted acid hydrolysis of proteins rapid protein degradation for peptide mass mapping and tandem mass spectrometric analysis of peptides has been reported [116].
7.4. Supercritical Solvent Extraction
Efficiency of traditional solid liquid extraction process can be improved by applying pressure and/or temperature to improve extraction yields. Solvent properties including density, diffusivity and viscosity can be controlled with an application of pressure and temperature thus allowing the use of environmentally friendly solvents (e.g., water). Application of pressure and temperature can also improves penetration of solvent and assist in disruption of cellular matrix. For example, CO2 at a temperature and pressure above the critical point (31.06 °C and 7.38 MPa) becomes supercritical fluid. Supercritical CO2 has low viscosity which improves diffusivity and extraction yields [117,118,119]. Supercritical CO2 can be used for the extraction of polar and neutral compounds which can eliminate the use of organic solvents. Extraction efficiency of water can be improved by employing high temperature and pressure. Use of pressure in the range of 3.5 to 20 MPa and temperature in the range of 50–200 °C for liquids is also known as pressurised liquid extraction or accelerated solvent extraction, high pressure solvent extraction, pressurized fluid extraction, and enhanced solvent extraction (ESE) [120,121,122]. The temperature and pressure conditions employed in PLE are in the range of 50 to 200 °C and 3.5 to 20 MPa respectively. Application of pressure with solvents for the extraction of bioactive carbohydrates has been reported extensively [123,124].
8. Conclusions
Bioactive peptides and carbohydrates have huge potential for use as functional foods and exist already in several food and pharmaceutical products as techno-functional and bioactive ingredients. There are requirements for chemical and biotechnological methods concerning efficient, sustainable and economic drying, extraction and bio-refinery procedures for protein/peptide and carbohydrate isolation, hydrolysate generation, purification, up-scaling of production and compound identification methods. Downstream processing for high valued products such as peptides currently involves one or several energy-expensive procedures including drying, cellular disruption and extraction of bioactive molecules. Quality protein/peptide and co-products production/cost relation studies with different drying, extraction and purification methods are needed. Biomass pre-treatment methods including sonic-treatments and centrifugation need to be fully explored to ensure processing costs remain viable and maximum yields of bioactive compounds are obtained.
Peptides, proteins and carbohydrates can contribute to increase the production of healthy and sustainably produced food. At present, novel processing technology utilization is limited by a number of factors including: (1) affordable technologies for the optimized processing of protein/peptide-rich biomass; (2) lack of sustainability and high costs associated with pre-treatment and processing and extraction of proteins; (3) costs associated with the isolation and downstream processing of valuable protein/peptide isolates, hydrolysates and co-products; (4) of health and safety legislation. The bioactivities of existing bioactive peptides and carbohydrates should be examined in different assay systems as often known peptides with specific bioactivities can have multifunctional attributes. In addition, the toxicity and absorption, distribution, metabolism and excretion (ADME) profiles of known peptides with health beneficial activities should be examined.
Application of novel extraction technologies alone or in combination with other conventional techniques demonstrates the possibility of enhancing extraction yield of bioactives while preserving biological activities. However, the scale up to industrial applications still needs to be explored and optimized for large scale production of bioactives.
Acknowledgments
This work was supported by the Marine Functional Foods Research Initiative (NutraMara programme) which is a programme for marine based functional food development. This programme (Grant-Aid Agreement No. MFFRI/07/01) is carried out under the Sea Change Strategy with the support of the Marine Institute and the Department of Agriculture, Food and the Marine funded under the National Development Plan 2007–2013.
