Abstract
Pre-eclampsia (PE) complicates 2%–8% of all pregnancies and is an important cause of perinatal morbidity and mortality worldwide. In order to reduce these complications and to develop possible treatment modalities, it is important to identify women at risk of developing PE. The use of biomarkers in early pregnancy would allow appropriate stratification into high and low risk pregnancies for the purpose of defining surveillance in pregnancy and to administer interventions. We used formal methods for a systematic review and meta-analyses to assess the accuracy of all biomarkers that have been evaluated so far during the first and early second trimester of pregnancy to predict PE. We found low predictive values using individual biomarkers which included a disintegrin and metalloprotease 12 (ADAM-12), inhibin-A, pregnancy associated plasma protein A (PAPP-A), placental growth factor (PlGF) and placental protein 13 (PP-13). The pooled sensitivity of all single biomarkers was 0.40 (95% CI 0.39–0.41) at a false positive rate of 10%. The area under the Summary of Receiver Operating Characteristics Curve (SROC) was 0.786 (SE 0.02). When a combination model was used, the predictive value improved to an area under the SROC of 0.893 (SE 0.03). In conclusion, although there are multiple potential biomarkers for PE their efficacy has been inconsistent and comparisons are difficult because of heterogeneity between different studies. Therefore, there is an urgent need for high quality, large-scale multicentre research in biomarkers for PE so that the best predictive marker(s) can be identified in order to improve the management of women destined to develop PE.
Keywords: pre-eclampsia, early pregnancy biomarkers, meta-analysis
1. Introduction
Pre-eclampsia (PE) is an important cause of perinatal morbidity and mortality and complicates 2%–8% of pregnancies [1]. Worldwide, PE is responsible for more than 50,000 maternal deaths annually [2,3]. It is characterized by de novo hypertension and proteinuria after 20 weeks of gestation. However, PE continues to cause diagnostic dilemmas due to the heterogeneity of its clinical presentations. Clinical phenotypes range from early-onset severe hypertension accompanied by fetal growth restriction and its consequences to late-onset mild hypertension with a normally grown (or even macrosomic) fetus and few long-term complications. The concept that PE may involve several subtypes is now emerging in the literature. It is thought that the end clinical presentation may be due to the maternal response to abnormal placentation or placental function [4].
As PE cannot be predicted by previous obstetric history and risk factors alone [5], much research has focused on the identification of women at high risk of developing PE. This would allow more intensive monitoring of this high risk group as well as targeted prophylactic intervention, timely diagnosis and treatment. The identification of PE biomarkers in early pregnancy would enable appropriate stratification of a pregnancy into high and low risk, such that a positive predictive test would allow specific therapeutic interventions. Maternal deaths due to PE might thus be avoided more easily as the ultimate long term goal [6]. However, on a pragmatic basis, the identification of PE biomarkers would lead to increased maternal surveillance of high risk pregnancies and improve perinatal outcomes.
Due to the complex pathophysiology and aetiology of PE, a wide range of potential biomarkers have been investigated [7]. These biomarkers can be classified under different categories and many novel biomolecules have been identified. In addition to the predictive value of biomarkers, the identification of these entities (e.g., metabolomic or proteiomic molecules) may elucidate the underlying mechanism for the pathogenesis of PE. Although no single biomarker has been deemed suitable for clinical application at present [8] various novel biomarkers or combinations of biomarkers with other well recognized clinical parameters are promising. To this end, we conducted a systematic review and meta-analyses of biomarkers during the first half of pregnancy for the prediction of PE.
2. Results
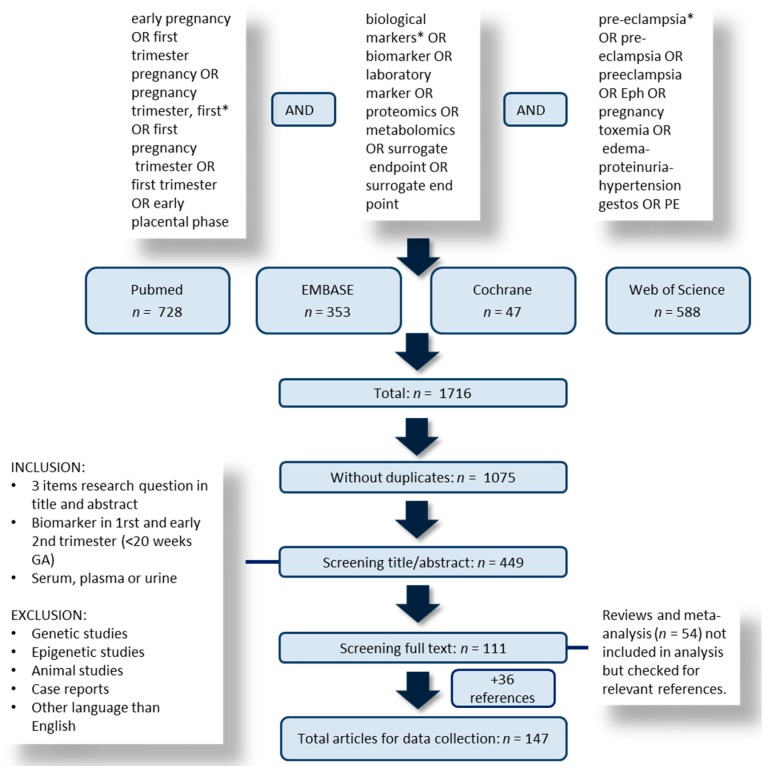
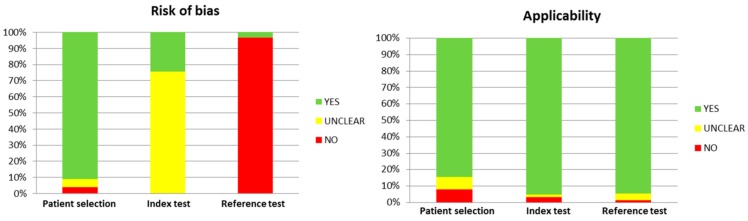
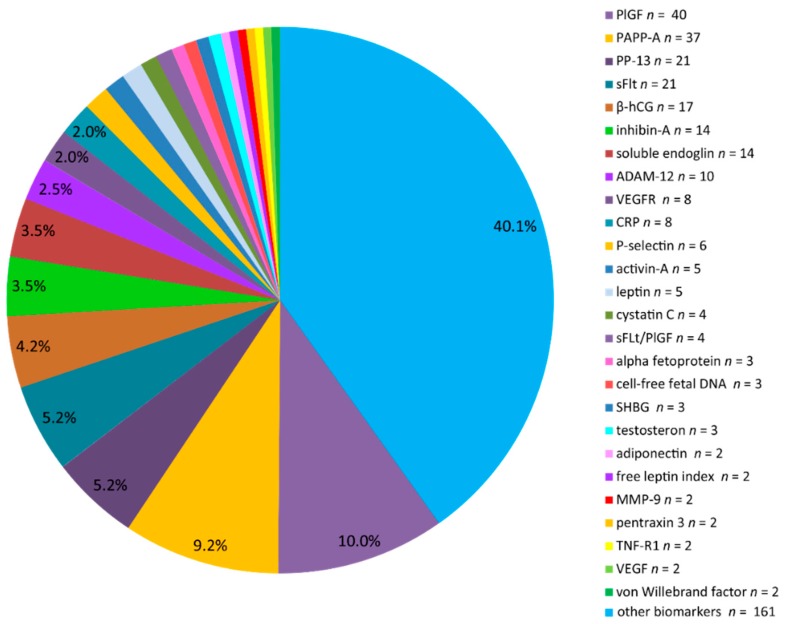
Of the 1716 identified articles, 147 articles were included following full screening. The study selection process is illustrated in Figure 1, while the overall result of the QUADAS-2 quality assessment is shown in Figure 2. Figure 3 demonstrates the frequency of the different laboratory biomarkers in all included studies (401 laboratory biomarkers were described in 147 studies). Placental growth factor (PlGF), pregnancy associated plasma protein A (PAPP-A), soluble fms-like tyrosine kinase (sFLT) and placental protein 13 (PP-13) were the most commonly studied biomarkers.
