Abstract
The story of high mobility group protein B1 (HMGB1) in cancer is complicated and the function of HMGB1 in different cancers is uncertain. This review aims to retrieve literature regarding HMGB1 from English electronic resources, analyze and summarize the role of the HMGB1 signaling pathway in hepatocellular carcinoma (HCC), and provide useful information for carcinogenesis and progression of HCC. Results showed that HMGB1 could induce cell proliferation, differentiation, cell death, angiogenesis, metastasis, inflammation, and enhance immunofunction in in vitro and in vivo HCC models. HMGB1 and its downstream receptors RAGE, TLRs and TREM-1 may be potential anticancer targets. In conclusion, HMGB1 plays an important role in oncogenesis and represents a novel therapeutic target, which deserves further study.
Keywords: high mobility group protein B1, receptor for advanced glycation end product, toll-like receptor, triggering receptor expressed on myeloid cells-1, hepatocellular carcinoma
1. Introduction
According to the database from GLOBOCAN 2012, liver cancer has the fifth highest incidence rate and is the second most life-threatening cancer in the world. There were an estimated 14.1 million new cases and 8.2 million cancer deaths worldwide in 2012, among which there were 782,500 new patients and 745,500 deaths caused by liver cancer [1]. Of note, liver cancer ranked the second leading cause of cancer death in males and the sixth in females. Furthermore, hepatocellular carcinoma (HCC) is the primary liver cancer at a dominant rate which is due to the infections of hepatitis B (HBV) and hepatitis C (HCV), food contamination of aflatoxin, alcohol- or nonalcohol-related obesity, type 2 diabetes, cirrhosis, and smoking [1].
In this scenario, it is essential for scientists to pay great affords for HCC therapies. Targeting the hallmarks of cancer is usually one of the approaches to anchoring this issue. For HCC, hallmarks include sustaining proliferative signaling, evading growth suppressors, avoiding immune destruction, enabling replicative immortality, promoting inflammation, activating invasion and metastasis, inducing angiogenesis, mediating genome instability and mutation, resisting cell death and deregulating cellular energetic [2,3]. This means the more hallmarks, the more signaling pathways and cytokines are involved. Interestingly, however, high mobility group box 1 (HMGB1), a protein involving in many signaling pathways, has discovered and been investigated since 1973 [4].
HMGB1 is one of the HMGB family members (HMGB 1, 2, 3 and 4) with a molecular weight of 25 to 30 kDa [5], which consists 215 amino acids and three domains. The three domains include HMGB A box (9–79 aa), HMG B box (95–163 aa) and the C-terminal acidic tail (186–215 aa) [4]. In normal organs, HMGB1 acts as a positive factor to protect cells from injury. For instance, the model mice were most likely to be sensitive to liver ischemia/reperfusion [6], pancreatitis [7] and sepsis [8] if HMGB1 was knocked out in the liver, pancreas or macrophages, respectively. In contrast, HMGB1 functions as one of the damage-associated molecular patterns (DAMPs) in the sterile inflammation model by amplifying hepatic ischemia/reperfusion and acetaminophen-induced liver necrotic injury [9]. Moreover, HMGB1 has been demonstrated as a critical role in a number of cancers, including colorectal [10], breast [11,12], lung [13,14,15,16], prostate [17], cervical [18], skin [19], kidney [20,21], gastric [22,23,24,25,26], pancreatic [27,28,29], osteosarcoma [30] and leukemia [31]. As to the signal pathways of HMGB1, its receptors include receptor for advanced glycation end product (RAGE) [32,33], the toll-like receptors (TLRs, such as TLR-2, 4 and 9) [34,35,36,37], intergrin [38], α-synuclein filaments [39], proteoglycans (e.g., heparin sulfate [40]), CD24 [41], the T-cell immunoglobulin domain and mucin domain-3 (TIM-3) [42], the member of the G protein-coupled receptors CXCR4 [43], N-methyl-d-aspartate receptor (NMDAR) [44] and the triggering receptor expressed on myeloid cells-1 (TREM1) [45].
HMGB1 is expressed in all eukaryotic cells and highly conserved through evolution. There is 99% identity of the hmgb1 gene in mammals [46]. To date, few research papers have reported its mutant, whereas the modifications and regulations are critical for HMGB1 location and function: Acetylated HMGB1 in cancer cells participates DNA replication; ADP-ribosylation of HMGB1 regulates cell death; methylation of HMGB1 facilitates its translocation from nucleus to the cytoplasm; phosphorylation of HMGB1 affects both its DNA-binding activity and nucleo-cytoplasmic distribution and release. In addition, location and function of HMGB1 highly depend on its redox status. Disulfide HMGB1 exhibits cytokine-inducing activity while sulfonyl HMGB1 has nonimmune activity [4].
However, the functions of HMGB1 in cancer are complicated and paradoxical because of its difference of intracellular and extracellular locations. In recent research, it has been documented as a regulator for a number of DNA events, cell differentiation, inflammatory response, cell migration, cell proliferation, cell deaths, cellular senescence, microRNA biogenesis, immune response, tissue regeneration, antibacterial, and so on [4], whereas its actions and the underlying mechanisms on the oncogenesis and advance in HCC are still unclear.
Here, we reviewed the effects of HMGB1 on the oncogenesis and progression in HCC. The review aims to critically summarize the multiple functions of HMGB1, the receptors and the signaling pathways to unveil the hallmarks and the potential therapeutic targets of HCC.
2. HMGB1 and Cell Proliferation in HCC
For HCC, some cell cycle proteins and proliferation cytokines are central in the proliferation, such as cyclin D1 and proliferating cell nuclear antigen (PCNA). HMGB1 not only increased cyclin D1 and PCNA to induce the proliferation of HCC [47], but also regulates for tumor multiplicity and size, alpha-fetoprotein (AFP) level and advanced TNM stage in HCC [48], indicating HMGB1 could be used as a biomarker for HCC diagnosis which deserves further study [49].
