Abstract
The chronic obstructive pulmonary disease has become a disease of public health importance. Among the various risk factors, smoking remains the main culprit. In addition to airway obstruction, the presence of intrinsic positive end expiratory pressure, respiratory muscle dysfunction contributes to the symptoms of the patient. Perioperative management of these patients includes identification of modifiable risk factors and their optimisation. Use of regional anaesthesia alone or in combination with general anaesthesia improves pulmonary functions and reduces the incidence of post-operative pulmonary complications.
Keywords: Anaesthesia, chronic obstructive pulmonary disease, management, perioperative
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a chronic progressive inflammatory condition resulting in expiratory airflow limitation that is not reversible. The global initiative for COPD guideline (GOLD) defines COPD as follows: ‘COPD is a preventable and treatable disease with some significant extrapulmonary effects that may contribute to the severity in individual patients. The airway limitation (pulmonary component) is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases and is not fully reversible’.[1]
COPD is a disease of increasing public health importance around the world. GOLD estimates suggest that COPD will rise from the sixth to third most common cause of death world wide by 2020.[1]
COPD includes: (i) Emphysema a condition characterised by destruction and enlargement of the lung alveoli; (ii) chronic bronchitis a condition with chronic cough and phlegm; and (iii) small airway disease in which small bronchioles are narrowed. COPD is present only if chronic airflow obstruction occurs. Chronic bronchitis without chronic airflow obstruction is not included with COPD.[2]
Risk factors
Cigarette smoking: 80% of patients with COPD have significant exposure to tobacco smoking[3] and manifest with accelerated decline in the volume of air exhaled in the forced expiratory volume at 1 s (FEV1), in a dose response relationship to the intensity of cigarette smoking. Effects of passive smoking on COPD is unclear[2]
Increased airway responsiveness to various exogenous stimuli
Respiratory infections
Occupational exposures: Exposure to dust at work e.g. coal mining, gold mining and cotton textile dust
Ambient air pollution
Genetic: Severe anti-trypsin (α1 AT) deficiency is a proven genetic risk factor for COPD.[2] A recent study has found a genetic variant (FAM13A), associated with the development of COPD in the COPD gene study.[4]
PATHOPHYSIOLOGY
COPD is a combination of inflammatory small airway disease (obstructive bronchiolitis) and parenchymal destruction (emphysema) and affects central and peripheral airway, lung parenchyma and pulmonary vasculature. This leads to poorly reversible narrowing of the airway, remodelling of airway smooth muscle, increased numbers of goblet cells and mucus-secreting glands and pulmonary vasculature changes resulting in pulmonary hypertension.
Airway obstruction
The major site of obstruction is found in the smaller conducting airway (<2 mm in diameter). Processes contributing to obstruction in the small conducting airway include disruption of the epithelial barrier, interference with mucociliary clearance apparatus that results in accumulation of inflammatory mucous exudates in the small airway lumen, infiltration of the airway walls by inflammatory cells and deposition of connective tissue in the airway wall. Fibrosis surrounding the small airway appears to be a significant contributor. This remodelling and repair thickens the airway walls, reduces lumen calibre and restricts the normal increase in calibre produced by lung inflation.
Increased resistance of the small conducting airway and increased compliance of the lung as a result of emphysematous destruction, causes the prolonged time constant. This constant is reflected in measurements of the FEV1 and its ratio to forced vital capacity (FEV1/FVC), which are reliable screening tools because they are affected by both airway obstruction and emphysema.[5] In contrast to asthma, reduced FEV1 in COPD seldom shows large responses to inhaled bronchodilators, although improvement up to 15% are common. Maximal inspiratory flow can be well preserved in the presence of markedly reduced FEV1.[2]
Dynamic hyperinflation
In COPD there is ‘air trapping’ (increased residual volume and increased ratio of residual volume to total lung capacity) and progressive hyperinflation (increased total lung capacity). During spontaneous breathing, the high expiratory airway resistance, combined with expiratory flow limitation, low elastic recoil, high ventilatory demands and short expiratory time due to the increased respiratory rate, may not permit the respiratory system to reach the elastic equilibrium volume (i.e., passive functional residual capacity [FRC]) at end-expiration. This phenomenon is commonly referred to as dynamic hyperinflation.[6,7] Thus, an elastic threshold load (intrinsic positive end expiratory pressure [PEEPi]) is imposed on the inspiratory muscles at the beginning of inspiration and increases the amount of the inspiratory effort needed for gas flow.[8]
Auto positive end expiratory pressure [Intrinsic positive end expiratory pressure (PEEPi)]
Patients with severe COPD often breathe in a pattern that interrupts expiration before the alveolar pressure has decreased to atmospheric pressure. This incomplete expiration is due to a combination of factors which include flow limitation, increased work of respiration and increased airway resistance. This interruption leads to an increase of the end-expiratory lung volume above the FRC. This PEEP in the alveoli at rest has been termed auto-PEEP or PEEPi. During spontaneous respiration the intrapleural pressure will have to be decreased to a level which counteracts PEEPi before inspiratory flow can begin. Thus, COPD patients can have an increased inspiratory load added to their already increased expiratory load.[9]
Respiratory muscle dysfunction
Hyperinflation can push the diaphragm in to a flattened position with a number of adverse effects:
By decreasing the zone of apposition between the diaphragm and the abdominal wall, positive abdominal pressure during inspiration is not applied as effectively to the chest wall, hindering rib cage movement and impairing inspiration
Because the muscle fibres of the diaphragm are shorter than those of normally curved diaphragm they are less capable of generating inspiratory pressures than normal
The flattened diaphragm must generate greater tension to develop transpulmonary pressure required to produce tidal breathing.[2]
In COPD dynamic hyperinflation, excessive resistive load and high ventilatory demands are factors leading to respiratory muscle dysfunction.
