Abstract
A variety of approaches have been utilized to identify and characterize the molecules that mediate vesicular trafficking along the secretory pathway. Two approaches that have been particularly fruitful include the genetic dissection of the yeast secretory pathway and the biochemical characterization of proteins involved in the synaptic vesicle membrane trafficking in the mammalian nerve terminal. The recent convergence of these approaches suggests that common mechanisms may underlie a wide variety of vesicle-mediated transport steps. We discuss the results that support this possibility and propose a model for synaptic vesicle docking and fusion that incorporates evolutionarily conserved elements that may be part of a constitutive fusion machinery and specialized elements that may mediate regulatory events that are specific to the process of neurotransmitter release.
Full text
PDF
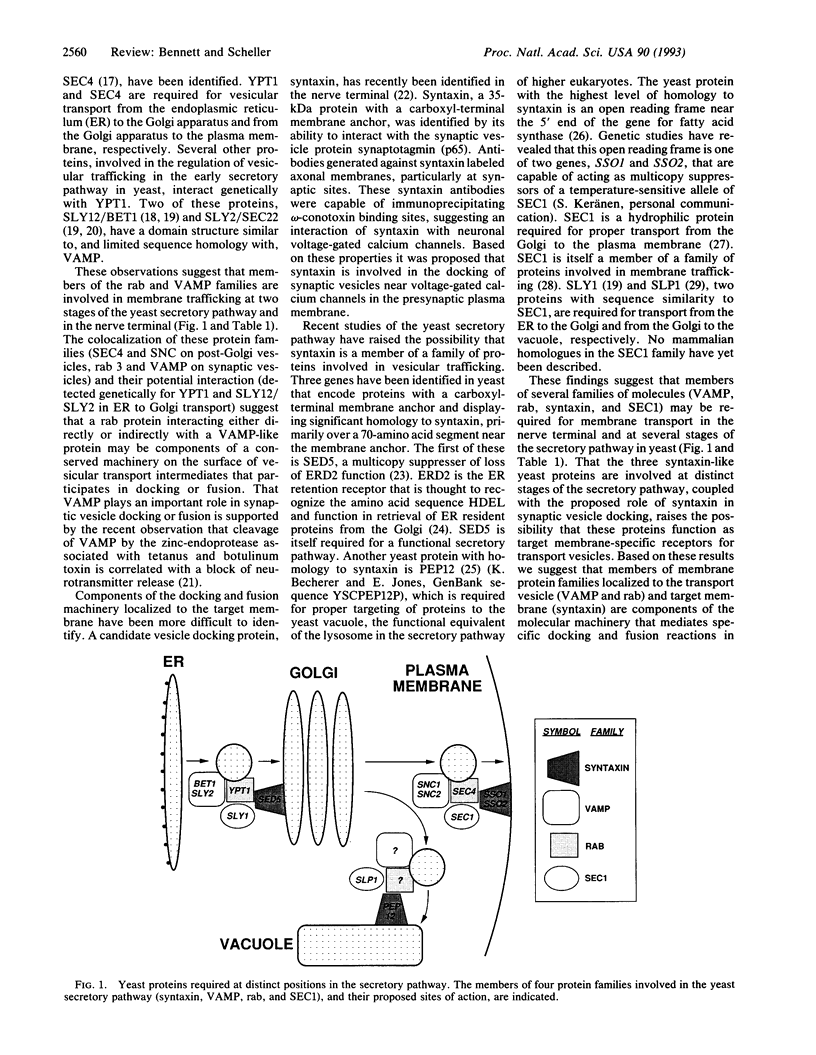


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalto M. K., Keränen S., Ronne H. A family of proteins involved in intracellular transport. Cell. 1992 Jan 24;68(2):181–182. doi: 10.1016/0092-8674(92)90462-l. [DOI] [PubMed] [Google Scholar]
- Aalto M. K., Ruohonen L., Hosono K., Keränen S. Cloning and sequencing of the yeast Saccharomyces cerevisiae SEC1 gene localized on chromosome IV. Yeast. 1991 Aug-Sep;7(6):643–650. doi: 10.1002/yea.320070613. [DOI] [PubMed] [Google Scholar]
- Bailly E., McCaffrey M., Touchot N., Zahraoui A., Goud B., Bornens M. Phosphorylation of two small GTP-binding proteins of the Rab family by p34cdc2. Nature. 1991 Apr 25;350(6320):715–718. doi: 10.1038/350715a0. [DOI] [PubMed] [Google Scholar]
- Bajjalieh S. M., Peterson K., Linial M., Scheller R. H. Brain contains two forms of synaptic vesicle protein 2. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2150–2154. doi: 10.1073/pnas.90.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajjalieh S. M., Peterson K., Shinghal R., Scheller R. H. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 1992 Aug 28;257(5074):1271–1273. doi: 10.1126/science.1519064. [DOI] [PubMed] [Google Scholar]
- Barnekow A., Jahn R., Schartl M. Synaptophysin: a substrate for the protein tyrosine kinase pp60c-src in intact synaptic vesicles. Oncogene. 1990 Jul;5(7):1019–1024. [PubMed] [Google Scholar]
- Baumert M., Maycox P. R., Navone F., De Camilli P., Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989 Feb;8(2):379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Calakos N., Kreiner T., Scheller R. H. Synaptic vesicle membrane proteins interact to form a multimeric complex. J Cell Biol. 1992 Feb;116(3):761–775. doi: 10.1083/jcb.116.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992 Jul 10;257(5067):255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Block M. R., Glick B. S., Wilcox C. A., Wieland F. T., Rothman J. E. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Bucci C., Parton R. G., Mather I. H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992 Sep 4;70(5):715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Buckley K., Kelly R. B. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985 Apr;100(4):1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C. C., Trimble W. S., Lienhard G. E. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992 Jun 15;267(17):11681–11684. [PubMed] [Google Scholar]