Author Contributions
Both authors contributed equally to this publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pimenta D.C., Lebrun I. Cryptides: Buried secrets in proteins. Peptides. 2007;28:2403–2410. doi: 10.1016/j.peptides.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Renukuntla J., Vadlapudi A.D., Patel A., Boddu S.H., Mitra A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013;447:75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes M. Bioactive peptides and their potential use for the prevention of diseases associated with Alzheimers’ Disease and Mental Health Disorders: Food for Thought? Ann. Psychiatry Ment. Health. 2014;2:1017–1025. [Google Scholar]
- 4.Lafarga T., Hayes M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014;98:227–239. doi: 10.1016/j.meatsci.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Lordan S., Stanton C., Ross R.P. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic disease. Mar. Drugs. 2011;9:1056–1100. doi: 10.3390/md9061056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhlig T., Kyprianou T., Martinelli F.G., Oppici C.A., Heiligers D., Hills D., Calvo X.R., Verhaert P. The emergence of peptides in the pharmaceutical business: From exploration to exploitation. EuPA Open Prot. 2014;4:58–69. doi: 10.1016/j.euprot.2014.05.003. [DOI] [Google Scholar]
- 7.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific opinion on the substantiation of health claims related to bonito protein peptide and maintenance of normal blood pressure (ID 1716) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010;8:1730–1744. [Google Scholar]
- 8.Hayes M., Carney B., Slater J., Bruck W. Mining marine shellfish wastes for bioactive molecules: Chitin and chitosan; Part A extraction methods. Biotechnol. J. 2008;3:871–877. doi: 10.1002/biot.200700197. [DOI] [PubMed] [Google Scholar]
- 9.Hayes M., Carney B., Slater J., Bruck W. Mining marine shellfish waste for bioactive molecules: Chitin and chitosan, Part B: Applications. Biotechnol. J. 2008;3:878–889. doi: 10.1002/biot.200800027. [DOI] [PubMed] [Google Scholar]
- 10.O’Sullivan L., Murphy B., McLoughlin P., Duggan P., Lawlor P.G., Hughes H., Gardiner G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs. 2010;8:2038–2064. doi: 10.3390/md8072038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vuyst L., Degeest B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 1999;23:153–177. doi: 10.1111/j.1574-6976.1999.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 12.Dhingra D., Michael M., Rajput H., Patil R.T. Dietary Fibre in foods: A review. J. Food Sci. Technol. 2012;49:255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Udenigwe C.C., Aluko R. Food-Protein derived bioactive peptides: Production, processing and potential health benefits. J. Food Sci. 2012;71:R11–R23. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann R., Meisel H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007;18:163–169. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Mora L., Hayes M. Cardioprotective cryptides derived from fish and other food sources: Generation, application and future markets. J. Agric. Food Chem. 2015;63:1319–1331. doi: 10.1021/jf505019z. [DOI] [PubMed] [Google Scholar]
- 16.Li E.C.K., Heran B.S., Wright J.M. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst. Rev. 2014;8 doi: 10.1002/1465/858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacourciere Y., Brunner H., Irwin R. Effects of modulators of the renin angiotensin aldosterone system on cough. J. Hypertens. 1994;12:1387–1393. Losartan Cough Study Group. [PubMed] [Google Scholar]
- 18.Stuknyté M., Cattaneo S., Masotti F., de Noni I. Occurrence and fate of ACE-inhibitor peptides in cheeses and in their digestates following in vitro static gastrointestinal digestion. Food Chem. 2015;168:27–33. doi: 10.1016/j.foodchem.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 19.Tidona F., Criscione A., Guastella A.M., Zuccaro A., Bordonaro S., Marletta D. Bioactive peptides in dairy products. Ital. J. Anim. Sci. 2009;8:315–340. [Google Scholar]