Gas exchange impairment
Non-uniform ventilation and ventilation-perfusion mismatch are characteristics of COPD, due to the heterogenous nature of the disease process within airway and lung parenchyma. Ventilation/perfusion (V/Q) mismatching causes reduction in PaO2: but shunting is minimal. This finding explains the effectiveness of modest elevation of inspired oxygen in treating hypoxaemia due to COPD. PaO2 usually remains near normal until FEV1 is decreased to 50% of predicted and elevation of PaCO2 is not seen until FEV1 is <25%.[2] Hypercapnia, if present, reflects both V/Q mismatching and alveolar hypoventilation, the later resulting from both respiratory muscle dysfunction and increased ventilatory requirements.[10]
Pulmonary hypertension severe enough to cause corpulmonale and right heart failure is seen when there is marked decrease in FEV1 <25% along with chronic hypoxaemia PaO2 < 55 mm Hg.[2]
Extrapulmonary effects
It also has significant extrapulmonary effects, the so-called systemic effects of COPD. Weight loss, nutritional abnormalities and skeletal muscle dysfunction are well-recognised systemic effects of COPD.[11] Cardiovascular dysfunction is usually related to acute and chronic blood gas derangement, dynamic hyperinflation and increased right ventricular afterload.[12] Pulmonary hypertension is increasingly being recognised as a contributing factor to the morbidity, and mortality associated with COPD. Approximately, 10–30% of patients with moderate to severe COPD have elevated pulmonary pressures and severe acidosis occurs in <5% of patients.[13] Left ventricular dysfunction is commonly associated as these patients are frequently old and suffer from several risk factors for coronary artery disease.[14]
PRE-OPERATIVE EVALUATION AND OPTIMISATION
Pre-operative evaluation aims at finding out the degree of impairment, identification of modifiable risk factors and their optimisation which can influence the perioperative outcome. Signs and symptoms of COPD are listed in Table 1.
Table 1.
Signs and symptoms in patients with COPD

Upper respiratory infections should be ruled out and treated as a cause of increased secretions and airway hyper-reactivity. History of smoking and presence of wheeze, prolonged expiration increases the risk of post-operative pulmonary complications (PPCs).[15,16,17] A positive cough test and smoking more than 40 pack years were found to be among the best predictors of PPCs.[18] Age over 60 years, spirometric changes (FEV1 <1 L), duration of anaesthesia (>3 h), surgeries of upper torso, and use of nasogastric tube pre-operatively increase the incidence of respiratory events.[19]
Simple bedside pulmonary function tests using Wrights respirometer, De Bono's Whistle still have some utility in clinical assessment of severity of disease.[20] A study carried out by Viecili et al. found a good correlation between maximum voluntary breath holding time and pulmonary function tests (FEV1 and FVC), with lower breath holding times in patients with COPD compared with that of controls.[21] Electrocardiogram helps to rule out ischaemic heart disease in these patients. Although not regularly recommended in stable COPD patients, (NICE guidelines) chest X-ray is useful to rule out lower respiratory infection and occult malignancy in patients showing recent deterioration in symptoms. The presence of extensive bullous disease on a chest X-ray highlights the risk of pneumothorax.[22] FEV1, FVC and the ratio of the two will help us differentiate between obstructive and restrictive pathology. A reduction in PEFR indicates early airway obstruction and a reduction in FEF 25–75% indicates small airway obstruction.[23] The incidence of PPCs (except atelectasis) most often parallels the severity of respiratory impairment (moderate, if FEV1-50–80%; severe, if FEV1 <50%). In patients undergoing lung resection, a FEV1 below 60% has been shown to carry a two to threefold increased risk for operative mortality and major respiratory complications.[24]
Presence of pulmonary hypertension worsens prognosis. Patients with mean pulmonary artery pressure >50 mm Hg, pulmonary artery systolic pressure >60 mm Hg, Right ventricular systolic pressure >70 mm Hg, presence of mixed pulmonary hypertension (those with pulmonary capillary wedge pressure >15 mm Hg and pulmonary vascular resistance (PVR) >3 Wood units) are at high risk of developing perioperative cardiac and pulmonary complications.[25] Arterial blood gas measurement is useful in patients with marked symptoms, PaCO2>45 mm Hg and PaO2 <60 mm Hg (on room air) are both associated with a worse prognosis. Clinical assessment of functional status with simple and safe tests such as stair climbing and the 6 min walk test correlates well with more formal exercise testing. A more objective way of assessing functional ability of patient is cardiopulmonary exercise testing (CPET). Peak VO2, the ratio of minute ventilation to CO2 production (VE/VCO2) slope, and partial pressure of end tidal carbon di oxide (PETCO2) are primary CPET variables used for assessing the severity and prognosis for both COPD and interstitial lung disease patients. Peak oxygen uptake (VO2) values >15 ml/min/kg predict no increase in operative risk for pneumonectomy or lobectomy compared with the lowest-risk patients, even for patients whose resting pulmonary function or predicted post-operative pulmonary function is poor, whereas peak VO2 values <10 ml/min/kg is associated with poor prognosis.[26,27]
Poor nutritional status with a serum albumin level <3.5 g/dl is a strong predictor of PPCs. A blood urea nitrogen level of <8 or >21 mg/dL was also associated with an increased risk of PPCs.[28]
Pre-operative optimisation of these patients include cessation of smoking, improvement of pulmonary functions using bronchodilators and steroids, pre-operative chest physiotherapy and training of patient with lung expansion manuoevers.[29] Reversible components of COPD such as bronchospasm, infections, and pulmonary oedema should be actively looked for and treated aggressively.