- Charriaut-Marlangue C., Otani S., Creuzet C., Ben-Ari Y., Loeb J. Rapid activation of hippocampal casein kinase II during long-term potentiation. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10232–10236. doi: 10.1073/pnas.88.22.10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Griff I. C., Rothman J. E. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990 May 18;61(4):709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- Creutz C. E. The annexins and exocytosis. Science. 1992 Nov 6;258(5084):924–931. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- Dascher C., Ossig R., Gallwitz D., Schmitt H. D. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991 Feb;11(2):872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakle K. A., Bernstein M., Emr S. D. Characterization of a component of the yeast secretion machinery: identification of the SEC18 gene product. Mol Cell Biol. 1988 Oct;8(10):4098–4109. doi: 10.1128/mcb.8.10.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink L. A., Peterson M. R., Scheller R. H. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993 Jan 15;72(1):153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Elferink L. A., Trimble W. S., Scheller R. H. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989 Jul 5;264(19):11061–11064. [PubMed] [Google Scholar]
- Feany M. B., Lee S., Edwards R. H., Buckley K. M. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992 Sep 4;70(5):861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst J. E., Rodgers L., Riggs M., Wigler M. SNC1, a yeast homolog of the synaptic vesicle-associated membrane protein/synaptobrevin gene family: genetic interactions with the RAS and CAP genes. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4338–4342. doi: 10.1073/pnas.89.10.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991 Mar 8;64(5):915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Goud B., McCaffrey M. Small GTP-binding proteins and their role in transport. Curr Opin Cell Biol. 1991 Aug;3(4):626–633. doi: 10.1016/0955-0674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Hardwick K. G., Pelham H. R. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992 Nov;119(3):513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977 Jan;85(1):23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus P., Marquèze-Pouey B., Scherer H., Betz H. Synaptoporin, a novel putative channel protein of synaptic vesicles. Neuron. 1990 Oct;5(4):453–462. doi: 10.1016/0896-6273(90)90084-s. [DOI] [PubMed] [Google Scholar]
- Leube R. E., Kaiser P., Seiter A., Zimbelmann R., Franke W. W., Rehm H., Knaus P., Prior P., Betz H., Reinke H. Synaptophysin: molecular organization and mRNA expression as determined from cloned cDNA. EMBO J. 1987 Nov;6(11):3261–3268. doi: 10.1002/j.1460-2075.1987.tb02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V., Orci L., Glick B. S., Block M. R., Rothman J. E. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988 Jul 15;54(2):221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- Mohamed A. H., Chirala S. S., Mody N. H., Huang W. Y., Wakil S. J. Primary structure of the multifunctional alpha subunit protein of yeast fatty acid synthase derived from FAS2 gene sequence. J Biol Chem. 1988 Sep 5;263(25):12315–12325. [PubMed] [Google Scholar]