Figure 1.
Flowchart of selection process. GA: gestational age.
Figure 2.
QUADAS-2 Quality score. QUADAS: Quality Assessment of Diagnostic Accuracy Studies.
Figure 3.
Distribution of studied laboratory biomarkers (n = 401) in included articles (n = 147). PlGF: Placental growth factor; PAPP-A: Pregnancy associated plasma protein A; PP-13: Placental protein 13; ADAM-12: a disintegrin and metalloprotease 12; CRP: C-reactive protein; sFlt: Soluble fms-like tyrosine kinase-1; MMP-9: Matrix metallopeptidase 9; TNF-R1: Tumour-necrosis factor receptor-1; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; SHBG: Sex hormone-binding globulin.
We were able to extract sensitivity and specificity from 36 studies for all PE, 10 studies for EOPE and 7 studies for LOPE; we performed a meta-analyses of all single biomarkers and of the reported combination of biomarkers separately. We performed separate meta-analyses for the following biomarkers (>2 studies available): a disintegrin and metalloprotease 12 (ADAM-12), inhibin-A, PAPP-A, PlGF, PP-13. The characteristics of the included studies are shown in Table 1.
Table 1.
General characteristics of the included studies in the meta-analyses. GH: gestational hypertension; SGA: small for gestational age. PTB: preterm birth. The outcomes used were in line with the definitions from International Society for the study of Hypertension (ISSHP) [9].
| Study | Year | GA of Test (Weeks) | Biomarker (s) | Outcome | Study Design A | Low/High Risk | Location | n (Total) | n (PE) | Level of Evidence |
|---|---|---|---|---|---|---|---|---|---|---|
| Anderson et al. [10] | 2011 | 11–16 | α-1-microglobulin and fetal hemoglobin | PE | Nested case control (in prospective study) 1 | LR | UK | 96 | 60 | 3b |
| Akolekar et al. [11] | 2008 | 11–14 | PlGF, PAPP-A | EOPE, LOPE, GH | Nested case-control (in trisomy 21 screening cohort) 2 | LR + HR | UK | 824 | 127 | 3b |
| Akolekar et al. [12] | 2013 | 11–14 | PlGF, PAPP-A | PE | Prospective cohort (in screening) | LR + HR | UK | 58,703 | 1245 | 1b |
| Audibert et al. [13] | 2010 | 11–13 | PAPP-A, ADAM-12, PlGF, hCG, inhibin-A, PP-13, protein-A, inhibin-A | PE, EOPE, LOPE, GH | Prospective cohort (trisomy 21 screening cohort) 3 | LR + HR | Canada | 893 | 40 | 1b |
| Bills et al. [14] | 2009 | First trimester | VEGF(165)b, sFLT, sEng | PE, EOPE, LOPE | Case-control 4 | LR + HR | UK | 70 | 25 | 3b |
| Bosio et al. [15] | 2001 | 10–14 | P-selectin | PE, GH | Nested case-control (in longitudinal cohort) 5 | LR + HR | Ireland | 70 | 20 | 3b |
| Boucoiran et al. (1) [16] | 2013 | 12–18 | PlGF, sFlt-1, inhibin A | PE, GH, SGA | Prospective cohort (nested in RCT) 6 | LR + HR | Canada | 793 | 34 | 1b |
| Boucoiran et al. (2) [17] | 2013 | 11-14 and 18-22 | PlGF, PP-13, ADAM-12 | EOPE, LOPE, GH | Prospective cohort (trisomy 21 screening cohort) 7 | LR + HR | Canada | 893 | 40 | 1b |
| Brameld et al. [18] | 2008 | 12 + 3 | PAPP-A, free-hCG | PE | Retrospective cohort (trisomy 21 screening cohort) 8 | LR | Australia | 22,125 | 660 | 2b |
| Chafetz et al. [19] | 2007 | 9–12 | PP-13 | PE, PTB, SGA | Nested case control in prospective cohort (MOMS-study) 9 | LR | USA | 425 | 47 | 3b |
| Cohen et al. [20] | 2014 | 10–13 | PAPP-A, α fetoprotein, free β-hCG | PE | Nested case control (retrospective cohort) 10 | LR + HR | USA | 2199 | 148 | 3b |
| Cowans et al. [21] | 2011 | 11–14 | PP-13 | EOPE, LOPE | Nested case control (in cohort of trisomy screening) 11 | HR | UK | 234 | 37 | 3b |
| Deurloo et al. [22] | 2013 | 9–14 | ADAM-12, PP-13 | PE, GH, SGA | Nested case control (in cohort of trisomy screening 12 | LR + HR | The Netherlands | 220 | 17 | 3b |
| Dugoff et al. [23] | 2004 | 10–14 | PAPP-A | PE, PTB, SGA | Prospective study (FASTER trial, trisomy screening cohort) 13 | LR | USA | 34,271 | 764 | 1b |
| Giguere et al. [24] | 2014 | 10–18 | PlGF, sFlt, PAPP-A, inhibin-A | PE | Nested case-control (in prospective cohort) 14 | LR | Canada | 648 | 216 | 3b |
| Goetzinger et al. [25] | 2013 | 11–14 | ADAM-12, PAPP-A | PE, EOPE, LOPE | Prospective cohort 15 | LR + HR | USA | 578 | 54 | 1b |
| Gonen et al. [26] | 2008 | 6–10 | PP-13 | PE, GH | Prospective cohort 16 | LR + HR | Israel | 1239 | 20 | 1b |
| Ghosh et al. [27] | 2013 | 11–14 | PlGF | EOPE | Prospective study (screening antenatal care) 17 | LR + HR | India | 1206 | 9 | 1b |
| Hedley et al. [28] | 2010 | 10–14 | PAPP-A, free leptin index | PE | Nested case control (in First Trimester Screening Study) 18 | LR | Denmark | 415 | 126 | 3b |
| Kang et al. [29] | 2008 | 11 and 16 | PAPP-A, AFP, uE3, hCG, inhibin-A | PE | Retrospective cohort (trisomy 21 screening cohort) 19 | LR + HR | Korea | 3076 | 32 | 2b |
| Kenny et al. [30] | 2014 | 14–16 | Multiple | PE, EOPE, preterm and term PE | Prospective cohort 20 | LR | Australia/UK/Ireland | 5623 | 278 | 1b |
| Khalil et al. [31] | 2010 | 11–14 | PP-13 | PE, EOPE, PE + SGA | Nested case-control (in antenatal clinic cohort) 21 | HR | UK | 252 | 42 | 3b |
| Kuc et al. [32] | 2013 | 9–14 | PAPP-A, free -hCG, ADAM-12, PlGF | EOPE, LOPE | Nested case control (in screening cohort) 22 | LR + HR | The Netherlands | 667 | 167 | 3b |
| Kusanovic et al. [33] | 2009 | 6–15 | PlGF, soluble endoglin, sVEGFR-1 | EOPE, LOPE | Prospective cohort 23 | LR | Chile | 1622 | 62 | 3b |
| Myatt et al. [34] | 2012 | 9–13 | ADAM-12, PAPP-A, PP-13, sFLT, endoglin | PE | Nested case control (in cohort of RCT) 24 | LR | USA | 683 | 174 | 2b |
| Myers et al. [35] | 2013 | 14–16 | PlGF, soluble endoglin, sFLT-1 | preterm PE (<37 week) | Prospective cohort 25 | LR | Australia/UK/Ireland | 235 | 47 | 1b |
| Nicolaides et al. [36] | 2006 | 11–14 | PP-13 | EOPE | Nested case control (in screening cohort) 26 | LR + HR | UK | 433 | 10 | 3b |
| Odibo et al. [37] | 2011 | 11–14 | PP13, PAPP-A | PE, EOPE | Prospective cohort (trisomy 21 screening cohort) 27 | LR + HR | USA | 452 | 42 | 1b |
| Park et al. [38] | 2014 | 11–14 | PAPP-A, PlGF, inhibin-A, sFLT | LOPE | Prospective cohort 28 | LR | Korea | 262 | 8 | 1b |
| Poon et al. (1) [39] | 2009 | 11–14 | PAPP-A | PE, EOPE, LOPE | Prospective cohort (trisomy 21 screening cohort) 29 | LR + HR | UK | 8051 | 156 | 1b |
| Poon et al. (2) [40] | 2009 | 11–14 | PAPP-A, MMP-9, TNF-R1 | EOPE, LOPE, GH, SGA, PTB | Nested case-control (in trisomy 21 screening cohort) 30 | LR + HR | UK | 1138 | 128 | 3b |