Cessation of smoking is an important measure to reduce perioperative pulmonary complications in COPD patients. Maximum benefit is obtained if smoking is stopped at least 8 weeks before surgery with some studies showing increased risk with cessation <8 weeks before surgery is associated with increased risk of post-operative complications, whereas other study showed decreased incidence of complications with >4 weeks of cessation.[30,31] However, these data are largely from observational studies in cardiac surgery, and studies in other surgical specialities have failed to confirm the findings. Most centres therefore now advocate stopping smoking regardless of the interval before surgery. Nicotine replacement therapy, varenicline or amfebutamone are options for motivated patients, but should only be prescribed alongside behavioural support. Varenicline (Champix) is a partial agonist at the α4 β2 neuronal nicotinic acetylcholine receptor and has been shown to reduce withdrawal and craving by preventing nicotine binding to the receptor.[22]
Anticholinergic bronchodilators such as ipratropium, act by inhibiting cyclic guanosine monophosphate formation and block vagus nerve-mediated bronchoconstriction, which is an important component of bronchospasm in patients with COPD, and hence are more useful in patients with COPD.[32]
Suzuki et al., in their study, observed longer duration of improvement in pulmonary functions with tiotropium bromide compared to ipratropium in patients having moderate to severe lung cancer with COPD.[33] Addition of formoterol and budesonide to tiotropium further improves pulmonary functions in perioperative period.[34] Addition of short acting β2 adrenoreceptor agonists such as salbutamol, terbutaline helps in reversing bronchospasm and may be added to anticholinergics in patients remaining symptomatic even after anticholinergic treatment. Levalbuterol, an enantiomer of albuterol, is thought to produce less tachyarrhythmias based on limited evidence. Routine administration of oral steroids is not necessary in patients with COPD and is indicated only in resistant cases. Only 20–25% of patients with COPD will respond to corticosteroids.[35] Pre-operative use of inhaled corticosteroids have not shown to increase risk of respiratory infections or affect wound healing.[36,37] Methylxanthines inhibit phosphodiesterase enzyme thus preventing degradation of cyclic AMP, which aids bronchodilatation. Oral theophylline is occasionally used in the treatment of severe COPD or in those unable to use inhaled therapy; intravenous aminophylline is used during perioperative management of these patients. It also enhances diaphragmatic contraction. However caution has to be excised during its use to prevent the toxic effects of methylxanthines such as, tachyarrhythmias, seizures and rhabdomyolysis, which may occur because of narrow therapeutic index. In addition, the inhaled anaesthetics, particularly halothane, may sensitize the heart to the toxic effects of theophylline.[38] Doxofylline, a next generation methylxanthine has shown similar or better efficacy than theophylline in relieving spasm both in adult and paediatric age group with lesser cardiovascular, nervous and gastrointestinal side effects due to its reduced affinity towards adenosine A1 and A2 receptors.[39,40] The role of oral or inhaled mucolytic therapy in COPD is controversial, though the recent evidence favours its use in COPD.[41,42] It may be helpful, particularly in those with increased secretions with minimal side effects and its continuation is found to reduce the incidence post-operative complications in these patients.
Patients with COPD have fewer PPCs when a programme of intensive chest physiotherapy is initiated pre-operatively.[43] Of the different available modalities (coughing and deep breathing, incentive spirometry, PEEP and continuous positive airway pressure), there is no clearly proven superior method. It is possible to improve exercise tolerance with a physiotherapy programme, even in the patient with the most severe COPD. Of patients with COPD, those with excessive sputum benefit the most from chest physiotherapy. A comprehensive programme of pulmonary rehabilitation, featuring physiotherapy, exercise, nutrition and education, can improve the functional capacity of patients with severe COPD.[35] Prophylactic use of antibiotics without bacteriological confirmation of infection is not recommended but every lung infection should be properly treated before surgery. Benzodiazepine pre-medication should be used cautiously, since it can blunt response to hypoxia and hypercarbia in addition to reduction in respiratory drive.
INTRAOPERATIVE MANAGEMENT
The primary goal of anaesthesia in these patients is to maintain oxygenation and ventilation and minimise airway manipulation to prevent complications.