- 20.Amado I.R., Vázquez J.A., González P., Esteban-Fernánedz D., Carrera M., Pineiro C. Identification of the major ACE-inhibitory peptides produced by enzymatic hydrolysis of a protein concentrate from cuttlefish wastewater. Mar. Drugs. 2014;12:1390–1405. doi: 10.3390/md12031390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu C.-F., Li G.-Z., Peng H.-B., Zhang F., Chen Y., Li Y. Effect of marine collagen peptides on markers of metabolic nuclear receptors in Type 2 Diabetic patients with/without hypertension. Biomed. Environ. Sci. 2010;23:113–120. doi: 10.1016/S0895-3988(10)60040-2. [DOI] [PubMed] [Google Scholar]
- 22.Lafarga T., O’Connor P., Hayes M. Identification of novel dipeptidyl peptidase IV and angiotensin I converting enzyme inhibitory peptides from meat proteins using in silico analysis. Peptides. 2014;59:53–62. doi: 10.1016/j.peptides.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Mallikarjun G.K.G., Gowda L.R., Rao A.G., Prakash V. Angiotensin-I-converting enzyme inhibitory peptide derived from glycinin, the 11S globulin of soybean (Glycine max) J. Agric. Food Chem. 2006;54:4568–4573. doi: 10.1021/jf060264q. [DOI] [PubMed] [Google Scholar]
- 24.Girgih A.T., Alashi A., He R., Malomo S., Aluko R.E. Preventative treatment effects of a hemp seed (Cannabis sativa L) meal protein hydrolysate against high blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2014;53:1237–1246. doi: 10.1007/s00394-013-0625-4. [DOI] [PubMed] [Google Scholar]
- 25.Sharifi N., Souri E., Ali Ziai S., Amin G., Amanlou M. Discovery of new angiotensin converting enzyme (ACE) inhibitors from medicinal plants to treat hypertension using an in vitro assay. DARU J. Pharm. Sci. 2014;21:1–74. doi: 10.1186/2008-2231-21-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J.-E., Bae I.Y., Lee H.G., Yang C.-B. Tyr-Pro-Lys, an angiotensin I converting enzyme inhibitory peptide derived from Broccoli (Brassica oleracea Italica) Food Chem. 2006;99:143–148. doi: 10.1016/j.foodchem.2005.06.050. [DOI] [Google Scholar]
- 27.Shamloo M., Eck P., Beta T. Angiotensin Converting Enzyme Inhibitory Peptides derived from cereals. J. Hum. Nutr. Food Sci. 2015;3:1057–1067. [Google Scholar]
- 28.Wu H., Xu N., Sun X., Yu H., Zhou C. Hydrolysis and purification of ACE inhibitory peptides from the marine microalga. Isochrysis Galbana. J. Appl. Phycol. 2015;27:351–361. doi: 10.1007/s10811-014-0347-x. [DOI] [Google Scholar]
- 29.Paiva L., Lima E., Neto A., Baptista J. Investigation of Azorean macroalgae for angiotensin-I converting enzyme (ACE) inhibitory peptides: Extraction, purification and antihypertensive evaluation. Planta Med. 2014:80–82. doi: 10.1055/s-0034-1394601. [DOI] [Google Scholar]
- 30.Li G.-H., Le G.-W., Shi Y.-H., Shrestha S. Angiotensin I converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmaceutical effects. Nutr. Res. 2004;24:469–486. doi: 10.1016/S0271-5317(04)00058-2. [DOI] [Google Scholar]
- 31.Rawendra R.D., Aisha C.C.I., Hsu J.L. A novel angiotensin converting enzyme inhibitory peptide derived from proteolytic digest of Chinese soft-shelled turtle egg white proteins. J. Proteom. 2013;94:359–369. doi: 10.1016/j.jprot.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Fujita H., Yoshikawa M. LKPNM: A pro-drug type ACE inhibitory peptide derived from fish protein. Immunopharmacology. 1999;44:123–127. doi: 10.1016/S0162-3109(99)00118-6. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki T., Seki E., Osajima K. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolysate on mild hypertensive subjects. J. Hum. Hypertens. 2000;14:519–523. doi: 10.1038/sj.jhh.1001065. [DOI] [PubMed] [Google Scholar]
- 34.Sato M., Hosokawa T., Yamaguchi T., Nakano T., Muramoto K., Kahara T., Funayama K., Kobayashi A., Nakano T. Angiotensin I-converting enzyme inhibitory peptides derived from Wakame (Undaria pinnatifida) and their antihypertensive rats. J. Agric. Food Chem. 2002;50:6245–6252. doi: 10.1021/jf020482t. [DOI] [PubMed] [Google Scholar]
- 35.Peptide Nori S. [(accessed on 8 October 2012)]. Available online: http://en.item.rakuten.com/kenkoex/noripepu30/