The signaling pathways for HMGB1 on the proliferation in HCC may involve its downstream, RAGE and TLRs. On the one hand, HMGB1 could increase the cellular proliferation by HMGB1/RAGE/NF-κB pathway. Knockdown of RAGE by siRNA inhibited cell growth in HCC in vitro [50,51]. Furthermore, the serum level of RAGE in the primary hepatocellular carcinoma (PHC) tissue was higher than that of the adjacent para-neoplastic liver samples [51], indicating that a critical role and a novel potential target of RAGE in HCC [50,52]. On the other hand, under the condition of hypoxia caused by the rapid growth of HCC, HMGB1 was translocated from the nucleus to the cytosol and bound mitochondrial DNA (mtDNA) in the cytoplasm of hypoxic tumor cells, inducing tumor growth by activating TLR-9 signaling pathways both in vitro and in vivo [53].
3. HMGB1 and Angiogenesis in HCC
Recently, HMGB1 has been recognized as a pro-angiogenesis factor leading to the generation of vascular endothelial growth factor (VEGF) in colon cancer [54,55], while RAGE was identified as the requirement for cell angiogenesis in HCC [56]. Since RAGE is known as one of the receptors of HMGB1, this indirectly indicates that HMGB1 may induce angiogenesis by RAGE in HCC. Unfortunately, however, to date, the direct evidence of HMGB1 in the angiogenesis of HCC has not been reported.
4. HMGB1 and Cell Death in HCC
Autophagy and apoptosis are recognized as both the programmed cell deaths. In HCC, the release of HMGB1 from nuclei to cytoplasm was reported as an inducer for cell autophagic cell death, which may be associated with ROS and/or Beclin-1. In contrast, when this pathway was inhibited by administration of antioxidant N-acetyl-cysteine (NAC), the cell death was altered from autophagic to apoptotic [57]. Such a role of HMGB1 might be partially proved by another research area: Ethyl pyruvate (VP) induced apoptosis by decreasing HMGB1 [32]. However, interestingly, autophagic cell death could exist in an HMGB1-independent way since there were no significantly changes of baseline and glucocorticoid-induced hepatic gene expression with HMGB1 ablation [58,59]. HMGB1 also induced HCC cell apoptosis by inhibition of p38-dependent mitochondrial pathway [60], as HMGB1 could be released from necrotic cells [61]. This may result from the complicate multiple functions of HMGB1 partially in a cross-talk way [4,61] or the different targeting strategies between the different studies [62].
5. HMGB1 and Cell Differentiation in HCC
As a nuclear non-histone protein response to various stimuli, HMGB1 is elucidated to be involved in the differentiation of HCC. HMGB1 was a higher level in HCC tissues than that in the normal tissues. For moderately differentiated cancer cells, the localization of HMGB1 was perinuclear. In contrast, in the low differentiated cancer cells, HMGB1 normally resided in the nucleus [63]. In addition, the role of HMGB1 in HCC differentiation was associated with its downstream receptor. Indeed, similar to HMGB1, the high level of RAGE was required for hepatitis as well as HCC. Furthermore, RAGE exhibited a higher level in well- and moderately differentiated HCC but declined as tumors dedifferentiated to poorly differentiated HCC [64]. As HCC resistance to hypoxia was found to have higher levels of RAGE, RAGE transfectant significantly prolonged cell availability under hypoxia, indicating the early stage of oncogenesis with less oxygen and nutrition may acquire resistance by HMGB1/RAGE axis [64].
6. HMGB1 and Metastasis in HCC
To date, strategies for HCC radiotherapie, chemotherapie and surgical resection are inefficient due to high recurrence rate and metastasis. However, the recurrence after tumor rectomy at least partially stems from the distant metastasis to other tissues and organs [65]. HMGB1 was attributed to the one of the causes for HCC metastasis [49], and down-regulation of HMGB1 by siRNA led to the decrease of migration and invasion [66]. HMGB1 repressed the matrix metalloproteinase (MMP) inhibitors RECK and TIMP3, which induced the expression of MMPs to enable metastases [67]. The main signaling pathways at least include: (1) The activation of HMGB1 required the up-stream signaling pathway of HSP70/Beclin-1 [68]. In contrast, HMGB1 could be inhibited by PPARγ agonists [50]; (2) HMGB1 activated RAGE/NF-кB [63,69] and/or TLR-4/caspase-1 [36] for HCC metastasis; (3) HMGB1 also mediated the activation of metastasis by inducing IL6/Stat3-miR-21 axis [67]. Of note, HMGB1-mediated metastasis of HCC is one of the inflammatory responses to hypoxia stress [36].
7. HMGB1 and Inflammatory Response in HCC
HCC is definitely associated with hepatitis B and hepatitis C and other sterile liver injury such as alcoholic fatty liver disease (AFLD) [1]. With regard to inflammation response, HMGB1 functions as an inducer to activate macrophages and leukocytes, as well as a number of inflammatory cytokines [61]. In HBV transgenic mice, HMBG1 may be involved in the amplification of the cytotoxic T lymphocytes (CTLs)-initiated liver damage [70], while in sterile inflammation, HMGB1 can be released from necrotic cells and triggered neutrophil-mediated liver injury [9], suggesting that treatment of HMGB1 inhibitors may be a potential strategy for fighting against hepatitis-caused HCC. In HCC, p53 promotes inflammation-associated hepatocarcinogenesis by inducing HMGB1 release although p53 is usually thought as a tumor suppression factor [71]. The constant activation of p53 could induce pro-tumorigenic inflammation, at least in part, via inducing HMGB1 release. By contrast, application of HMGB1 inhibitors when restoring p53 in cancer therapy might protect against pro-tumorigenic effects while leaving p53-mediated clearance of cancer cells intact [71], indicating the active function of the p53-binding domain in HMGB1 protein. In addition, RAGE, NF-кB and TREM-1 play a great role in HMGB1-induced inflammation [45,72,73]. Therefore, HMGB1 is an important target for the alternation from hepatitis to tumor or early hepatocarcinogenesis.