Type of anaesthesia
Choice of anaesthesia depends on the patient factors (clinical state) and surgical factors (type and duration of procedure). General anaesthesia and endotracheal intubation is associated with increased morbidity. The fall in FRC and atelectasis noted in normal patients may also be seen in patients with chronic bronchitis.[30] However, in patients with emphysema, the PEEPi, loss of elastic recoil, chest wall stiffness and relaxation of abdominal muscles result in maintenance of alveolar patency preventing atelectasis.[18] Diaphragmatic position, presence of alveoli at the upper flat end of the alveolar compliance curve, all increase the work of breathing and hence depression of ventilation under anaesthesia can seriously hamper ventilation when not assisted. Duration of surgery more than 2.5–4 h is an independent risk factor for development of post-operative complications, so also is the use of long acting muscle relaxants such as pancuronium, and opioids such as morphine.[44] There is increased risk of bronchospasm, laryngospasm, hypoxemia and barotrauma during general anaesthesia in these patients. Regional anaesthesia, including central neuraxial block eliminates the need for airway manipulation and was associated with 50% reduction in PPCs in a study.[45] These factors make regional anaesthesia a popular choice where ever feasible in these patients. Combined spinal epidural anaesthesia has been successfully used as a sole anaesthetic technique in major abdominal surgeries in patients with COPD.[46] In spite of conflicts in opinion with regards to benefits of epidural analgesia in COPD patients,[28] recent evidence favours use thoracic epidural anaesthesia and analgesia in these patients to reduce post-operative complications.[45,47] Use of interscalene block in patients with COPD remains a concern due to ipsilateral phrenic nerve paralysis and loss of sympathetic tone due to stellate ganglion block resulting in bronchospasm.[48] Hence it is contraindicated in patients who cannot tolerate 25% reduction in pulmonary function.[49]
Laparoscopic surgeries may reduce the incidence of post operative respiratory muscle dysfunction, and reduce morbidity. However, abdominal insufflation with carbon di oxide should raise concern during intraoperative ventilator management of the patient.[5]
Management of patients under general anaesthesia
During management of patients under general anaesthesia, tracheal intubation should be avoided by using the laryngeal mask airway or similar device where possible. Propofol, ketamine, or volatile anaesthetics are the induction agents of choice; barbiturates may sometimes provoke bronchospasm. Adjuvants to increase the depth of anaesthesia and blunt airway reflexes before intubation such as lidocaine or opioids may be useful. However, laryngotracheal lidocaine may be less useful, as it might transiently increase airway resistance. Volatile anaesthetics are useful for maintenance of anaesthesia due to their excellent bronchodilating properties with the possible exception of desflurane.[5] Pre-oxygenation should be used in any patient who is hypoxic on air before induction. In patients with severe COPD and hypoxia, continuous positive airway pressure (CPAP) during induction may be used to improve the efficacy of pre-oxygenation and reduce the development of atelectasis.[22] Haemodynamic compromise can occur following general anaesthesia due to fall in pre-load which is secondary to increased intrathoracic pressure.[30]
Management of ventilation during general anaesthesia should be aimed at reducing the dynamic hyperinflation, PEEPi, and air trapping. Harmful effects of air trapping include hypotension, barotrauma and volume trauma to the lungs, hypercapnia, and acidosis. Measures to reduce air trapping include use of smaller tidal volumes and lower respiratory rates, with more time for expiration.[15] Shortening the inspiratory time increases the peak inspiratory flow thereby facilitating better ventilation of all alveoli.[5] Use of low tidal volumes during major abdominal surgeries reduces the risk of post-operative complications.[50] Application of external PEEP has been shown to decrease the effort of triggering breaths in patients breathing spontaneously on assisted modes of ventilation. Even during controlled ventilation, external PEEP that is less than or equal to PEEPi, is not found to significantly increase the alveolar pressure and a study demonstrated reduction in end inspiratory plateau pressure with application of PEEP in patients with COPD, implying less air trapping.[51] Pressure controlled ventilation, by virtue of its decelerating flow reduces peak inspiratory pressure and allows for more uniform distribution of tidal volume and improvement of static and dynamic compliance.[52] In spite of limited evidence regarding the role of pressure controlled ventilation in COPD patients, it has been suggested as an alternative when other measures to prevent air trapping fail.[15]
Residual neuromuscular blockade remains a major predictor of PPCs. Intraoperative use of drugs (volatile anaesthetics and antibiotics) potentiating effects of muscle relaxants, metabolic abnormalities further complicate the situation.[18] Hence, neuromuscular blockade monitoring, use of short acing or intermediate acting muscle relaxants is warranted.
Cabrini et al., in their review observed that non-invasive ventilation (using bilevel positive airway pressure or CPAP) either prophylactically or therapeutically in patients with severe respiratory limitation combined with regional technique would serve as an alternative when tracheal intubation is best avoided.[53]
Restrictive fluid administration decreases risk of pulmonary oedema, it has been accepted in thoracic surgery and showed good outcomes after major abdominal interventions.[18,54]
Intraoperative problems
Bronchospasm
Perioperative bronchospasm in patients with reactive airway disease is relatively uncommon. In patients with well-controlled asthma and COPD the incidence is approximately 2%. The overall incidence of bronchospasm during general anaesthesia is approximately 0.2%.[55]
Bronchospasm during anaesthesia usually manifests as prolonged expiration. Expiratory wheeze may be auscultated in the chest or heard in the breathing circuit due to movement of the gas through narrowed airway. Breath sounds may be reduced or absent. With intermittent positive pressure ventilation, peak airway pressures are increased, tidal volumes reduced, or both. In severe bronchospasm, wheeze may be quiet or absent due to cessation of movement of air. Such a chest is often termed as silent chest and denotes the severest form of COPD. With capnography, narrowed airway and prolonged expiration result in a delayed rise in end-tidal carbon dioxide, producing a characteristic ‘shark fin’ appearance [Figure 1]. One has to rule out the other causes of wheeze or increased peak airway pressures during anaesthesia [Table 2].
Figure 1.
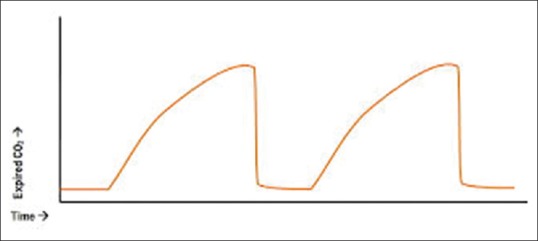
Characteristic shark fin appearance due to bronchospasm in capnograph
Table 2.