- 36.Siltari A., Kivimaki A.S., Ehlers P.I., Korpela R., Vapaatalo H. Effects of milk casein derived tripeptides on endothelial enzymes in vitro; a study with synthetic tripeptides. Arzneimittelforschung. 2012;62:477–481. doi: 10.1055/s-0032-1321846. [DOI] [PubMed] [Google Scholar]
- 37.Sekiya S. Antihypertensive effects of tryptic hydrolysate of casein on normotensive and hypertensive volunteers. J. Jpn. Soc. Nutr. 1992;45:513–517. doi: 10.4327/jsnfs.45.513. [DOI] [Google Scholar]
- 38.Nakano D., Ogura K., Miyakoshi M., Ishii F., Kawanishi H., Kurumazuka D., Kuak C.J., Ikemura K., Takaoka M., Moriguchi S., et al. Antihypertensive effect of angiotensin-I-converting enzyme inhibitory peptides from a sesame protein hydrolysate in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006;2:1118–1126. doi: 10.1271/bbb.70.1118. [DOI] [PubMed] [Google Scholar]
- 39.Lau C.C., Abdullah N., Shuib A.S. Novel angiotensin I converting enzyme inhibitory peptides derived from an edible mushroom, Pleurotus cystidiosin, O. K. Miller identified by LC-MS/MS. BMC Complement. Altern. Med. 2013;13:313–323. doi: 10.1186/1472-6882-13-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tokunaga K., Yoshida C., Suzuki K., Maruyama H., Futamura Y., Araki Y., Mishima S. Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biol. Pharm. Bull. 2004;27:89–192. doi: 10.1248/bpb.27.189. [DOI] [PubMed] [Google Scholar]
- 41.Nagaoka S., Fujimura W., Morikawa K., Nakamura A., Kanamaru Y., Hori G., Yamamoto K., Takamura M., Oda M., Kzuo S. Lactostatin (LLAEK) and CSPHP: New cholesterol lowering peptides derived from food proteins. In: Huang Y.S., Yanagita T., Knap H.R., editors. Dietary Fats and Risk of Chronic Disease. AOCS Press; Urbana, IL, USA: 2006. [Google Scholar]
- 42.Nagai T., Suzuki N., Nagashima T. Antioxidant activities and angiotensin I converting enzyme inhibitory activities of enzymatic hydrolysates from commercially available Kamaboko species. Food Sci. Technol. 2006;12:335–346. [Google Scholar]
- 43.Guillerminet F. Hydrolysed collagen improves bone metabolism and biochemical parameters in ovariectomized mice: An in vitro and an in vivo study. Bone. 2010;46:827–834. doi: 10.1016/j.bone.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 44.Bourseau P., Vandanjon L., Jaouen P., Chaplain-Derouiniot M., Massé A., Guérard F., Chabeaud A., Fouchereau-Péron M., Le Gal Y., Johansson I. Fractionation of fish protein hydrolysates by ultrafiltration and nanofiltration: Impact on peptidic populations. Desalination. 2009;244:303–320. doi: 10.1016/j.desal.2008.05.026. [DOI] [Google Scholar]
- 45.Boutin Y., Paradis M.-E., Couture P., Lamarche B. Immunological effect of fish protein supplementation on healthy adults. J. Nat. Prod. 2012;5:37–44. [Google Scholar]
- 46.Fitzgerald A.J., Rai P.S., Marchbank T., Taylor G.W., Ghosh S., Ritz B.W., Planford R.J. Reparative properties of a commercial fish protein hydrolysate preparation. Gut. 2005;54:775–781. doi: 10.1136/gut.2004.060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D., Wang L.-J., Zhu F.-X., Zhu J.-Y., Chen X.D., Zou L., Saito M., Li L.-T. In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese salt-fermented soybean food) Food Chem. 2008;10:1421–1428. doi: 10.1016/j.foodchem.2007.09.072. [DOI] [Google Scholar]
- 48.Liu Y., Zhou Y., Nirasawa S., Tatsumi E., Cheng Y., Li L. In vivo anti-fatigue activity of sufu with fortification of isoflavone. Pharmacogn. Mag. 2014;10:367–373. doi: 10.4103/0973-1296.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pins J., Keenan J. The antihypertensive effects of a hydrolyzed whey protein isolate supplement (BioZate 1): A pilot study. Cardiovasc. Drugs Ther. 2002;6:68–69. [Google Scholar]
- 50.Yamada S., Nagaoka H., Terajima M., Tsuda N., Hayashi Y., Yamauchi M. Effect of fish collagen peptides on collagen post-translational modifications and mineralization in osteoblastic cell culture systems. Dent. Mater. J. 2013;32:88–95. doi: 10.4012/dmj.2012-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arihara K. Strategies for designing novel functional meat products. Meat Sci. 2006;74:219–229. doi: 10.1016/j.meatsci.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 52.Tigerstedt R., Bergman P.G. Niere und Kreishaf. Skand. Arch. Physiol. 1898;8:223–235. doi: 10.1111/j.1748-1716.1898.tb00272.x. [DOI] [Google Scholar]