8. HMGB1 and Immune Function in HCC
The immune system including the innate and adaptive parts protects the body against pathogens, destroy cancer cells and foreign substances. In some case, HMGB1 regulates innate and adaptive immune responses by TLRs and RAGE pathways [4,74], guiding repair and immune protection at the site of tissue injury. In injury tissue, HMGB1 is passively released by necrotic cells and actively secreted by monocytes or macrophages cells [5,46]. HMGB1 is also secreted in the CTLs-killed cells [75]. In addition, HMGB1 attracts inflammatory leukocytes into paracetaminophen-mediated massive necrotic liver [76]. Of note, HMGB1 may interact with its receptor, TLR-9 and enhance the TLR-9-dependent immunostimulatory effect of CpG DNAs on macrophages and dendritic cells (DCs) [5]. These implicate the crucial role of HMGB1 in innate immunity. On the other side, HMGB1 mediates T-cell-dependent acquired immune response acting as an adaptive immune adjuvant [5]. HMGB1-containing proteins may also be used as a tumor-derived autophagosome vaccine for antitumor [77].
However, the role of HMGB1 in liver cancer immunity needs to be further studied. For the patients who suffered from HCC and had undergone transarterial chemoembolization (TACE) therapy, though the level of HMGB1 was found to increase after TACE, the level of HMGB1 was no different between the “progression group” and “no progression group” patients after TACE for 24 h. In contrast, the soluble receptor of advanced glycation end products (sRAGE) were significantly higher in the non-progression group than that in the progression group [33], indicating the clinical prognosis value of sRAGE was superior to HMGB1 for patients undergoing TACE therapy.
9. Discussion
HMGB1 has attracted researchers since it was discovered in 1973, because it plays a critical role in various diseases and disorders, especially in inflammatory, immune responses, and hypoxia in cancer microenvironment. However, little is known about its role in HCC.
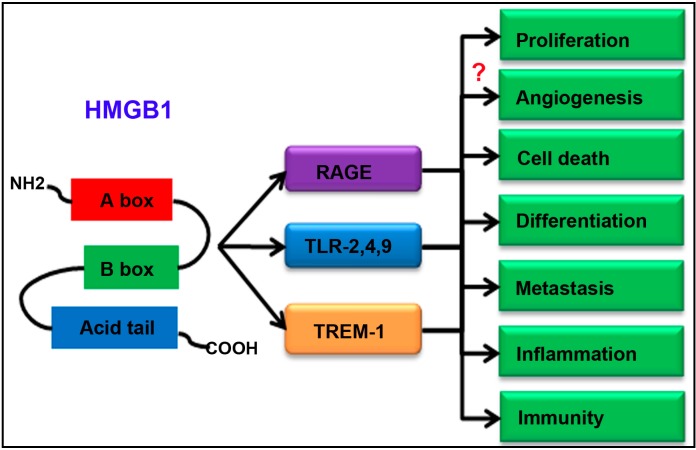
In this review, we summarized HMGB1 in oncogenesis and progression in HCC. Indeed, HMGB1 can induce cell proliferation, angiogenesis and metastasis, cell death, differentiation and inflammation (Table 1). While HMGB1 enhancing immunofunction after TACE may be not prognostic value for TACE [33]. As to the underlying signaling pathways, HMGB1 receptors in HCC include RAGE, TLRs and TREM-1 [45,72,73], though there are a number of receptors of HMGB1 in other cancers. The schematic diagram of HMGB1 signaling pathways in HCC is illustrated in Figure 1.
Table 1.
HMGB1 and its roles in HCC.
| Location of HMGB1 | Function | Receptor | References |
|---|---|---|---|
| recombinant | induce proliferation | NA | [47] |
| NA | promote progression | NA | [48] |
| NA | promote metastasis | NA | [49] |
| NA | induce proliferation and metastasis, block apoptosis | RAGE | [50] |
| NA | induce proliferation | RAGE | [51] |
| NA | induce proliferation and invasion | RAGE | [52] |
| cytosol | induce proliferation | TLR-9 | [53] |
| NA | induce angiogenesis | RAGE | [56] |
| cytosol | induce autophagic cell death | NA | [57] |
| NA | induce proliferation and reduce apoptosis | RAGE | [32] |
| NA | induce apoptosis | NA | [60] |
| perinuclear | induce differentiation | NA | [63] |
| nucleus | block differentiation | NA | [63] |
| NA | induce differentiation | RAGE | [64] |
| NA | induce metastasis | NA | [66] |
| NA | induce metastasis | NA | [67] |
| NA | induce metastasis | RAGE | [68] |
| NA | induce metastasis | RAGE | [69] |
| NA | induce metastasis | TLR-4 and RAGE | [36] |
| NA | induce metastasis | NA | [67] |
| NA | induce inflammation | NA | [71] |
| NA | induce inflammation | TREM-1 | [45] |
| NA | induce inflammation | RAGE | [72] |
| NA | induce inflammation | TREM-1 and RAGE | [73] |
| NA | induce immunity | RAGE | [33] |
| NA | induce proliferation and metastasis | NA | [82] |
| serum | induce carcinogenesis | NA | [83] |
| NA | induce carcinogenesis | NA | [84] |
| NA | induce proliferation and metastasis | NA | [85] |
| serum | induce metastasis | TLR-4 | [86] |
| recombinant | induce proliferation and metastasis, reduce apoptosis | TLR-2 | [87] |
Figure 1.