Differential diagnosis for intraoperative wheeze and increased peak airway pressures

Intraoperatively, bronchospasm occurs most commonly during the induction and maintenance stages of anaesthesia and is less often encountered in the emergence and recovery stages.[56]
Bronchospasm during the induction stage is most commonly caused by airway irritation, often related to intubation. During the maintenance stage of anaesthesia, bronchospasm may result from an anaphylactic or serious allergic reaction. Following endotracheal intubation, wheezing is more likely to occur when barbiturates are used as anaesthetic induction agents, compared with propofol, ketamine or volatile anaesthetics.[57,58]
To attenuate the bronchoconstrictive reflex due to endotracheal intubation or suctioning, prophylactic treatments with lidocaine (intravenous or inhaled) and/or a beta2-adrenergic agonist are recommended in asthmatic and COPD patients with bronchospastic response.[18]
Auto-positive end expiratory pressure
Auto-PEEP becomes even more important during mechanical ventilation. It is directly proportional to tidal volume and inversely proportional to expiratory time. The presence of PEEPi is not detected by the manometer of standard anaesthesia ventilators. It can be measured by end-expiratory flow interruption, a feature available on newer generation of intensive care ventilators. Auto-PEEP has been found to develop in most COPD patients during one-lung anaesthesia.[59] Paradoxically it has been found that small amount of added PEEP (e.g., 5 cm H2O) can decrease PEEPi and hyperinflation in many ventilated COPD patients.[53]
Haemodynamic instability in chronic obstructive pulmonary disease patients
Along with direct pressure exerted on the heart by hyper-inflated lungs, the elevation of intrathoracic pressure results in decreased systemic venous return and may be transmitted to the pulmonary artery, raising PVR, and leading to right heart strain. Movement of interventricular septum into left ventricle impairs its filling. This explains the cardiovascular instability that commonly occurs.[15,22]
Ways of reducing intraoperative PEEPi and haemodynamic instability:[22]
Allowing more time for exhalation. Reducing the respiratory rate or the I:E ratio (typically to 1:3–1:5), with a long expiratory time to allow complete exhalation and reduce ‘breath-stacking’ and PEEPi. If bronchospasm is severe, only 3–4 breaths/min may be possible with full expiration-it is useful to either auscultate or listen at the end of the disconnected endotracheal tube to confirm that expiration has finished, before commencing the next breath. Rarely, to facilitate this, it is necessary to apply manual external pressure to the chest
Application of PEEP. There is no consensus on application of (external) PEEP, but many advocate trying to match the applied PEEP to the estimated PEEPi
Bronchospasm should be treated promptly either by inhaled bronchodilators or by deepening anaesthesia with propofol or increased concentrations of inhalation anaesthetics.
Atelectasis
The lack of atelectasis formation and preservation of FRC in COPD patients may be explained by three complementary mechanisms:[60,61,62]
Chronic airflow obstruction that generates intrinsic positive end-expiratory pressure (PEEPi)
Loss of lung elastic recoil and the increased stiffness of the chest wall
Relaxation of the abdominal muscular tone (facilitated with analgesia) promoting caudal displacement of the diaphragm. As a rule, most COPD patients exhibit weaker hypoxic pulmonary vasoconstriction (HPV) and blunted ventilatory responses to CO2 and hypoxia.[18]
POST-OPERATIVE COMPLICATIONS
Multiple logistic regression identified composite scoring systems, such as the American Society of Anesthesiologists physical status, along with pulmonary factors are the best pre-operative predictors of PPCs, probably because they include both pulmonary and non-pulmonary factors [Table 3]. During the intraoperative period, avoiding general anaesthesia with tracheal intubation, shortening the duration of surgery and anaesthesia may decrease the risk of prolonged Intensive Care Unit stay.[63]
Table 3.
Post-operative complications

Smoking status is a consistent univariate risk factor for variety of pulmonary and cardiac adverse events.[64]
On the 2nd and 3rd day following major surgery, recurrent nocturnal hypoxaemic episodes have been attributed to sleep disturbances and abnormal breathing patterns that may be aggravated by the administration of analgesic and sedative drugs.[65] Besides abnormal ventilatory patterns, circulatory failure, sepsis and hypermetabolic conditions may also alter oxygen exchanges.[66,67]
Post-operative diaphragmatic dysfunction is attributed to three different mechanisms: (i) Direct phrenic nerve injuries (ii) reflex phrenic nerve inhibition (iii) muscular pump failure. In contrast to lower abdominal, orthopaedic and superficial surgery, upper abdominal and intra-thoracic procedures produce marked alterations in respiratory function.[18]
The pulmonary restrictive syndrome following thoracic and abdominal surgery is characterised by substantial reductions in lung volumes (−30% of FRC and total lung capacity, −40–60% of FEV1) with an elevated airway occlusion pressure and a shallow ‘thoracic’ breathing pattern.[68]
The minimally invasive approach confers major advantages in terms of shorter hospital stay, earlier ambulation and feeding, with lesser need for analgesic medications and better preservation of lung functional volume.[69]
Thoracic epidural anaesthesia using low concentrations of local anaesthetic agents and low doses of opiates effectively suppresses both the afferent nociceptive inputs and the efferent sympathetic output thus preserving the respiratory muscular activity and the HPV response. It has also shown to improve ventilatory mechanics resulting in decreased airway resistance, lower work of breathing and better preservation of the inspiratory and expiratory capacities allowing lung recruitment manoeuvres and voluntary drainage of bronchial secretions.[70,71]
CONCLUSION