- 53.Bock K.D., Gross F. Renin and Angiotensin Tachyphylaxis. Circ. Res. 1961;9:1044–1050. doi: 10.1161/01.RES.9.5.1044. [DOI] [PubMed] [Google Scholar]
- 54.Udenigwe C.C., Lin Y.-S., Hou W.C., Aluko R.E. Kinetics of the inhibition of renin and ACE enzyme by flaxseed protein hydrolysate fractions. J. Funct. Foods. 2009;1:199–207. doi: 10.1016/j.jff.2009.01.009. [DOI] [Google Scholar]
- 55.Li H., Aluko R.E. Identification and inhibitory properties of multifunctional peptides from pea protein hydrolysate. J. Agric. Food Chem. 2010;10:11471–11476. doi: 10.1021/jf102538g. [DOI] [PubMed] [Google Scholar]
- 56.Fitzgerald C., Aluko R.E., Hossain M., Rai D.K., Hayes M. Potential of a renin inhibitory peptide from the red seaweed Palmaria palmata as a functional food ingredient following confirmation and characterization of a hypotensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2014;62:8352–8356. doi: 10.1021/jf500983n. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi S., Tokiwano T., Suzuki N., Kodama I., Yoshizawa Y., Gotoh T. Renin inhibitory activity in rice and cereals. J. Biol. Macromol. 2010;10:83–91. [Google Scholar]
- 58.Campbell D.J. Renin inhibitors—Mechanisms of action. Aust. Prescr. 2009;32:132–135. [Google Scholar]
- 59.Campbell D.J., Aggarwal A., Esler M., Kaye D. Beta-blockers, angiotensin II and ACE inhibitors in patients with heart failure. Lancet. 2001;358:1609–1610. doi: 10.1016/S0140-6736(01)06660-0. [DOI] [PubMed] [Google Scholar]
- 60.Stafforini D.M., Zimmerman G.A. Unraveling the PAF-AH/Lp-PLA2 controversy. J. Lipid Res. 2014;55:1811–1813. doi: 10.1194/jlr.E052886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fitzgerald C., Gallagher E., O’Connor P., Prieto J., Mora-Soler L., Grealy M., Hayes M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the Zebrafish larvae assay. Peptides. 2013;50:119–124. doi: 10.1016/j.peptides.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 62.Drucker D.J. Therapeutic potential of dipeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes. Expert Opin. Investig. Drugs. 2003;12:87–100. doi: 10.1517/13543784.12.1.87. [DOI] [PubMed] [Google Scholar]
- 63.Sila A., Martinez-Alvarez O., Haddar A., Gómez-Gullén M.C., Momtero M.P., Bougatef A. Recovery, viscoelastic and functional properties of Barbel skin gelatin: Investigation of anti-DPP-IV and anti-prolyl endopeptidase activities of generated gelatin polypeptides. Food Chem. 2015;168:478–486. doi: 10.1016/j.foodchem.2014.07.086. [DOI] [PubMed] [Google Scholar]
- 64.Nongonierma A.B., FitzGerald R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by tryptophan containing dipeptides. Food Chem. 2013;4:1843–1849. doi: 10.1039/c3fo60262a. [DOI] [PubMed] [Google Scholar]
- 65.Wilson J., Hayes M., Carney B. Angiotensin-I-Converting enzyme and prolyl endopeptidase inhibitory peptides from natural sources with a focus on marine processing by-products. Food Chem. 2011;129:235–244. doi: 10.1016/j.foodchem.2011.04.081. [DOI] [PubMed] [Google Scholar]
- 66.Sørensen R., Kildal E., Stepaniak L., Pripp A.H., Søhaug T. Screening for peptides from fish and cheese inhibitory to prolyl endopeptidase. Food Nahrung. 2004;48:53–56. doi: 10.1002/food.200300358. [DOI] [PubMed] [Google Scholar]
- 67.Lawandi J., Gerber-Lemaire S., Juillerat-Jeanneret L., Moitessier N. Inhibitors of prolyl endopeptidase for the therapy of human disease. J. Med. Chem. 2010;53:3423–3438. doi: 10.1021/jm901104g. [DOI] [PubMed] [Google Scholar]
- 68.Pripp A.H. Quantitative structure-activity relationship of prolyl endopeptidase inhibitory peptides derived from β-casein using simple amino acid descriptors. J. Agric. Food Chem. 2006;54:224–228. doi: 10.1021/jf0521303. [DOI] [PubMed] [Google Scholar]
- 69.Park Y.S., Jang H.J., Lee K.H., Hahn T.R., Paik Y.S. Prolyl endopeptidase inhibitory activity of unsaturated fatty acids. J. Agric. Food Chem. 2006;54:1238–1242. doi: 10.1021/jf052521h. [DOI] [PubMed] [Google Scholar]