Schematic diagram of high mobility group protein B1 (HMGB1) signaling pathways in hepatocellular carcinoma (HCC). “?” represents that the more evidence is required.
However, there are conflicting roles of HMGB1 acting as both a tumor suppressor and an oncogenic factor in cancer. On the one hand, in the transcriptional level, p53, the tumor suppressor, could down-regulate the activity of the HMGB1 gene promoter by binding to CTF2. In contrast, CTF2 was proved both to up-regulate the promoter of p21 by binding to p53 and to down-regulate the promoter of p21 by binding to p73, a homologue of p53 [78], which indicated an opposite transcriptional regulation of HMGB1. On the other hand, for the role in oncogenesis and progression of tumor, HMGB1 also had paradox effects. For example, as to the pro-tumor roles, HMGB1 induced the tumorigenesis such as sustenance of cell proliferation, differentiation, angiogenesis, metastasis, inflammation, and enhanced immunofunction in in vitro and in vivo HCC models. However, according to the research, intercellular HMGB1 also could interact with RB, a tumor suppressor in breast cancer [79], and increase genome instability and autophagy [80]. This may depend on the context and the study conditions as well as HMGB1 location and modification [32,60,81]. However, whether the roles of HMGB1 in the carcinogenesis and progression in HCC are good or bad, is still to be studied comprehensively in the future.
Additionally, more details of HMGB1 about its up- and down-regulation, alteration between different signaling pathways as well as strategies must constitute future potential approaches.
10. Conclusions
In summary, HMGB1 plays a pivotal role in oncogenesis and progression in HCC which may be a potential target for therapies and is worthy of further study.
Acknowledgments
The study was financially supported by the Young Scientist Innovation Team Project of Hubei Colleges (T201510); the National Natural Science Foundation of China (81402994); the Natural Science Foundation of Hubei Province of China (2014CFB642; 2014CFB652); the Open Project of Hubei Key Laboratory of Wudang Local Chinese Medicine Research, Hubei University of Medicine (WDCM001); the Foundation for Innovative Research Team of Hubei University of Medicine (2014CXG03); the Key Discipline Project of Hubei Province (2014XKJSXJ18); the Scientific and Technological Project of Education Department of Hubei Province (B2015471).
Author Contributions
Xuanbin Wang, Longchao Xiang, Hongliang Li, Jingxuan Zhang, Nian Yang, Fei Li, Ye Wang, Qiufang Zhang and Fang Li collected materials and wrote the manuscript together. Xuanbin Wang, Ping Chen, Yibin Feng and Fengjun Cao contributed to the manuscript design, revision and proofreading.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Wang X., Wang N., Cheung F., Lao L., Li C., Feng Y. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: Current progress on pharmacological actions and mechanisms. J. Integr. Med. 2015;13:142–164. doi: 10.1016/S2095-4964(15)60171-6. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Kang R., Chen R., Zhang Q., Hou W., Wu S., Cao L., Huang J., Yu Y., Fan X.G., Yan Z., et al. Hmgb1 in health and disease. Mol. Asp. Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi M.E., Manfredi A.A. High-mobility group box 1 (hmgb1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang H., Nace G.W., McDonald K.A., Tai S., Klune J.R., Rosborough B.R., Ding Q., Loughran P., Zhu X., Beer-Stolz D., et al. Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: A role for intracellular high-mobility group box 1 in cellular protection. Hepatology. 2014;59:1984–1997. doi: 10.1002/hep.26976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang R., Zhang Q., Hou W., Yan Z., Chen R., Bonaroti J., Bansal P., Billiar T.R., Tsung A., Wang Q., et al. Intracellular hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology. 2014;146:1097–1107. doi: 10.1053/j.gastro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanai H., Matsuda A., An J., Koshiba R., Nishio J., Negishi H., Ikushima H., Onoe T., Ohdan H., Yoshida N., et al. Conditional ablation of hmgb1 in mice reveals its protective function against endotoxemia and bacterial infection. Proc. Natl. Acad. Sci. USA. 2013;110:20699–20704. doi: 10.1073/pnas.1320808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huebener P., Pradere J.P., Hernandez C., Gwak G.Y., Caviglia J.M., Mu X., Loike J.D., Jenkins R.E., Antoine D.J., Schwabe R.F. The hmgb1/rage axis triggers neutrophil-mediated injury amplification following necrosis. J. Clin. Investig. 2015;125:539–550. doi: 10.1172/JCI76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriwaka Y., Luo Y., Ohmori H., Fujii K., Tatsumoto N., Sasahira T., Kuniyasu H. Hmgb1 attenuates anti-metastatic defense of the lymph nodes in colorectal cancer. Pathobiology. 2010;77:17–23. doi: 10.1159/000272950. [DOI] [PubMed] [Google Scholar]
- 11.Stoetzer O.J., Fersching D.M.I., Salat C., Steinkohl O., Gabka C.J., Hamann U., Braun M., Feller A.M., Heinemann V., Siegele B., et al. Circulating immunogenic cell death biomarkers hmgb1 and rage in breast cancer patients during neoadjuvant chemotherapy. Tumor Biol. 2013;34:81–90. doi: 10.1007/s13277-012-0513-1. [DOI] [PubMed] [Google Scholar]