| Roes et al. [41] | 2004 | 6–15 | Inhibin-A | PE | Case control 31 | LR | The Netherlands | 55 | 19 | 3b |
| Schneuer et al. [42] | 2012 | 11–13 | PP-13 | PE, EOPE, LOPE, SGA | Prospective cohort (trisomy 21 screening cohort) 32 | LR + HR | Australia | 2678 | 71 | 1b |
| Spencer et al. [43] | 2006 | 11–14 | PP-13, PAPP-A | PE, EOPE, LOPE | Nested case-control (in trisomy 21 screening cohort) 33 | LR | UK | 534 | 88 | 3b |
| Spencer et al. [44] | 2008 | 11–14 | Inhibin-A and activin-A | PE, EOPE, LOPE | Nested case-control (in trisomy 21 screening cohort) 34 | LR | UK | 304 | 64 | 3b |
| Tidwell et al. [45] | 2001 | 5–15 | PlGF | EOPE, LOPE | Case control 35 | LR | Taiwan | 39 | 14 | 3b |
| Thilaganathan et al. [46] | 2010 | 14.7 (CO), 16.3 (PE) | cystatin-C, CRP | PE | Nested case-control (in antenatal clinic cohort) 36 | LR | UK | 170 | 45 | 3b |
| Xu et al. [47] | 2014 | First trimester | Chemerin | PE | Prospective cohort (antenatal care)37 | LR | China | 518 | 41 | 1b |
| Youssef et al. [48] | 2011 | 11–14 | PAPP-A, PlGF, sFlt-1, P-selectin, NGAL | LOPE | Prospective cohort 38 | LR + HR | Italy | 528 | 13 | 1b |
| Yu et al. [49] | 2011 | 12–16 | PlGF, inhibin-A, activin-A | PE | Nested case-control (in antenatal clinic cohort) 39 | LR | China | 124 | 31 | 3b |
| Zong et al. [50] | 2012 | 13–16 | Htr-A1 (High-Temperature Requirement A1) | PE | Prospective cohort (clinical cohort) 40 | LR | China | 1396 | 100 | 1b |
A Characteristics of the study population are mentioned below; 1 Exclusion criteria diabetes, prepregnancy hypertension and premature delivery; 2 Controls: did not develop any pregnancy complications and resulted in the live birth of phenotypically normal neonates; 3 Exclusion: multiparous, multiple gestation, major fetal chromosomal/structural anomaly; 4 Exclusion: pregnancy induced hypertension, fetal growth restriction, intrauterine death, preterm birth (PTB); 5 Controls: normal obstetric outcome. Matched for body mass index (BMI); 6 Exclusion: vitamin C and/or vitamin E supplements, history of major medical complications, major fetal defects, repeated spontaneous abortion, use of an illicit drug or warfarin treatment during the current pregnancy; 7 Inclusion: nulliparous women with singleton pregnancies without major fetal chromosomal or structural anomaly; 8 Exclusion: Women who had a previous fetus with a chromosomal abnormality and women with insulin-dependent diabetes mellitus; 9 Exclusion: AIDS or hepatitis, cases of major fetal anomaly, fetal death and women with placenta previa, placenta accrete, or placental abruption; 10 General population, singleton pregnancies; 11 A priori high risk pregnancies; 12 General population; 13 Inclusion: singleton pregnancy, exclusion: diabetes and chromosomal abnormalities; 14 Exclusion: chronic hepatic or renal diseases, pregnancies with major fetal abnormalities and those ending in termination, miscarriage or fetal death <24 weeks; 15 Exclusion : known aneuploidy and major congenital malformations; 16 Exclusion: miscarriages; 17 Exclusion: congenital abnormalities or medication use; 18 Randomly selected controls; 19 Exclusion: multifetal gestation, diabetes, chromosomal or structural abnormalities; 20 Exclusion: increased risk factors of PE, SGA or PTB, known major fetal anomaly or abnormal karyotype, intervention that may modify pregnancy outcome such as treatment with aspirin or progesterone; 21 Inclusion: history of PE in a previous pregnancy, chronic hypertension, chronic renal disease, antiphospholipid syndrome, systemic lupus erythematosus, pregestational diabetes mellitus, obesity (BMI ≥ 30 kg/m2). Exclusion: multiple pregnancy, cases of major fetal anomaly, miscarriage or fetal death, HIV or hepatitis, placenta previa or placental abruption; 22 Exclusion: multiple pregnancy, delivery <24 weeks, chromosomal abnormalities; 23 Inclusion: pregnancies in which a single live fetus was delivered after 37 complete weeks of gestation with birth weight above the 10th centile and without fetal anomalies; 24 Inclusion: nulliparous, low risk women; 25 Exclusion: increased risk of PE, SGA or PTB, known major fetal anomaly or abnormal karyotype, intervention that may modify pregnancy outcome such as treatment with aspirin or progesterone; 26 Gestational age matched controls; 27 Inclusion : singleton pregnancies. Exclusion: spontaneous miscarriage prior to 20 weeks, loss to follow-up or fetal anomalies diagnosed in the second trimester; 28 Exclusion: high risk pregnancies; 29 Definition controls: randomly selected women without reported pregnancy-associated hypertension; 30 Definition controls: had blood collected and stored on the same day, which did not develop any pregnancy complications and resulted in the live birth of phenotypically normal neonates; 31 Unknown in- and exclusion criteria; 32 Inclusion: singleton pregnancies; 33,34 Gestational age matched controls; 35 Exclusion: multiparity, chronic hypertension, diabetes, multiple gestation, connective tissue disorder, any long-term use of medicine other than prenatal vitamins, and miscarriage before viability; 36 Exclusion: diabetes, connective tissue disease, renal disorders, essential hypertension; 37 Exclusion: previous systemic disorders or drug use, chronic hypertension, diabetes, renal disorders, recent or present fever or infectious disease, malignancies, autoimmune diseases and multiple pregnancies; 38 Exclusion: early-onset PE, multiple gestations, pregnancies with fetal chromosomal or major structural anomaly, miscarriages; 39 Unknown inclusion/exclusion criteria; 40 Exclusion: cases showing intrahepatic cholestasis of pregnancy, abortion, peripartum cardiomyopathy, and other complications. PlGF: Placental growth factor; PAPP-A: Pregnancy associated plasma protein A; ADAM-12: A disintegrin and metalloprotease 12; CRP: C-reactive protein; hCG: human chorionic gonadotropin; PP-13: Placental protein 13; VEGF: Vascular endothelial growth factor; sFLT: Soluble fms-like tyrosine kinase-1; sEng: Soluble endoglin; sVEGR-1: Soluble endothelial growth factor-1; uE3: Oestradiol; MMP-9: Matrix metallopeptidase 9; TNF-R1: Tumor-necrosis factor receptor-1; NGAL: Neutrophil gelatinase-associated lipocalin; (1): publication 1 by same author in same year; (2): publication 2 by same author in same year.