Anaesthetic management of patients with COPD requires proper insight into the patients condition. Objective methods, such as CPET are very useful in assessing the functional status non-invasively. Intraoperative management involves taking proper measures to minimize airway manipulation, and prevention of air trapping and PEEPi during intraoperative ventilation. Recent evidences favour the use of regional techniques for anaesthetic and analgesic management in these patients. Thoracic epidural analgesia is shown to reduce the incidence of post-operative complications in upper abdominal and thoracic surgeries in patients with COPD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Reilly J, Jr, Silverman EK, Shapiro SD. Chronic obstructive pulmonary disease. In Harrison's Principles of Internal Medicine. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. 16th ed. New York: Mc Graw Hill publication; 2005. pp. 1547–60. [Google Scholar]
- 3.Vijayan VK. Chronic obstructive pulmonary disease. Indian J Med Res. 2013;137:251–69. [PMC free article] [PubMed] [Google Scholar]
- 4.Cho MH, Boutaoui N, Klanderman BJ, Sylvia JS, Ziniti JP, Hersh CP, et al. Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat Genet. 2010;42:200–2. doi: 10.1038/ng.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maddali MM. Chronic obstructive lung disease: Perioperative management. Middle East J Anaesthesiol. 2008;19:1219–39. [PubMed] [Google Scholar]
- 6.Rossi A, Ganassini A, Polese G, Grassi V. Pulmonary hyperinflation and ventilator-dependent patients. Eur Respir J. 1997;10:1663–74. doi: 10.1183/09031936.97.10071663. [DOI] [PubMed] [Google Scholar]
- 7.Tobin MJ, Jubran A, Laghi F. Patient-ventilator interaction. Am J Respir Crit Care Med. 2001;163:1059–63. doi: 10.1164/ajrccm.163.5.2005125. [DOI] [PubMed] [Google Scholar]
- 8.Fleury B, Murciano D, Talamo C, Aubier M, Pariente R, Milic-Emili J. Work of breathing in patients with chronic obstructive pulmonary disease in acute respiratory failure. Am Rev Respir Dis. 1985;131:822–7. doi: 10.1164/arrd.1985.131.6.822. [DOI] [PubMed] [Google Scholar]
- 9.Slinger P. Don’t Make Things Worse with Your Ventilator Settings: How You Manage the Lungs During the Perioperative Period Affects Postoperative Outcomes. IARS 2013 Review Course Lectures. 2013. [Last accessed on 2015 Aug 18]. pp. 38–46. Available from: http://www.iars.org/assets/1/7/RCL-16.pdf .
- 10.Younes M. Mechanisms of ventilatory failure. Curr Pulmonol. 1993;14:242–92. [Google Scholar]
- 11.Agustí AG. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:367–70. doi: 10.1513/pats.200504-026SR. [DOI] [PubMed] [Google Scholar]
- 12.MacNee W. Pathophysiology of corpulmonale in chronic obstructive pulmonary disease. Part One. Am J Respir Crit Care Med. 1994;150:833–52. doi: 10.1164/ajrccm.150.3.8087359. [DOI] [PubMed] [Google Scholar]
- 13.Elwing J, Panos RJ. Pulmonary hypertension associated with COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:55–70. doi: 10.2147/copd.s1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemaire F, Teboul JL, Cinotti L, Giotto G, Abrouk F, Steg G, et al. Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology. 1988;69:171–9. doi: 10.1097/00000542-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Edrich T, Sadovnikoff N. Anesthesia for patients with severe chronic obstructive pulmonary disease. Curr Opin Anaesthesiol. 2010;23:18–24. doi: 10.1097/ACO.0b013e328331ea5b. [DOI] [PubMed] [Google Scholar]
- 16.Saad IA, De Capitani EM, Toro IF, Zambon L. Clinical variables of preoperative risk in thoracic surgery. Sao Paulo Med J. 2003;121:107–10. doi: 10.1590/S1516-31802003000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smetana GW, Lawrence VA, Cornell JE American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: Systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–95. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 18.Licker M, Schweizer A, Ellenberger C, Tschopp JM, Diaper J, Clergue F. Perioperative medical management of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:493–515. [PMC free article] [PubMed] [Google Scholar]
- 19.Degani-Costa LH, Faresin SM, dos Reis Falcão LF. Preoperative evaluation of the patient with pulmonary disease. Braz J Anesthesiol. 2014;64:22–34. doi: 10.1016/j.bjane.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Young RC., Jr Practical evaluation of lung function in the physician's office. J Natl Med Assoc. 1966;58:245–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Viecili RB, Silva DR, Sanches PR, Mu×ller AF, da Silva DP, Sérgio Brreto SM. Real-time measurement of maximal voluntary breath-holding time in patients with obstructive ventilatory defects and normal controls. J Pulm Respir Med. 2014;2:1–3. [Google Scholar]
- 22.Lumb A, Biercamp C. Chronic obstructive pulmonary disease and anaesthesia. Contin Educ Anaesth Crit Care Pain. 2014;14:1–5. [Google Scholar]
- 23.Ranu H, Wilde M, Madden B. Pulmonary function tests. Ulster Med J. 2011;80:84–90. [PMC free article] [PubMed] [Google Scholar]
- 24.Whalen F, Sprung J, Burkle CM, Schroeder DR, Warner DO. Recent smoking behavior and postoperative nausea and vomiting. Anesth Analg. 2006;103:70–5. doi: 10.1213/01.ane.0000221435.14002.4c. [DOI] [PubMed] [Google Scholar]
- 25.Minai OA, Yared JP, Kaw R, Subramaniam K, Hill NS. Perioperative risk and management in patients with pulmonary hypertension. Chest. 2013;144:329–40. doi: 10.1378/chest.12-1752. [DOI] [PubMed] [Google Scholar]
- 26.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 27.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, et al. EACPR/AHA Joint Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2012;33:2917–27. doi: 10.1093/eurheartj/ehs221. [DOI] [PubMed] [Google Scholar]
- 28.Bapoje SR, Whitaker JF, Schulz T, Chu ES, Albert RK. Preoperative evaluation of the patient with pulmonary disease. Chest. 2007;132:1637–45. doi: 10.1378/chest.07-0347. [DOI] [PubMed] [Google Scholar]
- 29.Yoder MA, Sharma S. Perioperative Pulmonary Management. [Last updated on 2013 Oct 02; Last accessed on 2015 Jun 20]. Available from: http://www.emedicine.medscape.com/article/284983-overview#a6 .