- 70.Lee S.H., Jun M., Choi J.Y., Uang E.J., Hur J.M. Plant phenolics as prolyl endopeptidase inhibitors. Arch. Pharm. Res. 2007;30:827–833. doi: 10.1007/BF02978832. [DOI] [PubMed] [Google Scholar]
- 71.Martorell P., Bataller E., Llopis S., Gonzalez N., Alvarez B., Montón F. A cocoa peptide protects Caenorhabditis elegans from oxidative stress and B-amyloid peptide toxicity. PLoS ONE. 2013;1:e63283. doi: 10.1371/journal.pone.0063283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu H., Wang W., Wallack M., Li H., Carreras I. Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioural impairment and brain amyloid pathology in murine models of Alzheimer’s disease. Mol. Psychiatry. 2015;20:1–8. doi: 10.1038/mp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krushner P., Gorrell M. DPP-4 inhibitors in type 2 diabetes: Importance of selective enzyme inhibition and implications for clinical use. J. Fam. Pract. 2010;59:1–2. [Google Scholar]
- 74.Li-Chan E.C., Hunag S.L., Jao C.L., Ho K.P., Hsu K.C. Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012;60:973–978. doi: 10.1021/jf204720q. [DOI] [PubMed] [Google Scholar]
- 75.Green B.D., Gault V.A., O’Harte F.P., Flatt P.F. Structurally modified analogues of glucagon-like peptide-I (GLP-1) and glucose dependent insulinotropic polypeptide (GIP) as future anti-diabetic agents. Curr. Pharm. Des. 2010;10:3651–3662. doi: 10.2174/1381612043382774. [DOI] [PubMed] [Google Scholar]
- 76.Ahn C.-B., Lee K.-H., Je J.-Y. Enzymatic production of bioactive protein hydrolysates from tuna liver: Effect of enzymes and molecular weight on bioactivity. Int. J. Food Sci. Technol. 2010;45:562–568. doi: 10.1111/j.1365-2621.2009.02166.x. [DOI] [Google Scholar]
- 77.Kannan A., Hettiarachchy N.S., Mahedevan M. Peptides derived from rice bran protect cells from obesity and Alzheimer’s disease. Int. J. Biomed. Res. 2012;3:131–135. doi: 10.7439/ijbr.v3i3.299. [DOI] [Google Scholar]
- 78.Rees T.M., Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today. 2003;39:75–83. doi: 10.1358/dot.2003.39.1.740206. [DOI] [PubMed] [Google Scholar]
- 79.Kihara T., Shimohama S. Alzheimer’s disease and acetylcholine receptors. Acta Neurobiol. Exp. 2004;64:99–105. doi: 10.55782/ane-2004-1495. [DOI] [PubMed] [Google Scholar]
- 80.Liu L., Wang L., Cheng Y., Saito M., Yamaki K., Qiao Z., Li L. Isoflavone content and anti-acetylcholinesterase activity in commercial Douchi a traditional Chinese, salt-fermented Soybean food. Jpn. Agric. Res. Q. 2009;43:301–307. doi: 10.6090/jarq.43.301. [DOI] [Google Scholar]
- 81.Chen J., Quan M.H., Cheng Y.Q., Sun J., Li L.T. Acetylcholinesterase inhibitory activity of Chinese sufu (fermented tofu) ethanol-extract. Food Chem. 2012;134:1263–1266. doi: 10.1016/j.foodchem.2012.02.141. [DOI] [PubMed] [Google Scholar]
- 82.Hayes M., Valverde J. Nutraceuticals derived from marine sources: Extraction and application of bioactive carbohydrates, proteins and peptides as functional ingredients. In: Brar S.K., Kaur S., Dhillon G.S., editors. Nutraceutical and Functional Foods: Natural Remedy. INRS; Québec, QC, Canada: 2013. pp. 39–49. Chapter 13. [Google Scholar]
- 83.Gupta S., Abu-Ghannam N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011;22:315–319. doi: 10.1016/j.tifs.2011.03.011. [DOI] [Google Scholar]
- 84.Bixler H.J., Porse H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2011;23:321–335. doi: 10.1007/s10811-010-9529-3. [DOI] [Google Scholar]
- 85.Elsabee M.Z., Abdou E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. 2010;33:1819–1841. doi: 10.1016/j.msec.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 86.Hayes M. Chitin, chitosan and their derivatives from marine rest raw materials: Potential food and pharmaceutical applications. In: Hayes M., editor. Marine Bioactive Compounds: Sources, Characterization and Application. Springer; New York, NY, USA: 2012. [Google Scholar]
- 87.Wang D., Han J., Yu Y., Li X., Wang Y., Tim H., Guo S., Jin S., Luo T., Qin S. Chitosan oligosaccharide decreases very low density lipoprotein tryglyceride and increases high-density lipoprotein cholesterol in high fat diet fed rats. Exp. Biol. Med. 2011;236:1064–1069. doi: 10.1258/ebm.2011.011032. [DOI] [PubMed] [Google Scholar]