2.1. PE
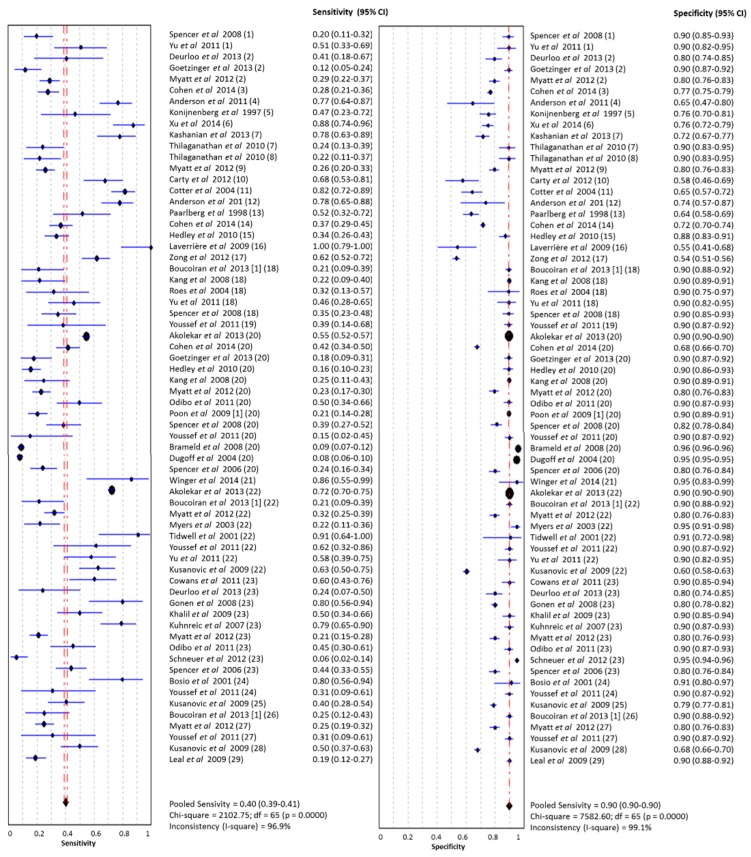
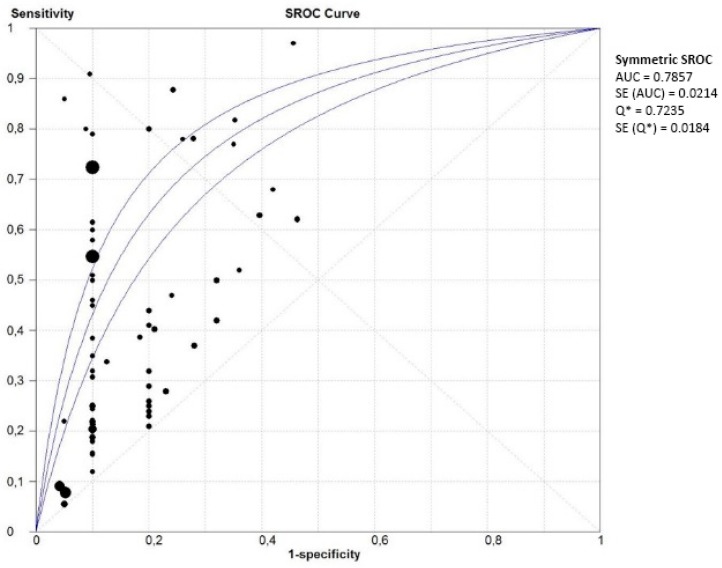
In studies which analysed women with PE without sub-classifying into EOPE and LOPE, the pooled sensitivity of all single biomarkers (n = 66) was 0.40 (95% CI 0.39–0.41, I2 96.9%) at a false positive rate of 10% (Figure 4). The area under the SROC was 0.786 (SE 0.02) (Figure 5). The pooled sensitivity, specificity and area under the SROC of the separate meta-analyses of ADAM-12, inhibin-A, PAPP-A, PlGF and PP-13 are shown in Table 2. All these meta-analyses showed a high heterogeneity (I2 > 50%).
Figure 4.
Meta-analysis of single laboratory biomarkers in PE (both EOPE and LOPE). Legend: (1) activin-A; (2) ADAM-12; (3) α fetoprotein; (4) α-1-macroglobulin; (5) anti-CD63 (GP53, lysosomal secretion); (6) chemerin; (7) C-reactive protein; (8) cystatin C; (9) endoglin; (10) E-selectin; (11) fetal DNA; (12) fetal hemoglobin (ratio); (13) fibronectin; (14) free β-hCG; (15) free leptin index; (16) GRP78 (glucose regulated protein) ratio C-term/full length; (17) Htr-A1 (High-Temperature Requirement A1); (18) inhibin-A; (19) NGAL (neutrophil gelatinase-associated lipocalin); (20) PAPP-A; (21) PBMC (peripheral blood mononuclear cell) miRNA; (22) PlGF; (23) PP-13; (24) P-selectin; (25) soluble endoglin; (26) sFLT/PlGF ratio; (27) sFlt-1; (28) sVEGFR-1 (vascular endothelial growth factor); (29) TNF-R1 (tumor necrosis factor receptor). PE: Pre-eclampsia; EOPE: early-onset PE; LOPE: late-onset PE.
Figure 5.
Summary of receiver operating characteristics curve of single laboratory biomarkers in PE (both EOPE and LOPE).
Table 2.
Meta-analyses of single laboratory biomarkers.
| All PE | Pooled Sensitivity (95% CI) | Pooled Specificity (95% CI) | Area Under SROC (SE) | EOPE | Pooled Sensitivity (95% CI) | Pooled Specificity (95% CI) | Area Under SROC (SE) | LOPE | Pooled Sensitivity (95% CI) | Pooled Specificity (95% CI) | Area Under SROC (SE) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ADAM-12 (n = 3) | 0.26 (021–0.32) | 0.84 (0.82–0.86) | 0.671 (0.093) | ADAM-12 (n = 3) | - | - | - | - | - | - | - |
| Inhibin-A (n = 5) | 0.32 (0.25–0.39) | 0.90 (0.89–0.91) | 0.957 (0.046) | Inhibin-A (n = 5) | - | - | - | - | - | - | - |
| PAPP-A (n = 14) | 0.30 (0.29–0.32) | 0.92 (0.92–0.92) | 0.744 (0.071) | PAPP-A (n = 4) | 0.26 (0.19–0.34) | 0.90 (0.89–0.90) | 0.907 (0.150) | PAPP-A (n = 4) | 0.19 (0.14–0.24) | 0.89 (0.89–0.90) | 0.781 (0.173) |
| PlGF (n = 8) | 0.65 (0.63–0.67) | 0.89 (0.89–0.89) | 0.849 (0.068) | PlGF (n = 3) | 0.37 (0.27–0.48) | 0.79 (0.78–0.81) | 0.796 (0.179) | - | - | - | - |
| PP-13 (n = 9) | 0.37 (0.33–0.41) | 0.88 (0.87-0.89) | 0.882 (0.0450) | PP-13 (n = 9) | 0.59 (0.48–0.69) | 0.92 (0.91–0.93) | 0.898 (0.064) | - | - | - | - |
2.2. Early-Onset PE
In the group of studies which categorized EOPE separately (n = 17), the pooled sensitivity of all single biomarkers was 0.37 (95% CI 0.32–0.41, I2 82.4%) with a specificity of 0.88 (95% CI 0.87–088, I2 98.8%). The area under the SROC was 0.794 (SE 0.05). The pooled sensitivity, specificity and area under the SROC of the separate meta-analyses of PAPP-A, PlGF and PP-13 are shown in Table 2.
2.3. Late-Onset PE
In late-onset PE, (n = 14), the pooled sensitivity of all single biomarkers was 0.22 (95% CI 0.19–0.25, I2 82.2%) with a specificity of 0.89 (95% CI 0.88–089, I2 97.4%). The area under the SROC was 0.763 (SE 0.106). The pooled sensitivity, specificity and area under the SROC of the separate meta-analyses of PAPP-A is shown in Table 2.