- 30.Henzler D, Dembinski R, Kuhlen R, Rossaint R. Anesthetic considerations in patients with chronic pulmonary diseases. Minerva Anestesiol. 2004;70:279–84. [PubMed] [Google Scholar]
- 31.Vaporciyan AA, Merriman KW, Ece F, Roth JA, Smythe WR, Swisher SG, et al. Incidence of major pulmonary morbidity after pneumonectomy: Association with timing of smoking cessation. Ann Thorac Surg. 2002;73:420–5. doi: 10.1016/s0003-4975(01)03443-9. [DOI] [PubMed] [Google Scholar]
- 32.Woods BD, Sladen RN. Perioperative considerations for the patient with asthma and bronchospasm. Br J Anaesth. 2009;103(Suppl 1):i57–65. doi: 10.1093/bja/aep271. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki H, Sekine Y, Yoshida S, Suzuki M, Shibuya K, Takiguchi Y, et al. Efficacy of perioperative administration of long-acting bronchodilator on postoperative pulmonary function and quality of life in lung cancer patients with chronic obstructive pulmonary disease. Preliminary results of a randomized control study. Surg Today. 2010;40:923–30. doi: 10.1007/s00595-009-4196-1. [DOI] [PubMed] [Google Scholar]
- 34.Bölükbas S, Eberlein M, Eckhoff J, Schirren J. Short-term effects of inhalative tiotropium/formoterol/budenoside versus tiotropium/formoterol in patients with newly diagnosed chronic obstructive pulmonary disease requiring surgery for lung cancer: A prospective randomized trial. Eur J Cardiothorac Surg. 2011;39:995–1000. doi: 10.1016/j.ejcts.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Slinger P. Intraoperative management of the patient with severe lung disease. South Afr J Anaesth Analg. 2013;19:34–7. [Google Scholar]
- 36.Silvanus MT, Groeben H, Peters J. Corticosteroids and inhaled salbutamol in patients with reversible airway obstruction markedly decrease the incidence of bronchospasm after tracheal intubation. Anesthesiology. 2004;100:1052–7. doi: 10.1097/00000542-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Smetana GW. Preoperative pulmonary evaluation: Identifying and reducing risks for pulmonary complications. Cleve Clin J Med. 2006;73(Suppl 1):S36–41. doi: 10.3949/ccjm.73.suppl_1.s36. [DOI] [PubMed] [Google Scholar]
- 38.Doherty GM, Chisakuta A, Crean P, Shields MD. Anesthesia and the child with asthma. Paediatr Anaesth. 2005;15:446–54. doi: 10.1111/j.1460-9592.2005.01602.x. [DOI] [PubMed] [Google Scholar]
- 39.Sankar J, Lodha R, Kabra SK. Doxofylline: The next generation methylxanthine. Indian J Pediatr. 2008;75:251–4. doi: 10.1007/s12098-008-0054-1. [DOI] [PubMed] [Google Scholar]
- 40.Lal D, Manocha S, Ray A, Vijayan VK, Kumar R. Comparative study of the efficacy and safety of theophylline and doxofylline in patients with bronchial asthma and chronic obstructive pulmonary disease. J Basic Clin Physiol Pharmacol. 2015;26:443–51. doi: 10.1515/jbcpp-2015-0006. [DOI] [PubMed] [Google Scholar]
- 41.Poole PJ. Role of mucolytics in the management of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:123–8. doi: 10.2147/copd.2006.1.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies L, Calverley PM. The evidence for the use of oral mucolytic agents in chronic obstructive pulmonary disease (COPD) Br Med Bull. 2010;93:217–27. doi: 10.1093/bmb/ldp050. [DOI] [PubMed] [Google Scholar]
- 43.Warner DO. Preventing postoperative pulmonary complications: The role of the anesthesiologist. Anesthesiology. 2000;92:1467–72. doi: 10.1097/00000542-200005000-00037. [DOI] [PubMed] [Google Scholar]
- 44.Smetana GW. Preoperative pulmonary assessment of the older adult. Clin Geriatr Med. 2003;19:35–55. doi: 10.1016/s0749-0690(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 45.Van Lier F, van der Geest PJ, Hoeks SE, van Gestel YR, Hol JW, Sin DD, et al. Epidural analgesia is associated with improved health outcomes of surgical patients with chronic obstructive pulmonary disease. Anesthesiology. 2011;115:315–21. doi: 10.1097/ALN.0b013e318224cc5c. [DOI] [PubMed] [Google Scholar]
- 46.Santhosh MCB, Pai RB, Rao RP. Anaesthetic management of nephrectomy in a chronic obstructive pulmonary disease patient with recurrent spontaneous pneumothorax. Rev Bras Anestesiol. 2014 doi: 10.1016/j.bjane.2014.02.002. [Ahead of print] [In Press] [DOI] [PubMed] [Google Scholar]
- 47.Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: A population-based cohort study. Lancet. 2008;372:562–9. doi: 10.1016/S0140-6736(08)61121-6. [DOI] [PubMed] [Google Scholar]
- 48.Wakatsuki M, Havelock T. Anaesthesia in Patients with Chronic Obstructive Pulmonary Disease. [Last accessed on 2015 Aug 20]. Available from: http://www.aagbi.org/sites/default/files/106-Anaesthesia-and-COPD.pdf .