- 12.Fersching D.M.I., Stotzer O.J., Siegele B., Nagel D., Holdenrieder S. Nucleosomes, rage and hmgb1 in predicting response to neoadjuvant chemotherapy in breast cancer patients. Tumor Biol. 2010;31:99. [Google Scholar]
- 13.Wang C.H., Fei G.R., Liu Z.M., Li Q.C., Xu Z.G., Ren T. Hmgb1 was a pivotal synergistic effecor for cpg oligonucleotide to enhance the progression of human lung cancer cells. Cancer Biol. Ther. 2012;13:727–736. doi: 10.4161/cbt.20555. [DOI] [PubMed] [Google Scholar]
- 14.Liu P.L., Tsai J.R., Hwang J.J., Chou S.H., Cheng Y.J., Lin F.Y., Chen Y.L., Hung C.Y., Chen W.C., Chen Y.H., et al. High-mobility group box 1-mediated matrix metalloproteinase-9 expression in non-small cell lung cancer contributes to tumor cell invasiveness. Am. J. Respir. Cell Mol. Biol. 2010;43:530–538. doi: 10.1165/rcmb.2009-0269OC. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X.H., Wang H.M., Wang J. Expression of hmgb1 and nf-kappa b p65 and its significance in non-small cell lung cancer. Wspolczesna Onkol. 2013;17:350–355. doi: 10.5114/wo.2013.35291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang G.H., Jia C.Q., Tian H., Xiao W., Li Y., Wang A.H., Dong L., Lin D.J. Serum high mobility group box protein 1 as a clinical marker for non-small cell lung cancer. Respir. Med. 2009;103:1949–1953. doi: 10.1016/j.rmed.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Gnanasekar M., Thirugnanam S., Ramaswamy K. Short hairpin RNA (shRNA) constructs targeting high mobility group box-1 (HMGB1) expression leads to inhibition of prostate cancer cell survival and apoptosis. Int. J. Oncol. 2009;34:425–431. doi: 10.3892/ijo_00000166. [DOI] [PubMed] [Google Scholar]
- 18.Pang X.A., Zhang Y., Wei H., Zhang J., Luo Q.S., Huang C.L., Zhang S.L. Expression and effects of high-mobility group box 1 in cervical cancer. Int. J. Mol. Sci. 2014;15:8699–8712. doi: 10.3390/ijms15058699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittal D., Saccheri F., Venereau E., Pusterla T., Bianchi M.E., Rescigno M. Tlr4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. Embo J. 2010;29:2242–2252. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi T., Sakazume K., Tonooka A., Zaitsu M., Takeshima Y., Mikami K., Uekusa T. Cytosolic hmgb1 expression in human renal clear cell cancer indicates higher pathological T classifications and tumor grades. Urol. J. 2013;10:960–965. [PubMed] [Google Scholar]
- 21.Wu F., Zhao Z.H., Ding S.T., Wu H.H., Lu J.J. High mobility group box 1 protein is methylated and transported to cytoplasm in clear cell renal cell carcinoma. Asian J. Cancer Prev. 2013;14:5789–5795. doi: 10.7314/APJCP.2013.14.10.5789. [DOI] [PubMed] [Google Scholar]
- 22.Song B., Song W.G., Li Z.J., Xu Z.F., Wang X.W., Wang C.X., Liu J. Effect of hmgb1 silencing on cell proliferation, invasion and apoptosis of mgc-803 gastric cancer cells. Cell Biochem. Funct. 2012;30:11–17. doi: 10.1002/cbf.1811. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Zhu J.S., Yang Y.C., Zhou Z., Chen W.X., Chen N.W. Enhancing the therapeutic effects of ethyl pyruvate on gastric cancer through knockdown of yap1 expression. Eur. J. Inflamm. 2013;11:123–132. [Google Scholar]
- 24.Akaike H., Kono K., Sugai H., Takahashi A., Mimura K., Kawaguchi Y., Fujii H. Expression of high mobility group box chromosomal protein-1 (hmgb-1) in gastric cancer. Anticancer Res. 2007;27:449–457. [PubMed] [Google Scholar]
- 25.Zhang J., Kou Y.B., Zhu J.S., Chen W.X., Li S. Knockdown of hmgb1 inhibits growth and invasion of gastric cancer cells through the nf-.B pathway in vitro and in vivo. Int. J. Oncol. 2014;44:1268–1276. doi: 10.3892/ijo.2014.2285. [DOI] [PubMed] [Google Scholar]
- 26.Chung H.W., Lee S.G., Kim H., Hong D.J., Chung J.B., Stroncek D., Lim J.B. Serum high mobility group box-1 (hmgb1) is closely associated with the clinical and pathologic features of gastric cancer. J. Transl. Med. 2009;7:38–48. doi: 10.1186/1479-5876-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soreide K., Sund M. Epidemiological-molecular evidence of metabolic reprogramming on proliferation, autophagy and cell signaling in pancreas cancer. Cancer Lett. 2015;356:281–288. doi: 10.1016/j.canlet.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Wittwer C., Boeck S., Heinemann V., Haas M., Stieber P., Nagel D., Holdenrieder S. Circulating nucleosomes and immunogenic cell death markers hmgb1, srage and dnase in patients with advanced pancreatic cancer undergoing chemotherapy. Int. J. Cancer. 2013;133:2619–2630. doi: 10.1002/ijc.28294. [DOI] [PubMed] [Google Scholar]
- 29.Kang R., Tang D.L. Autophagy in pancreatic cancer pathogenesis and treatment. Am. J. Cancer Res. 2012;2:383–396. [PMC free article] [PubMed] [Google Scholar]
- 30.Li B., Jiang S.D., Zheng X.F., Ni B.B., Yang Y.H., Chen J.W., Chen K., Jiang L.S. Expression of the inflammatory molecule hmgb1 in human osteosarcoma and its clinical relevance. Eur. J. Inflamm. 2013;11:61–73. [Google Scholar]