2.4. Combination of Biomarkers
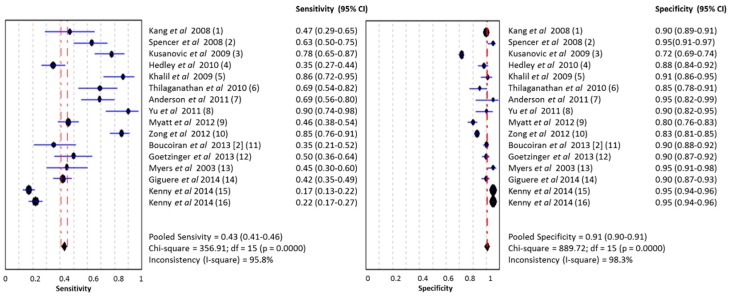
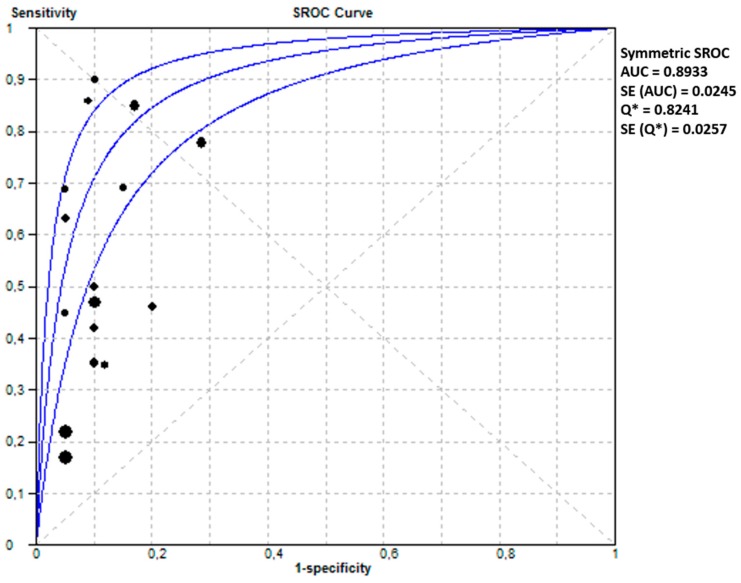
From 13 studies, we extracted a pooled sensitivity of 0.43 (95% CI 0.41–0.46, I2 95.8) and a pooled sensitivity of 0.91 (95% CI 0.90–0.91, I2 98.3) and an area under the SROC of 0.893 (SE 0.03) for a combination model of clinical characteristics, laboratory biomarkers and/or uterine artery Doppler pulsatility index (UA-PI). The forest plot and SROC of these studies are shown in Figure 6 and Figure 7, respectively.
Figure 6.
Meta-analysis of combination of laboratory and clinical makers in PE (both EOPE and LOPE). Legend: (1) PAPP-A, AFP, uE3, hCG (total or free β), inhibin-A; (2) mean PI + activin-A; (3) PlGF/sEng-ratio; (4) PAPP-A and free leptin index; (5) PP-13, UA-PI, AIx-75 (measure of arterial stiffness); (6) cystatin-C, CRP, uterine artery resistance index; (7) HbF ratio and A1M; (8) activin-A, inhibin-A, PlGF and UA-PI; (9) African American race, systolic blood pressure, BMI, education level, ADAM12, PAPP-A, PlGF; (10) BMI, education mother and HtrA1; (11) maternal characteristics, PlGF; (12) maternal characteristics, ADAM12; (13) maternal characteristics, PlGF; (14) sFLT-1, PlGF, PAPP-A, inhibin A, BMI, MAP; (15) PlGF, MAP, BMI, high fruit intake, uterine artery Doppler resistive index (UA-RI) * validation cohort; (16) PlGF, MAP, BMI, high fruit intake, UA-RI * training cohort.
Figure 7.
Summary of receiver operating characteristics curve of combination model of laboratory and makers in PE (both EOPE and LOPE).
3. Discussion
There is extensive literature on biomarkers in relation to PE and despite our focused strategy, we identified 401 biomarkers from the included publications. We then conducted a systematic review and meta-analyses using studies where we were able to extract comparable data for AUC and with more than two studies for each biomarker.
We examined single biomarkers in research conducted with different study cohorts, i.e., EOPE, LOPE or PE in general. Five biomarkers were highlighted: ADAM-12, inhibin-A, PAPP-A, PlGF and PP-13. ADAM12 is part of the ADAM protein family, which are involved in cell-to-cell and cell-to-matrix interactions in neural and muscle development as well as fertilization [51,52,53]. PAPP-A is part of the first trimester Down’s syndrome screening test and is a large zinc glycoprotein produced by placental trophoblasts [54]. PlGF and sFLT are both angiogenic factors. PlGF is a polypeptide growth factor mainly expressed in placental trophoblasts and regulate the early development of placental villi [55] while sFLT induces endothelial cell dysfunction [56].
Prediction models utilizing a combination of biomarkers and clinical parameters improved the predictive value in studies examining PE (without distinction of EOPE and LOPE) with an area under the SROC of 0.893. However, the majority of combined models include evaluation of clinical history or assessment of uterine artery Doppler waveforms. This limits the potential of solely using laboratory-based biomarkers.
A limitation of this study is that our search strategy lead to significant number of missed articles that were found subsequently by other means, such as through the reference lists of articles that have been already identified. This may be due to our limited search terms and the wide variation in terminology used for studies on PE. Previous meta-analyses on early pregnancy biomarkers for PE have concentrated on either biochemical markers alone [42] or in combination with ultrasound indices [57,58,59].
Due to the low population prevalence of PE, despite >200 studies on candidate biomarkers in the literature, none (nor any combination) have been identified with specificity and sensitivity that are useful for clinical practice [60]. The systematic review from the World Health Organization (WHO) concluded that there is no cost effective or reliable screening test (clinical, biophysical, or biochemical) for PE [61]. Perhaps this finding reflects that different types of biomarkers could point to different preventative strategies. For example, pregnancies associated with raised ADAM levels may need to be treated with aspirin, while those linked to raised PlGF levels may need to be treated with calcium.
The low predictive values using a single biomarker may be due to the heterogeneity between most studies such that we were unable to extract comparable data. Despite using the International Society for the study of Hypertension (ISSHP) definition in our review, there is a wide variation in the clinical manifestations and categorization of PE, such as early or late gestation, maternal or placental disease and mild or severe degree of PE. These could have introduced additional variability between the studies. Furthermore, many studies were conducted using different biomarkers, study population and definition of PE phenotype, i.e., EOPE, LOPE or PE as one entity. We identified 147 articles but only 36 of these could be included in the meta-analyses. For each biomarker analyzed, the number of studies was even lower.
Many publications used PE, without sub-classification into EOPE and LOPE. This resulted in a poorly defined phenotype of PE which may further contribute to the low predictive value in these studies. As EOPE and LOPE have distinct and different pathogenesis mechanisms, it is likely that they are characterized by different biomarkers. Therefore, it is important to stratify study populations appropriately for accurate identification of biomarkers.
A possible source of bias arises from the over-representation of case-control studies in the reviewed literature. Furthermore, some studies were only conducted in women at high-risk of developing PE. Biomarkers which only have a high predictive value in EOPE may be another cause of overestimation. On the other hand, it is difficult to conduct studies focusing on LOPE as the phenotype is generally less severe than EOPE.
A well-designed study for biomarkers to predict PE in early pregnancy should be conducted in clearly defined populations, such as those with EOPE. The classic WHO screening criteria by Wilson and Jungner [62] can be adapted for biomarker studies [63,64]. These include: clearly defined clinical population and setting for use, set inclusion and/or exclusion criteria, focused outcome of interest, prospective specimen collection, aim for positive biomarker results in case and negative biomarker results in control, random selection of case and control subjects, accurate definition of true positive and true negative rates, clinically acceptable minimal test performance, favourable comparison with current risk stratification strategy, defined procedures for sample collection, processing, storage and retrieval, blind sampling, consideration of null hypothesis and alternative hypotheses, adequate sample size and that there is a policy present for early termination of the study if appropriate.Identification of women at risk of PE pre-eclampsia is the first step to effective intervention and prevention. However, currently there are no reliable biomarker tests for PE that have been accepted for wide clinical use and some countries have banned the use of biomarker screening in early gestation due to the possibility of inaccurate predictive test and its ethical implications. It is vital to develop a screening tool which is clinically relevant due to the serious consequences of incorrect risk stratification and inappropriate medication or pregnancy surveillance.