- Newman A. P., Graf J., Mancini P., Rossi G., Lian J. P., Ferro-Novick S. SEC22 and SLY2 are identical. Mol Cell Biol. 1992 Aug;12(8):3663–3664. doi: 10.1128/mcb.12.8.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A. P., Shim J., Ferro-Novick S. BET1, BOS1, and SEC22 are members of a group of interacting yeast genes required for transport from the endoplasmic reticulum to the Golgi complex. Mol Cell Biol. 1990 Jul;10(7):3405–3414. doi: 10.1128/mcb.10.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990 May 17;345(6272):260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Petrenko A. G., Perin M. S., Davletov B. A., Ushkaryov Y. A., Geppert M., Südhof T. C. Binding of synaptotagmin to the alpha-latrotoxin receptor implicates both in synaptic vesicle exocytosis. Nature. 1991 Sep 5;353(6339):65–68. doi: 10.1038/353065a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal L., Meldolesi J. Alpha-latrotoxin and related toxins. Pharmacol Ther. 1989;42(1):115–134. doi: 10.1016/0163-7258(89)90024-7. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992 Oct 29;359(6398):832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Semenza J. C., Hardwick K. G., Dean N., Pelham H. R. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 1990 Jun 29;61(7):1349–1357. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Lottspeich F., Greengard P., Mehl E., Jahn R. A synaptic vesicle protein with a novel cytoplasmic domain and four transmembrane regions. Science. 1987 Nov 20;238(4830):1142–1144. doi: 10.1126/science.3120313. [DOI] [PubMed] [Google Scholar]
- Thomas L., Hartung K., Langosch D., Rehm H., Bamberg E., Franke W. W., Betz H. Identification of synaptophysin as a hexameric channel protein of the synaptic vesicle membrane. Science. 1988 Nov 18;242(4881):1050–1053. doi: 10.1126/science.2461586. [DOI] [PubMed] [Google Scholar]
- Trimble W. S., Cowan D. M., Scheller R. H. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Scheller R. H. Molecular biology of synaptic vesicle-associated proteins. Trends Neurosci. 1988 Jun;11(6):241–242. doi: 10.1016/0166-2236(88)90098-7. [DOI] [PubMed] [Google Scholar]
- Ushkaryov Y. A., Petrenko A. G., Geppert M., Südhof T. C. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992 Jul 3;257(5066):50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Wada Y., Kitamoto K., Kanbe T., Tanaka K., Anraku Y. The SLP1 gene of Saccharomyces cerevisiae is essential for vacuolar morphogenesis and function. Mol Cell Biol. 1990 May;10(5):2214–2223. doi: 10.1128/mcb.10.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walent J. H., Porter B. W., Martin T. F. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992 Sep 4;70(5):765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- Wendland B., Miller K. G., Schilling J., Scheller R. H. Differential expression of the p65 gene family. Neuron. 1991 Jun;6(6):993–1007. doi: 10.1016/0896-6273(91)90239-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Wilcox C. A., Flynn G. C., Chen E., Kuang W. J., Henzel W. J., Block M. R., Ullrich A., Rothman J. E. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989 Jun 1;339(6223):355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P., Hull M., Huber L. A., Mâle P., Goud B., Mellman I. Reversible phosphorylation--dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J. 1992 Dec;11(12):4379–4389. doi: 10.1002/j.1460-2075.1992.tb05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]