- 49.Long TR, Wass CT, Burkle CM. Perioperative interscalene blockade: An overview of its history and current clinical use. J Clin Anesth. 2002;14:546–56. doi: 10.1016/s0952-8180(02)00408-7. [DOI] [PubMed] [Google Scholar]
- 50.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–37. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 51.Caramez MP, Borges JB, Tucci MR, Okamoto VN, Carvalho CR, Kacmarek RM, et al. Paradoxical responses to positive end-expiratory pressure in patients with airway obstruction during controlled ventilation. Crit Care Med. 2005;33:1519–28. doi: 10.1097/01.CCM.0000168044.98844.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lohser J, Ishikawa S. Clinical management of one lung ventilation. In: Slinger P, Blank RS, Campos J, Cohen E, McRae K, editors. Principles and Practice of Anesthesia for Thoracic Surgery. New York: Springer; 2011. pp. 83–98. [Google Scholar]
- 53.Cabrini L, Nobile L, Plumari VP, Landoni G, Borghi G, Mucchetti M, et al. Intraoperative prophylactic and therapeutic non-invasive ventilation: A systematic review. Br J Anaesth. 2014;112:638–47. doi: 10.1093/bja/aet465. [DOI] [PubMed] [Google Scholar]
- 54.Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–106. doi: 10.1213/01.ANE.0000148691.33690.AC. [DOI] [PubMed] [Google Scholar]
- 55.Olsson GL. Bronchospasm during anaesthesia. A computer-aided incidence study of 136,929 patients. Acta Anaesthesiol Scand. 1987;31:244–52. doi: 10.1111/j.1399-6576.1987.tb02560.x. [DOI] [PubMed] [Google Scholar]
- 56.Westhorpe RN, Ludbrook GL, Helps SC. Crisis management during anaesthesia: Bronchospasm. Qual Saf Health Care. 2005;14:e7. doi: 10.1136/qshc.2002.004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown RH, Wagner EM. Mechanisms of bronchoprotection by anesthetic induction agents: Propofol versus ketamine. Anesthesiology. 1999;90:822–8. doi: 10.1097/00000542-199903000-00025. [DOI] [PubMed] [Google Scholar]
- 58.Goff MJ, Arain SR, Ficke DJ, Uhrich TD, Ebert TJ. Absence of bronchodilation during desflurane anesthesia: A comparison to sevoflurane and thiopental. Anesthesiology. 2000;93:404–8. doi: 10.1097/00000542-200008000-00018. [DOI] [PubMed] [Google Scholar]
- 59.Slinger PD, Hickey DR. The interaction between applied PEEP and iPEEP during one-lung ventilation. J Cardiothorac Vasc Anesth. 1998;12:133–6. doi: 10.1016/s1053-0770(98)90318-4. [DOI] [PubMed] [Google Scholar]
- 60.Gunnarsson L, Tokics L, Lundquist H, Brismar B, Strandberg A, Berg B, et al. Chronic obstructive pulmonary disease and anaesthesia: Formation of atelectasis and gas exchange impairment. Eur Respir J. 1991;4:1106–16. [PubMed] [Google Scholar]
- 61.Kleinman BS, Frey K, VanDrunen M, Sheikh T, DiPinto D, Mason R, et al. Motion of the diaphragm in patients with chronic obstructive pulmonary disease while spontaneously breathing versus during positive pressure breathing after anesthesia and neuromuscular blockade. Anesthesiology. 2002;97:298–305. doi: 10.1097/00000542-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Warner DO. Diaphragm function during anesthesia: Still crazy after all these years. Anesthesiology. 2002;97:295–7. doi: 10.1097/00000542-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Wong DH, Pharm D. Factors associated with postoperative pulmonary complications in patients with severe chronic obstructive pulmonary disease. Anesth Analg. 1995;80:276–84. doi: 10.1097/00000539-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 64.Warner DO. Perioperative abstinence from cigarettes: Physiologic and clinical consequences. Anesthesiology. 2006;104:356–67. doi: 10.1097/00000542-200602000-00023. [DOI] [PubMed] [Google Scholar]
- 65.Rosenberg J, Rasmussen GI, Wøjdemann KR, Kirkeby LT, Jørgensen LN, Kehlet H. Ventilatory pattern and associated episodic hypoxaemia in the late postoperative period in the general surgical ward. Anaesthesia. 1999;54:323–8. doi: 10.1046/j.1365-2044.1999.00744.x. [DOI] [PubMed] [Google Scholar]
- 66.Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: Outcomes and effects on length of stay. Am J Med. 2003;115:515–20. doi: 10.1016/s0002-9343(03)00474-1. [DOI] [PubMed] [Google Scholar]
- 67.Licker M, de Perrot M, Spiliopoulos A, Robert J, Diaper J, Chevalley C, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–65. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 68.Hedenstierna G, Edmark L. The effects of anesthesia and muscle paralysis on the respiratory system. Intensive Care Med. 2005;31:1327–35. doi: 10.1007/s00134-005-2761-7. [DOI] [PubMed] [Google Scholar]
- 69.Johnson N, Barlow D, Lethaby A, Tavender E, Curr L, Garry R. Methods of hysterectomy: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2005;330:1478. doi: 10.1136/bmj.330.7506.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Groeben H, Schäfer B, Pavlakovic G, Silvanus MT, Peters J. Lung function under high thoracic segmental epidural anesthesia with ropivacaine or bupivacaine in patients with severe obstructive pulmonary disease undergoing breast surgery. Anesthesiology. 2002;96:536–41. doi: 10.1097/00000542-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Gruber EM, Tschernko EM, Kritzinger M, Deviatko E, Wisser W, Zurakowski D, et al. The effects of thoracic epidural analgesia with bupivacaine 0.25% on ventilatory mechanics in patients with severe chronic obstructive pulmonary disease. Anesth Analg. 2001;92:1015–9. doi: 10.1097/00000539-200104000-00039. [DOI] [PubMed] [Google Scholar]