- 88.Martínez-Villaluenga C., Frías J., Vidal-Valverde C., Gómez R. Raffinose family of oligosaccharides from lupin seeds as prebiotics: Application in dairy products. J. Food Prot. 2005;68:1246–1252. doi: 10.4315/0362-028x-68.6.1246. [DOI] [PubMed] [Google Scholar]
- 89.Fabek H. Ph.D Thesis. The University of Guelph; Guelph, ON, Canada: 2015. Understanding the Effect of Soluble Fibres on the Hydrolysis of Starch and the Diffusion of Glucose During Simulated Human Digestion. [Google Scholar]
- 90.Yeo S.-K., Ooi L.-G., Lim T.-J., Liong M.-T. Antihypertensive properties of plant based prebiotics. Int. J. Mol. Sci. 2009;10:3517–3530. doi: 10.3390/ijms10083517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khoury D.E., Cuda C., Luhovyy B.L., Anderson G.H. Beta Glucan: Health Benefits in Obesity and Metabolic Syndrome. J. Nutr. Metab. 2012;2012 doi: 10.1155/2012/851362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vester Boler B.M., Fahey G.C. Prebiotics of plant and microbial origin. In: Callaway T.R., Ricke S.C., editors. Direct Fed Microbials and Prebiotics for Animals: Science and Mechanisms of Action. Springer; Berlin, Germany: 2011. pp. 13–26. Chapter 2. [Google Scholar]
- 93.EFSA Scientific Opinion on the substantiation of health claims related to galacto-oligosaccharides (GOS) and reduction of gastro-intestinal discomfort (ID 763) and decreasing potentially pathogenic microorganisms (ID 765) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011;9 doi: 10.2903/j.efsa.2011.2060. [DOI] [Google Scholar]
- 94.Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F., Jahurul M.H.A., Ghafoor K., Norulaini N.A.N., Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- 95.Wijngaard H., Hossain M.B., Rai D.K., Brunton N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012;46:505–513. doi: 10.1016/j.foodres.2011.09.027. [DOI] [Google Scholar]
- 96.Quirós A., Chichón R., Recio I., López-Fandiño R. The use of high hydrostatic pressure to promote the proteolysis and release of bioactive peptides from ovalbumin. Food Chem. 2007;117:1734–1739. doi: 10.1016/j.foodchem.2006.10.050. [DOI] [Google Scholar]
- 97.Chicón R., Belloque J., Recio I., López-Fandiño R. Influence of high hydrostatic pressure on the proteolysis of β-lactoglobulin A by trypsin. J. Dairy Res. 2006;73:121–128. doi: 10.1017/S0022029905001664. [DOI] [PubMed] [Google Scholar]
- 98.López-Expósito I., Chicón R., Belloque J., Recio I., Alonso E., López-Fandiño R. Changes in the ovalbumin proteolysis profile by high pressure and its effect on IgG and IgE binding. J. Agric. Food Chem. 2008;56:11809–11816. doi: 10.1021/jf8023613. [DOI] [PubMed] [Google Scholar]
- 99.Garcia-Mora P., Penas E., Frias J., Gomez R., Martinez-Villaluenga C. High-pressure improves enzymatic proteolysis and the release of peptides with angiotensin I converting enzyme inhibitory and antioxidant activities from lentil proteins. Food Chem. 2015;2:224–232. doi: 10.1016/j.foodchem.2014.08.116. [DOI] [PubMed] [Google Scholar]
- 100.Ibañez E., Herrero M., Mendiola J., Castro-Puyana M. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro Algae, cyanobacteria, and invertebrates. In: Hayes M., editor. Marine Bioactive Compounds. Springer; Berlin, Germany: 2012. [Google Scholar]
- 101.Mason T.J., Riera E., Vercet A., Lopez-Buesa P. 13-Application of Ultrasound. In: Sun D.-W., editor. Emerging Technologies for Food Processing. Academic Press; London, UK: 2005. [Google Scholar]
- 102.Shirsath S., Sonawane S., Gogate P. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Proc. 2012;53:10–13. doi: 10.1016/j.cep.2012.01.003. [DOI] [Google Scholar]
- 103.Kadam S.U., Tiwari B.K., O’Donnell C.P. Effect of ultrasound pre-treatment on the drying kinetics of brown seaweed Ascophyllum nodosum. Ultrason. Sonochem. 2015;23:302–307. doi: 10.1016/j.ultsonch.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 104.Vilkhu K., Manasseh R., Mawson R., Ashokkumar M. Ultrasonic recovery and modification of food ingredients. In: Feng H., Barbosa-Canovas G., Weiss J., editors. Ultrasound Technologies for Food and Bioprocessing. Springer; New York, NY, USA: 2011. [Google Scholar]