4. Methods
4.1. Studies
We searched MEDLINE, EMBASE, Cochrane and ISI web of science from inception to 31st January 2015 using a combination of search terms with synonyms related to pre-eclampsia (“preeclampsia”, “pre-eclampsia”, “EPH”, “pregnancy toxemia”, “edema-proteinura-hypertension gestos”, “PE”), biomarkers (“biological markers”, “biomarker”, “biological markers”, “laboratory markers”, “proteomics”, “metabolomics”, “surrogate endpoint”, “surrogate end point”), and first trimester pregnancy (“early pregnancy”, “first trimester pregnancy”, “pregnancy trimester, first”, “first pregnancy trimester”, “first trimester”, “early placental phase”). There were no limitations made on publication date or patient sample size. We excluded publications which were not in English. Animal studies were not included.
4.2. Study Selection
Two independent reviewers (Pensée Wu and Caroline van den Berg) screened the title, abstract and key words of each article and made a record of the study design, biomarker type, and test period during pregnancy and study outcome. We included observational studies (cohort, cross-sectional and case-control) which assessed tests performed in the first or early second trimester of pregnancy for predicting pre-eclampsia in unselected women. The outcome definitions were as described in the definition of PE from International Society for the study of Hypertension in Pregnancy (ISSHP).[9] Comments, editorials, case series (as defined by the authors of the studies) or reports were excluded, as were biomarker tests performed after 20 weeks of gestation. Genetic markers were not included as they require a different methodological approach and meta-analytic techniques. Reviews were included in the original search, to check for additional references.
4.3. Quality Assessment
An adapted version of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool was used to determine the methodological quality of the selected studies as not all items of the tools were relevant to our review [65,66]. Summary scores were not calculated as their interpretation is difficult and may be potentially misleading [67].
4.4. Data Extraction and Synthesis
We extracted the study outcome measures which were shown in the articles (odds ratio, risk ratio, area under the curve (AUC), sensitivity and specificity). In multiple or duplicate publication of the same data set, we used the most complete or the most recent study. To perform the meta-analyses we only used studies where a sensitivity and specificity was reported. We performed these meta-analyses separately for the three different outcomes: PE, early-onset PE (EOPE, before 34 weeks of gestation) and late-onset PE (LOPE, after 34 weeks of gestation). Studies that used biomarkers in combination with clinical parameters were analyzed separately.
Meta-DiSc (version 1.4; Zamora et al., Madrid, Spain) [68] was used for statistical analyses. A pooled sensitivity and specificity was calculated, as well as a Summary of Receiver Operating Characteristics Curve (SROC). Raw data were used from each study, as adjustments for confounding effects varied between different studies. The inverse variance of the study was used to determine the weighting of studies in the meta-analyses. The random effects model was chosen due to the expected clinical and statistical heterogeneity among the studies. We assessed the heterogeneity of the results among studies through visual examination of Forest plots of AUC’s, and using the I2 test [69]. For all effect estimates, a value of p < 0.05 was considered to be statistically significant.
5. Conclusions
We found that PlGF was best at predicting EOPE as a single biomarker. However, a combination model performed better than a single biomarker if studying PE as a single entity. A combination model including clinical and uterine artery Doppler assessments, negates the attraction of using a laboratory-based biomarker(s) prediction strategy.
Despite multiple potential biomarkers for PE, the efficacy of these markers has been inconsistent between different studies. The IMPROvED (IMproved PRegnancy Outcomes by Early Detection) study is an international multicentre study screening 5000 women in five European countries with the aim of developing a clinically robust predictive blood test for PE, utilising novel metabolite and protein biomarkers [60]. We hope our study will contribute towards the ultimate goal of identifying the best predictive marker(s) and improve the management of women destined to develop PE.
Acknowledgments
The IMPROvED consortium has arisen from the IMPROvED project which has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement N° 310806.
Author Contributions
Pensée Wu and Caroline van den Berg performed the systematic review, analysed data and wrote the manuscript; Zarko Alfirevic, Shaughn O’Brien and Maria Röthlisberger interpreted the data; Philip Newton Baker, Louise C. Kenny and Karolina Kublickiene developed the study concept and design; Johannes J. Duvekot supervised the project, interpreted the data and wrote the manuscript. All authors made critical revisions of the manuscript for important intellectual content and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Steegers E.A., von Dadelszen P., Duvekot J.J., Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 2.Ghulmiyyah L., Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin. Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Duley L. Pre-eclampsia and the hypertensive disorders of pregnancy. Br. Med. Bull. 2003;67:161–176. doi: 10.1093/bmb/ldg005. [DOI] [PubMed] [Google Scholar]
- 4.Roberts J.M., Bell M.J. If we know so much about preeclampsia, why haven’t we cured the disease? J. Reprod. Immunol. 2013;99:1–9. doi: 10.1016/j.jri.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.North R.A., McCowan L.M., Dekker G.A., Poston L., Chan E.H., Stewart A.W., Black M.A., Taylor R.S., Walker J.J., Baker P.N., et al. Clinical risk prediction for pre-eclampsia in nulliparous women: Development of model in international prospective cohort. BMJ. 2011;342 doi: 10.1136/bmj.d1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shennan A.H., Redman C., Cooper C., Milne F. Are most maternal deaths from pre-eclampsia avoidable? Lancet. 2012;379:1686–1687. doi: 10.1016/S0140-6736(11)60785-X. [DOI] [PubMed] [Google Scholar]
- 7.Grill S., Rusterholz C., Zanetti-Dallenbach R., Tercanli S., Holzgreve W., Hahn S., Lapaire O. Potential markers of preeclampsia—A review. Reprod. Biol. Endocrinol. 2009;7 doi: 10.1186/1477-7827-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong Y., Tuuli M., Odibo A.O. First-trimester assessment of placenta function and the prediction of preeclampsia and intrauterine growth restriction. Prenat. Diagn. 2010;30:293–308. doi: 10.1002/pd.2475. [DOI] [PubMed] [Google Scholar]
- 9.Tranquilli A.L., Dekker G., Magee L., Roberts J., Sibai B.M., Steyn W., Zeeman G.G., Brown M.A. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the isshp. Pregnancy Hypertens. 2014;4:97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Anderson U.D., Olsson M.G., Rutardottir S., Centlow M., Kristensen K.H., Isberg P.E., Thilaganathan B., Akerstrom B., Hansson S.R. Fetal hemoglobin and α1-microglobulin as first- and early second-trimester predictive biomarkers for preeclampsia. Am. J. Obstet. Gynecol. 2011;204 doi: 10.1016/j.ajog.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 11.Akolekar R., Zaragoza E., Poon L.C., Pepes S., Nicolaides K.H. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 2008;32:732–739. doi: 10.1002/uog.6244. [DOI] [PubMed] [Google Scholar]
- 12.Akolekar R., Syngelaki A., Poon L., Wright D., Nicolaides K.H. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn. Ther. 2013;33:8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]