- 31.Jia L., Clear A., Liu F.T., Matthews J., Uddin N., McCarthy A., Hoxha E., Durance C., Iqbal S., Gribben J.G. Extracellular hmgb1 promotes differentiation of nurse-like cells in chronic lymphocytic leukemia. Blood. 2014;123:1709–1719. doi: 10.1182/blood-2013-10-529610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng P., Dai W., Wang F., Lu J., Shen M., Chen K., Li J., Zhang Y., Wang C., Yang J., et al. Ethyl pyruvate inhibits proliferation and induces apoptosis of hepatocellular carcinoma via regulation of the hmgb1-rage and akt pathways. Biochem. Biophys. Res. Commun. 2014;443:1162–1168. doi: 10.1016/j.bbrc.2013.12.064. [DOI] [PubMed] [Google Scholar]
- 33.Kohles N., Nagel D., Jungst D., Stieber P., Holdenrieder S. Predictive value of immunogenic cell death biomarkers hmgb1, srage, and dnase in liver cancer patients receiving transarterial chemoembolization therapy. Tumour Biol. 2012;33:2401–2409. doi: 10.1007/s13277-012-0504-2. [DOI] [PubMed] [Google Scholar]
- 34.Zhou T.B. Role of high mobility group box 1 and its signaling pathways in renal diseases. J. Recept. Signal Transduct. 2014;34:348–350. doi: 10.3109/10799893.2014.904875. [DOI] [PubMed] [Google Scholar]
- 35.Roh Y.S., Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J. Gastroenterol. Hepatol. 2013;28:38–42. doi: 10.1111/jgh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan W., Chang Y., Liang X., Cardinal J.S., Huang H., Thorne S.H., Monga S.P., Geller D.A., Lotze M.T., Tsung A. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55:1863–1875. doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conti L., Lanzardo S., Arigoni M., Antonazzo R., Radaelli E., Cantarella D., Calogero R.A., Cavallo F. The noninflammatory role of high mobility group box 1/toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J. 2013;27:4731–4744. doi: 10.1096/fj.13-230201. [DOI] [PubMed] [Google Scholar]
- 38.Friggeri A., Yang Y., Banerjee S., Park Y.J., Liu G., Abraham E. Hmgb1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am. J. Physiol. Cell Physiol. 2010;299:8. doi: 10.1152/ajpcell.00152.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J.X., Lu J.H., Liu L.F., Chen L.L., Durairajan S.S., Yue Z., Zhang H.Q., Li M. Hmgb1 is involved in autophagy inhibition caused by snca/alpha-synuclein overexpression: A process modulated by the natural autophagy inducer corynoxine b. Autophagy. 2014;10:144–154. doi: 10.4161/auto.26751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu D., Young J., Song D., Esko J.D. Heparan sulfate is essential for high mobility group protein 1 (hmgb1) signaling by the receptor for advanced glycation end products (rage) J. Biol. Chem. 2011;286:41736–41744. doi: 10.1074/jbc.M111.299685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen G.Y., Tang J., Zheng P., Liu Y. Cd24 and siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiba S., Baghdadi M., Akiba H., Yoshiyama H., Kinoshita I., Dosaka-Akita H., Fujioka Y., Ohba Y., Gorman J.V., Colgan J.D., et al. Tumor-infiltrating dcs suppress nucleic acid-mediated innate immune responses through interactions between the receptor tim-3 and the alarmin hmgb1. Nat. Immunol. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penzo M., Molteni R., Suda T., Samaniego S., Raucci A., Habiel D.M., Miller F., Jiang H.P., Li J., Pardi R., et al. Inhibitor of nf-kappa b kinases alpha and beta are both essential for high mobility group box 1-mediated chemotaxis. J. Immunol. 2010;184:4497–4509. doi: 10.4049/jimmunol.0903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedrazzi M., Averna M., Sparatore B., Patrone M., Salamino F., Marcoli M., Maura G., Cervetto C., Frattaroli D., Pontremoli S., et al. Potentiation of nmda receptor-dependent cell responses by extracellular high mobility group box 1 protein. PLoS ONE. 2012;7:31. doi: 10.1371/journal.pone.0044518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J., Li J., Salcedo R., Mivechi N.F., Trinchieri G., Horuzsko A. The proinflammatory myeloid cell receptor trem-1 controls kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. 2012;72:3977–3986. doi: 10.1158/0008-5472.CAN-12-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumitriu I.E., Baruah P., Manfredi A.A., Bianchi M.E., Rovere-Querini P. Hmgb1: Guiding immunity from within. Trends Immunol. 2005;26:381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 47.He X., Fan X., Zhou R., Wang H. Effect of hmgb1 on human hepatoma cell line-hepg2 proliferation. J. Cent. South Univ. Med. Sci. 2010;35:451–457. doi: 10.3969/j.issn.1672-7347.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Bi M.R., Zhu L.Y., Yan B.Z., Chen L.Y., Wang F.X., Ma Y.J., Yang B.S. Association of upregulated hmgb1 and c-iap2 proteins with hepatocellular carcinoma development and progression. Hepat. Mon. 2014;14:23552. doi: 10.5812/hepatmon.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao J., Ding Y., Huang J., Li Q., Liu Y., Ni W., Zhang Y., Zhu Y., Chen L., Chen B. The association of hmgb1 gene with the prognosis of hcc. PLoS ONE. 2014;9:89097. doi: 10.1371/journal.pone.0089097. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Yang Y., Zhao L.H., Huang B., Wang R.Y., Yuan S.X., Tao Q.F., Xu Y., Sun H.Y., Lin C., Zhou W.P. Pioglitazone, a ppargamma agonist, inhibits growth and invasion of human hepatocellular carcinoma via blockade of the rage signaling. Mol. Carcinog. 2014;12:22231. doi: 10.1002/mc.22231. [DOI] [PubMed] [Google Scholar]