- 105.Rastogi N.K. Opportunities and challenges in application of ultrasound in food processing. Crit. Rev. Food Sci. Nutr. 2011;51:705–722. doi: 10.1080/10408391003770583. [DOI] [PubMed] [Google Scholar]
- 106.Chemat F., Zill E.H., Khan M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 107.Wang J., Sun B., Lin Y., Zhang H. Optimisation of ultrasound-assisted enzymatic extraction of arabinoxylan from wheat bran. Food Chem. 2014;150:482–488. doi: 10.1016/j.foodchem.2013.10.121. [DOI] [PubMed] [Google Scholar]
- 108.Wu H., Zhu J., Diao W., Wang C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbite moschata) Carbohydr. Polym. 2014;113:314–324. doi: 10.1016/j.carbpol.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 109.Bashari M., Eibaid A., Wang J., Tian Y., Xu X., Jin Z. Influence of low ultrasound intensity on the degradation of dextran catalyzed by dextranase. Ultrason. Sonochem. 2013;20:155–161. doi: 10.1016/j.ultsonch.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 110.Guiseppi-Elie A., Choi S.H., Geckeler K.E. Ultrasonic processing of enzymes: Effect on enzymatic activity of glucose oxidase. J. Mol. Catal. B: Enzym. 2009;58:118–123. doi: 10.1016/j.molcatb.2008.12.005. [DOI] [Google Scholar]
- 111.Kaufmann B., Christen P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002;13:105–113. doi: 10.1002/pca.631. [DOI] [PubMed] [Google Scholar]
- 112.Routray W., Orsat V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012;5:409–424. doi: 10.1007/s11947-011-0573-z. [DOI] [Google Scholar]
- 113.Fishman M.L., Chau H.K., Cooke P.H., Yadav M.P., Hotchkiss A.T. Physico-chemical characterization of alkaline soluble polysaccharides from sugar beet pulp. Food Hydrocoll. 2009;23:1554–1562. doi: 10.1016/j.foodhyd.2008.10.015. [DOI] [Google Scholar]
- 114.Zeng W.-C., Zhang Z., Gao H., Jia L.-R., Chen W.-Y. Characterization of antioxidant polysaccharides from Auricularia auricular using microwave-assisted extraction. Carbohydr. Polym. 2012;89:694–700. doi: 10.1016/j.carbpol.2012.03.078. [DOI] [PubMed] [Google Scholar]
- 115.Rodriguez-Jasso R.M., Mussatto S.I., Pastrana L., Aguilar C.N., Teixeira J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011;86:1137–1144. doi: 10.1016/j.carbpol.2011.06.006. [DOI] [Google Scholar]
- 116.Yuan Y., Macquarrie D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015;129:101–107. doi: 10.1016/j.carbpol.2015.04.057. [DOI] [PubMed] [Google Scholar]
- 117.Zhong H., Marcus S.L., Li L. Microwave-assisted acid hydrolysis of proteins combined with liquid chromatography MALDI MS/MS for protein identification. J. Am. Soc. Mass Spectrom. 2005;16:471–481. doi: 10.1016/j.jasms.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 118.Herrero M., Cifuentes A., Ibañez E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006;98:136–148. doi: 10.1016/j.foodchem.2005.05.058. [DOI] [Google Scholar]
- 119.Herrero M., Mendiola J.A., Cifuentes A., Ibáñez E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A. 2010;1217:2495–2511. doi: 10.1016/j.chroma.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 120.Taylor L.T. Supercritical fluid chromatography for the 21st century. J. Supercrit. Fluids. 2009;47:566–573. doi: 10.1016/j.supflu.2008.09.012. [DOI] [Google Scholar]
- 121.Nieto A., Borrull F., Pocurull E., Marcé R.M. Pressurized liquid extraction: A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. Trends Anal. Chem. 2010;29:752–764. doi: 10.1016/j.trac.2010.03.014. [DOI] [Google Scholar]
- 122.Mustafa A., Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta. 2011;703:8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 123.Ruiz-Matute A.I., Ramos L., Martínez-Castro I., Sanz M. Fractionation of honey carbohydrates using pressurized liquid extraction with activated charcoal. J. Agric. Food Chem. 2008;56:8309–8313. doi: 10.1021/jf8014552. [DOI] [PubMed] [Google Scholar]
- 124.Subhedar P.B., Gogate P.R. Enhancing the activity of cellulase enzyme using ultrasonic irradiations. J. Mol. Catal. B: Enzym. 2014;101:108–114. doi: 10.1016/j.molcatb.2014.01.002. [DOI] [Google Scholar]