- 13.Audibert F., Boucoiran I., An N., Aleksandrov N., Delvin E., Bujold E., Rey E. Screening for preeclampsia using first-trimester serum markers and uterine artery doppler in nulliparous women. Am. J. Obstet. Gynecol. 2010;203 doi: 10.1016/j.ajog.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Bills V.L., Varet J., Millar A., Harper S.J., Soothill P.W., Bates D.O. Failure to up-regulate VEGF165b in maternal plasma is a first trimester predictive marker for pre-eclampsia. Clin. Sci. 2009;116:265–272. doi: 10.1042/CS20080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosio P.M., Cannon S., McKenna P.J., O’Herlihy C., Conroy R., Brady H. Plasma P-selectin is elevated in the first trimester in women who subsequently develop pre-eclampsia. BJOG. 2001;108:709–715. doi: 10.1016/S0306-5456(00)00170-4. [DOI] [PubMed] [Google Scholar]
- 16.Boucoiran I., Thissier-Levy S., Wu Y., Wei S.Q., Luo Z.C., Delvin E., Fraser W.D., Audibert F., Miros Study Group Risks for preeclampsia and small for gestational age: Predictive values of placental growth factor, soluble fms-like tyrosine kinase-1, and inhibin a in singleton and multiple-gestation pregnancies. Am. J. Perinatol. 2013;30:607–612. doi: 10.1055/s-0032-1329691. [DOI] [PubMed] [Google Scholar]
- 17.Boucoiran I., Suarthana E., Rey E., Delvin E., Fraser W.B., Audibert F. Repeated measures of placental growth factor, placental protein 13, and a disintegrin and metalloprotease 12 at first and second trimesters for preeclampsia screening. Am. J. Perinatol. 2013;30:681–688. doi: 10.1055/s-0032-1331025. [DOI] [PubMed] [Google Scholar]
- 18.Brameld K.J., Dickinson J.E., O’Leary P., Bower C., Goldblatt J., Hewitt B., Murch A., Stock R. First trimester predictors of adverse pregnancy outcomes. Aust. N. Z. J. Obstet. Gynaecol. 2008;48:529–535. doi: 10.1111/j.1479-828X.2008.00912.x. [DOI] [PubMed] [Google Scholar]
- 19.Chafetz I., Kuhnreich I., Sammar M., Tal Y., Gibor Y., Meiri H., Cuckle H., Wolf M. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am. J. Obstet. Gynecol. 2007;197 doi: 10.1016/j.ajog.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J.L., Smilen K.E., Bianco A.T., Moshier E.L., Ferrara L.A., Stone J.L. Predictive value of combined serum biomarkers for adverse pregnancy outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;181:89–94. doi: 10.1016/j.ejogrb.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Cowans N.J., Stamatopoulou A., Khalil A., Spencer K. PP13 as a marker of pre-eclampsia: A two platform comparison study. Placenta. 2011;32:S37–S41. doi: 10.1016/j.placenta.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Deurloo K.L., Linskens I.H., Heymans M.W., Heijboer A.C., Blankenstein M.A., van Vugt J.M. ADAM12s and PP13 as first trimester screening markers for adverse pregnancy outcome. Clin. Chem. Lab. Med. 2013;51:1279–1284. doi: 10.1515/cclm-2012-0566. [DOI] [PubMed] [Google Scholar]
- 23.Dugoff L., Hobbins J.C., Malone F.D., Porter T.F., Luthy D., Comstock C.H., Hankins G., Berkowitz R.L., Merkatz I., Craigo S.D., et al. First-trimester maternal serum papp-a and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (the faster trial) Am. J. Obstet. Gynecol. 2004;191:1446–1451. doi: 10.1016/j.ajog.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 24.Giguere Y., Masse J., Theriault S., Bujold E., Lafond J., Rousseau F., Forest J.C. Screening for pre-eclampsia early in pregnancy: Performance of a multivariable model combining clinical characteristics and biochemical markers. BJOG. 2015;122:402–410. doi: 10.1111/1471-0528.13050. [DOI] [PubMed] [Google Scholar]
- 25.Goetzinger K.R., Zhong Y., Cahill A.G., Odibo L., Macones G.A., Odibo A.O. Efficiency of first-trimester uterine artery doppler, A-disintegrin and metalloprotease 12, pregnancy-associated plasma protein A, and maternal characteristics in the prediction of preeclampsia. J. Ultrasound Med. 2013;32:1593–1600. doi: 10.7863/ultra.32.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonen R., Shahar R., Grimpel Y.I., Chefetz I., Sammar M., Meiri H., Gibor Y. Placental protein 13 as an early marker for pre-eclampsia: A prospective longitudinal study. BJOG. 2008;115:1465–1472. doi: 10.1111/j.1471-0528.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh S.K., Raheja S., Tuli A., Raghunandan C., Agarwal S. Is serum placental growth factor more effective as a biomarker in predicting early onset preeclampsia in early second trimester than in first trimester of pregnancy? Arch. Gynecol. Obstet. 2013;287:865–873. doi: 10.1007/s00404-012-2662-2. [DOI] [PubMed] [Google Scholar]
- 28.Hedley P.L., Placing S., Wojdemann K., Carlsen A.L., Shalmi A.C., Sundberg K., Tabor A., Christiansen M. Free leptin index and PAPP-A: A first trimester maternal serum screening test for pre-eclampsia. Prenat. Diagn. 2010;30:103–109. doi: 10.1002/pd.2337. [DOI] [PubMed] [Google Scholar]
- 29.Kang J.H., Farina A., Park J.H., Kim S.H., Kim J.Y., Rizzo N., Elmakky A., Jun H.S., Hahn W.B., Cha D.H. Down syndrome biochemical markers and screening for preeclampsia at first and second trimester: Correlation with the week of onset and the severity. Prenat. Diagn. 2008;28:704–709. doi: 10.1002/pd.1997. [DOI] [PubMed] [Google Scholar]
- 30.Kenny L.C., Black M.A., Poston L., Taylor R., Myers J.E., Baker P.N., McCowan L.M., Simpson N.A., Dekker G.A., Roberts C.T., et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: The screening for pregnancy endpoints (scope) international cohort study. Hypertension. 2014;64:644–652. doi: 10.1161/HYPERTENSIONAHA.114.03578. [DOI] [PubMed] [Google Scholar]
- 31.Khalil A., Cowans N.J., Spencer K., Goichman S., Meiri H., Harrington K. First-trimester markers for the prediction of pre-eclampsia in women with a-priori high risk. Ultrasound Obstet. Gynecol. 2010;35:671–679. doi: 10.1002/uog.7559. [DOI] [PubMed] [Google Scholar]
- 32.Kuc S., Koster M.P., Franx A., Schielen P.C., Visser G.H. Maternal characteristics, mean arterial pressure and serum markers in early prediction of preeclampsia. PLoS ONE. 2013;8:e63546. doi: 10.1371/journal.pone.0063546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusanovic J.P., Romero R., Chaiworapongsa T., Erez O., Mittal P., Vaisbuch E., Mazaki-Tovi S., Gotsch F., Edwin S.S., Gomez R., et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J. Matern. Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myatt L., Clifton R.G., Roberts J.M., Spong C.Y., Hauth J.C., Varner M.W., Jr., Thorp J.M., Mercer B.M., Peaceman A.M., Ramin S.M., et al. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet. Gynecol. 2012;119:1234–1242. doi: 10.1097/AOG.0b013e3182571669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers J.E., Kenny L.C., McCowan L.M., Chan E.H., Dekker G.A., Poston L., Simpson N.A., North R.A., Consortium S. Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: A predictive test accuracy study. BJOG. 2013;120:1215–1223. doi: 10.1111/1471-0528.12195. [DOI] [PubMed] [Google Scholar]
- 36.Nicolaides K.H. Some thoughts on the true value of ultrasound. Ultrasound Obstet. Gynecol. 2007;30:671–674. doi: 10.1002/uog.5156. [DOI] [PubMed] [Google Scholar]
- 37.Odibo A.O., Zhong Y., Goetzinger K.R., Odibo L., Bick J.L., Bower C.R., Nelson D.M. First-trimester placental protein 13, PAPP-A, uterine artery doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta. 2011;32:598–602. doi: 10.1016/j.placenta.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park H.J., Kim S.H., Jung Y.W., Shim S.S., Kim J.Y., Cho Y.K., Farina A., Zanello M., Lee K.J., Cha D.H. Screening models using multiple markers for early detection of late-onset preeclampsia in low-risk pregnancy. BMC Pregnancy Childbirth. 2014;14 doi: 10.1186/1471-2393-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poon L.C., Maiz N., Valencia C., Plasencia W., Nicolaides K.H. First-trimester maternal serum pregnancy-associated plasma protein-a and pre-eclampsia. Ultrasound Obstet. Gynecol. 2009;33:23–33. doi: 10.1002/uog.6280. [DOI] [PubMed] [Google Scholar]