- 51.Yaser A.M., Huang Y., Zhou R.R., Hu G.S., Xiao M.F., Huang Z.B., Duan C.J., Tian W., Tang D.L., Fan X.G. The role of receptor for advanced glycation end products (rage) in the proliferation of hepatocellular carcinoma. Int. J. Mol. Sci. 2012;13:5982–5997. doi: 10.3390/ijms13055982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takada M., Ku Y., Toyama H., Suzuki Y., Kuroda Y. Suppressive effects of tea polyphenol and conformational changes with receptor for advanced glycation end products (rage) expression in human hepatoma cells. Hepato Gastroenterol. 2002;49:928–931. [PubMed] [Google Scholar]
- 53.Liu Y., Yan W., Tohme S., Chen M., Fu Y., Tian D., Lotze M., Tang D., Tsung A. Hypoxia induced hmgb1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through toll-like receptor 9. J. Hepatol. 2015;63:114–121. doi: 10.1016/j.jhep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Beijnum J.R., Dings R.P., van der Linden E., Zwaans B.M., Ramaekers F.C., Mayo K.H., Griffioen A.W. Gene expression of tumor angiogenesis dissected: Specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339–2348. doi: 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- 55.Schlueter C., Weber H., Meyer B., Rogalla P., Roser K., Hauke S., Bullerdiek J. Angiogenetic signaling through hypoxia: Hmgb1: An angiogenetic switch molecule. Am. J. Pathol. 2005;166:1259–1263. doi: 10.1016/S0002-9440(10)62344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takino J., Yamagishi S., Takeuchi M. Glycer-ages-rage signaling enhances the angiogenic potential of hepatocellular carcinoma by upregulating vegf expression. World J. Gastroenterol. 2012;18:1781–1788. doi: 10.3748/wjg.v18.i15.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng P., Ni Z., Dai X., Wang B., Ding W., Rae Smith A., Xu L., Wu D., He F., Lian J. The novel bh-3 mimetic apogossypolone induces beclin-1- and ros-mediated autophagy in human hepatocellular carcinoma cells. Cell Death Dis. 2013;7:17. doi: 10.1038/cddis.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huebener P., Gwak G.Y., Pradere J.P., Quinzii C.M., Friedman R., Lin C.S., Trent C.M., Mederacke I., Zhao E., Dapito D.H., et al. High-mobility group box 1 is dispensable for autophagy, mitochondrial quality control, and organ function in vivo. Cell Metab. 2014;19:539–547. doi: 10.1016/j.cmet.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun X., Tang D. Hmgb1-dependent and -independent autophagy. Autophagy. 2014;10:1873–1876. doi: 10.4161/auto.32184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gwak G.Y., Moon T.G., Lee D.H., Yoo B.C. Glycyrrhizin attenuates hmgb1-induced hepatocyte apoptosis by inhibiting the p38-dependent mitochondrial pathway. World J. Gastroenterol. 2012;18:679–684. doi: 10.3748/wjg.v18.i7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikoletopoulou V., Markaki M., Palikaras K., Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta. 2013;12:13. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Huebener P., Gwak G.Y., Schwabe R.F. Comment on: Hmgb1-dependent and -independent autophagy. Autophagy. 2015;11:1187–1188. doi: 10.1080/15548627.2015.1054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kostova N., Zlateva S., Ugrinova I., Pasheva E. The expression of hmgb1 protein and its receptor rage in human malignant tumors. Mol. Cell. Biochem. 2010;337:251–258. doi: 10.1007/s11010-009-0305-0. [DOI] [PubMed] [Google Scholar]
- 64.Hiwatashi K., Ueno S., Abeyama K., Kubo F., Sakoda M., Maruyama I., Hamanoue M., Natsugoe S., Aikou T. A novel function of the receptor for advanced glycation end-products (rage) in association with tumorigenesis and tumor differentiation of hcc. Ann. Surg. Oncol. 2008;15:923–933. doi: 10.1245/s10434-007-9698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ashtari S., Pourhoseingholi M.A., Sharifian A., Zali M.R. Hepatocellular carcinoma in asia: Prevention strategy and planning. World J. Hepatol. 2015;7:1708–1717. doi: 10.4254/wjh.v7.i12.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang W., Wang Z., Li X., Li J., Huang Y., Fan X., Duan Y. Reduced high-mobility group box 1 expression induced by rna interference inhibits the bioactivity of hepatocellular carcinoma cell line hcclm3. Dig. Dis. Sci. 2012;57:92–98. doi: 10.1007/s10620-011-1944-z. [DOI] [PubMed] [Google Scholar]
- 67.Chen M., Liu Y., Varley P., Chang Y., He X.X., Huang H., Tang D., Lotze M.T., Lin J., Tsung A. High-mobility group box 1 promotes hepatocellular carcinoma progression through mir-21-mediated matrix metalloproteinase activity. Cancer Res. 2015;75:1645–1656. doi: 10.1158/0008-5472.CAN-14-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong W., Wang Z.Y., Chen G.X., Liu Y.Q., Gu X.Y., Liu W.W. Invasion potential of h22 hepatocarcinoma cells is increased by hmgb1-induced tumor nf-kappab signaling via initiation of hsp70. Oncol. Rep. 2013;30:1249–1256. doi: 10.3892/or.2013.2595. [DOI] [PubMed] [Google Scholar]
- 69.Chen R.C., Yi P.P., Zhou R.R., Xiao M.F., Huang Z.B., Tang D.L., Huang Y., Fan X.G. The role of hmgb1-rage axis in migration and invasion of hepatocellular carcinoma cell lines. Mol. Cell. Biochem. 2014;390:271–280. doi: 10.1007/s11010-014-1978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sitia G., Iannacone M., Muller S., Bianchi M.E., Guidotti L.G. Treatment with hmgb1 inhibitors diminishes ctl-induced liver disease in hbv transgenic mice. J. Leukoc. Biol. 2007;81:100–107. doi: 10.1189/jlb.0306173. [DOI] [PubMed] [Google Scholar]
- 71.Yan H.X., Wu H.P., Zhang H.L., Ashton C., Tong C., Wu H., Qian Q.J., Wang H.Y., Ying Q.L. P53 promotes inflammation-associated hepatocarcinogenesis by inducing hmgb1 release. J. Hepatol. 2013;59:762–768. doi: 10.1016/j.jhep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pusterla T., Nemeth J., Stein I., Wiechert L., Knigin D., Marhenke S., Longerich T., Kumar V., Arnold B., Vogel A., et al. Receptor for advanced glycation endproducts (rage) is a key regulator of oval cell activation and inflammation-associated liver carcinogenesis in mice. Hepatology. 2013;58:363–373. doi: 10.1002/hep.26395. [DOI] [PubMed] [Google Scholar]
- 73.Catanzaro R., Celep G., Illuzzi N., Milazzo M., Rastmanesh R., Yaduvanshi S.K., He F., Trushin M., Sapienza C., Srivastava N., et al. Anti-inflammatory and anti-mutagenic effect of the yhk phytocompound in hepatocytes: In view of an age-management liver-protecting approach. Rejuvenation Res. 2014;17:168–171. doi: 10.1089/rej.2013.1492. [DOI] [PubMed] [Google Scholar]
- 74.Tsung A., Zheng N., Jeyabalan G., Izuishi K., Klune J.R., Geller D.A., Lotze M.T., Lu L., Billiar T.R. Increasing numbers of hepatic dendritic cells promote hmgb1-mediated ischemia-reperfusion injury. J. Leukoc. Biol. 2007;81:119–128. doi: 10.1189/jlb.0706468. [DOI] [PubMed] [Google Scholar]
- 75.Ito N., DeMarco R.A., Mailliard R.B., Han J., Rabinowich H., Kalinski P., Stolz D.B., Zeh H.J., 3rd, Lotze M.T. Cytolytic cells induce hmgb1 release from melanoma cell lines. J. Leukoc. Biol. 2007;81:75–83. doi: 10.1189/jlb.0306169. [DOI] [PubMed] [Google Scholar]
- 76.Scaffidi P., Misteli T., Bianchi M.E. Release of chromatin protein hmgb1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 77.Li Y., Wang L.X., Pang P., Cui Z., Aung S., Haley D., Fox B.A., Urba W.J., Hu H.M. Tumor-derived autophagosome vaccine: Mechanism of cross-presentation and therapeutic efficacy. Clin. Cancer Res. 2011;17:7047–7057. doi: 10.1158/1078-0432.CCR-11-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uramoto H., Izumi H., Nagatani G., Ohmori H., Nagasue N., Ise T., Yoshida T., Yasumoto K., Kohno K. Physical interaction of tumour suppressor p53/p73 with ccaat-binding transcription factor 2 (ctf2) and differential regulation of human high-mobility group 1 (hmg1) gene expression. Biochem. J. 2003;371:301–310. doi: 10.1042/bj20021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiao Y., Wang H.C., Fan S.J. Growth suppression and radiosensitivity increase by hmgb1 in breast cancer. Acta Pharmacol. Sin. 2007;28:1957–1967. doi: 10.1111/j.1745-7254.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 80.Kang R., Zhang Q., Zeh H.J., 3rd, Lotze M.T., Tang D. Hmgb1 in cancer: Good, bad, or both? Clin. Cancer Res. 2013;19:4046–4057. doi: 10.1158/1078-0432.CCR-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang R., Tang D., Schapiro N.E., Loux T., Livesey K.M., Billiar T.R., Wang H., van Houten B., Lotze M.T., Zeh H.J. The hmgb1/rage inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2014;33:567–577. doi: 10.1038/onc.2012.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dong Y.D., Cui L., Peng C.H., Cheng D.F., Han B.S., Huang F. Expression and clinical significance of hmgb1 in human liver cancer: Knockdown inhibits tumor growth and metastasis in vitro and in vivo. Oncol. Rep. 2013;29:87–94. doi: 10.3892/or.2012.2070. [DOI] [PubMed] [Google Scholar]
- 83.Ganz M., Bukong T.N., Csak T., Saha B., Park J.K., Ambade A., Kodys K., Szabo G. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J. Transl. Med. 2015;13 doi: 10.1186/s12967-015-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang H.L., Yu L.X., Yang W., Tang L., Lin Y., Wu H., Zhai B., Tan Y.X., Shan L., Liu Q., et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J. Hepatol. 2012;57:803–812. doi: 10.1016/j.jhep.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 85.Liu Z., Dou C., Wang Y., Jia Y., Li Q., Zheng X., Yao Y., Liu Q., Song T. Highmobility group box 1 has a prognostic role and contributes to epithelial mesenchymal transition in human hepatocellular carcinoma. Mol. Med. Rep. 2015;12:5997–6004. doi: 10.3892/mmr.2015.4182. [DOI] [PubMed] [Google Scholar]
- 86.Yu L.X., Yan L., Yang W., Wu F.Q., Ling Y., Chen S.Z., Tang L., Tan Y.X., Cao D., Wu M.C., et al. Platelets promote tumour metastasis via interaction between tlr4 and tumour cell-released high-mobility group box1 protein. Nat. Commun. 2014;5:5256. doi: 10.1038/ncomms6256. [DOI] [PubMed] [Google Scholar]
- 87.Shi W., Su L., Li Q., Sun L., Lv J., Li J., Cheng B. Suppression of toll-like receptor 2 expression inhibits the bioactivity of human hepatocellular carcinoma. Tumour Biol. 2014;35:9627–9637. doi: 10.1007/s13277-014-2268-3. [DOI] [PubMed] [Google Scholar]