- 40.Poon L.C., Nekrasova E., Anastassopoulos P., Livanos P., Nicolaides K.H. First-trimester maternal serum matrix metalloproteinase-9 (MMP-9) and adverse pregnancy outcome. Prenat. Diagn. 2009;29:553–559. doi: 10.1002/pd.2234. [DOI] [PubMed] [Google Scholar]
- 41.Roes E.M., Gaytant M.A., Thomas C.M., Raijmakers M.T., Zusterzeel P.L., Peters W.H., Steegers E.A. First trimester inhibin-A concentrations and later development of preeclampsia. Acta Obstet. Gynecol. Scand. 2004;83 doi: 10.1111/j.1600-0412.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 42.Schneuer F.J., Nassar N., Khambalia A.Z., Tasevski V., Guilbert C., Ashton A.W., Morris J.M., Roberts C.L. First trimester screening of maternal placental protein 13 for predicting preeclampsia and small for gestational age: In-house study and systematic review. Placenta. 2012;33:735–740. doi: 10.1016/j.placenta.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Spencer K., Cowans N.J., Chefetz I., Tal J., Meiri H. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery doppler pulsatility index as markers of pre-eclampsia. Ultrasound Obstet. Gynecol. 2007;29:128–134. doi: 10.1002/uog.3876. [DOI] [PubMed] [Google Scholar]
- 44.Spencer K., Cowans N.J., Nicolaides K.H. Maternal serum inhibin-A and activin-a levels in the first trimester of pregnancies developing pre-eclampsia. Ultrasound Obstet. Gynecol. 2008;32:622–626. doi: 10.1002/uog.6212. [DOI] [PubMed] [Google Scholar]
- 45.Tidwell S.C., Ho H.N., Chiu W.H., Torry R.J., Torry D.S. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am. J. Obstet. Gynecol. 2001;184:1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 46.Thilaganathan B., Wormald B., Zanardini C., Sheldon J., Ralph E., Papageorghiou A.T. Early-pregnancy multiple serum markers and second-trimester uterine artery doppler in predicting preeclampsia. Obstet. Gynecol. 2010;115:1233–1238. doi: 10.1097/AOG.0b013e3181dd5137. [DOI] [PubMed] [Google Scholar]
- 47.Xu Q.L., Zhu M., Jin Y., Wang N., Xu H.X., Quan L.M., Wang S.S., Li S.S. The predictive value of the first-trimester maternal serum chemerin level for pre-eclampsia. Peptides. 2014;62:150–154. doi: 10.1016/j.peptides.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Youssef A., Righetti F., Morano D., Rizzo N., Farina A. Uterine artery doppler and biochemical markers (PAPP-A, PIGF, sFlt-1, P-selectin, NGAL) at 11 + 0 to 13 + 6 weeks in the prediction of late (>34 weeks) pre-eclampsia. Prenat. Diagn. 2011;31:1141–1146. doi: 10.1002/pd.2848. [DOI] [PubMed] [Google Scholar]
- 49.Yu J., Shixia C.Z., Wu Y., Duan T. Inhibin A, activin A, placental growth factor and uterine artery doppler pulsatility index in the prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 2011;37:528–533. doi: 10.1002/uog.8800. [DOI] [PubMed] [Google Scholar]
- 50.Zong L., Gou W., Shao W., Huang P., Li C. Changes in the level of serum high-temperature requirement A1 (HtrA1) during pregnancy and its relationship to preeclampsia. Hypertens Pregnancy. 2012;31:389–397. doi: 10.3109/10641955.2012.667472. [DOI] [PubMed] [Google Scholar]
- 51.Yang P., Baker K.A., Hagg T. A disintegrin and metalloprotease 21 (ADAM21) is associated with neurogenesis and axonal growth in developing and adult rodent CNS. J. Comp. Neurol. 2005;490:163–179. doi: 10.1002/cne.20659. [DOI] [PubMed] [Google Scholar]
- 52.Cho C., Turner L., Primakoff P., Myles D.G. Genomic organization of the mouse fertilin β gene that encodes an ADAM family protein active in sperm-egg fusion. Dev. Genet. 1997;20:320–328. doi: 10.1002/(SICI)1520-6408(1997)20:4<320::AID-DVG3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 53.White J.M. ADAMS: Modulators of cell–cell and cell–matrix interactions. Curr. Opin. Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Zhabin S.G., Gorin V.S., Judin N.S. Review: Immunomodulatory activity of pregnancy-associated plasma protein-a. J. Clin. Lab. Immunol. 2003;52:41–50. [PubMed] [Google Scholar]
- 55.Ghosh S., Raheja S., Tuli A., Raghunandan C., Agarwal S. Serum PLGF as a potential biomarker for predicting the onset of preeclampsia. Arch. Gynecol. Obstet. 2012;285:417–422. doi: 10.1007/s00404-011-1960-4. [DOI] [PubMed] [Google Scholar]
- 56.De Vivo A., Baviera G., Giordano D., Todarello G., Corrado F., D’Anna R. Endoglin, PLGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet. Gynecol. Scand. 2008;87:837–842. doi: 10.1080/00016340802253759. [DOI] [PubMed] [Google Scholar]
- 57.Zhu X.-L., Wang J., Jiang R.-Z., Teng Y.-C. Pulsatility index in combination with biomarkers or mean arterial pressure for the prediction of pre-eclampsia: Systematic literature review and meta-analysis. Ann. Med. 2015;47:414–422. doi: 10.3109/07853890.2015.1059483. [DOI] [PubMed] [Google Scholar]
- 58.Allen R.E., Rogozinska E., Cleverly K., Aquilina J., Thangaratinam S. Abnormal blood biomarkers in early pregnancy are associated with preeclampsia: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;182:194–201. doi: 10.1016/j.ejogrb.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 59.Kuc S., Wortelboer E.J., van Rijn B.B., Franx A., Visser G.H.A., Schielen P.C.J.I. Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: A systematic review. Obstet. Gynecol. Surv. 2011;66:225–239. doi: 10.1097/OGX.0b013e3182227027. [DOI] [PubMed] [Google Scholar]
- 60.Navaratnam K., Alfirevic Z., Baker P., Gluud C., Gruttner B., Kublickiene K., Zeeman G., Kenny L. A multi-centre phase IIa clinical study of predictive testing for preeclampsia: Improved pregnancy outcomes via early detection (IMPROVED) BMC Pregnancy Childbirth. 2013;13 doi: 10.1186/1471-2393-13-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conde-Agudelo A., Villar J., Lindheimer M. World health organization systematic review of screening tests for preeclampsia. Obstet. Gynecol. 2004;104:1367–1391. doi: 10.1097/01.AOG.0000147599.47713.5d. [DOI] [PubMed] [Google Scholar]
- 62.Wilson J., Jungner G. Principles and Practice of Screening. World Health Organization; Geneva, Switzerland: 1968. [Google Scholar]
- 63.Pepe M.S., Feng Z., Janes H., Bossuyt P.M., Potter J.D. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: Standards for study design. J. Natl. Cancer Inst. 2008;100:1432–1438. doi: 10.1093/jnci/djn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dancey J.E., Dobbin K.K., Groshen S., Jessup J.M., Hruszkewycz A.H., Koehler M., Parchment R., Ratain M.J., Shankar L.K., Stadler W.M., et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin. Cancer Res. 2010;16:1745–1755. doi: 10.1158/1078-0432.CCR-09-2167. [DOI] [PubMed] [Google Scholar]
- 65.Whiting P., Rutjes A., Reitsma J.B., Bossuyt P.M., Kleijnen J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 2003;3 doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., Leeflang M.M., Sterne J.A., Bossuyt P.M., Group Q. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 67.Whiting P., Harbord R., Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Med. Res. Methodol. 2005;5 doi: 10.1186/1471-2288-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zamora J., Abraira V., Muriel A., Khan K., Coomarasamy A. Meta-disc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006;6:31–31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reitsma J.B., Glas A.S., Rutjes A.W., Scholten R.J., Bossuyt P.M., Zwinderman A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Clin. Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]