Abstract
Chronic obstructive pulmonary disease (COPD) and bronchial asthma often complicate the surgical patients, leading to post-operative morbidity and mortality. Many authors have tried to predict post-operative pulmonary complications but not specifically in COPD. The aim of this review is to provide recent evidence-based guidelines regarding predictors and ventilatory strategies for mechanical ventilation in COPD and bronchial asthma patients. Using Google search for indexing databases, a search for articles published was performed using various combinations of the following search terms: ‘Predictors’; ‘mechanical ventilation’; COPD’; ‘COPD’; ‘bronchial asthma’; ‘recent strategies’. Additional sources were also identified by exploring the primary reference list.
Keywords: Bronchial asthma, chronic obstructive pulmonary disease, heliox, mechanical ventilation, risk
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a disease spectrum that includes bronchitis and emphysema. It is becoming a major health and economic problem worldwide; in 1990, it was the sixth most common cause of death which is expected to be third most common cause by 2020. Mortality associated with asthma is also substantial with 1–8 deaths per lakh worldwide; however, the actual magnitude of the problem in our country is not known.[1] So, it is advisable to optimise these patients pre-operatively to avoid complications in the post-operative period as many times mechanical ventilation can be more of a problem than a solution. Ventilating a COPD patient is often difficult because the disease may not have a reversible component. Further, quantification and management of dynamic hyperinflation (DH) at bedside is very difficult. In due course of time, it is becoming a major health problem as more and more number of patients with obstructive disease are presenting for surgeries.[2]
Obstructive lung disease
Obstructive lung diseases primarily include disorders of the airways such as COPD, asthma, bronchiectasis and bronchiolitis.[3]
PHYSIOLOGICAL CHANGES IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE RELEVANT TO MECHANICAL VENTILATION
Expiratory flow limitation
It is the principal physiologic alteration in COPD and is overcome by increasing the inspiratory flow and lung volume. Although the load is expiratory the compensation is essentially inspiratory, this combined with high respiratory drive leads to development of inspiratory muscle fatigue which is of central pathophysiological importance in the development of acute respiratory failure (ARF) in these patients.
Dynamic hyperinflation and auto-positive end-expiratory pressure
The airflow obstruction, low elastic recoil, high ventilatory demand and short expiratory time result in air trapping and consequent DH. In COPD patients with ARF, DH is the main factor explaining the increased intrathoracic pressure, increased work of breathing (WOB), ventilator dependency and weaning failure.[4,5] According to the waterfall theory, increasing pressure downstream from the site of small airway closure or collapse should not decrease expiratory flow until the downstream water (external positive end-expiratory pressure [PEEP]) reaches the critical pressure [Figure 1].[4] So, The external PEEP should be kept below 75% to 85% of auto-PEEP to avoid any worsening of hyperinflation or circulatory compromise.[6,7] Determination of dynamic pulmonary hyperinflation is, however, not easy to perform in an ICU. It requires insertion of an oesophageal balloon and assessment of the abdominal muscles that can be recruited during expiration.[8] It has been shown, though, that changes in inspiratory capacity (IC) replicate that of hyperinflation, the greater the IC, the lower the end-expiratory lung volume assuming a constant total lung capacity.[9,10]
Figure 1.
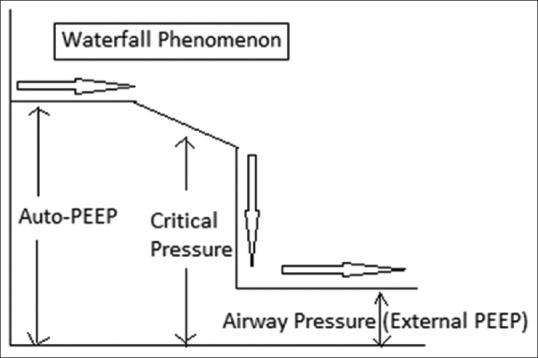
Waterfall phenomenon and its relation with critical pressure
Types of auto-positive end-expiratory pressure and their measurement
Static auto-positive end-expiratory pressure
It is measured only in patients without active respiratory effort using the end-expiratory occlusion on the ventilator. The auto-PEEP is then calculated by subtracting the external PEEP from the total PEEP [Figure 2].[11]
Figure 2.
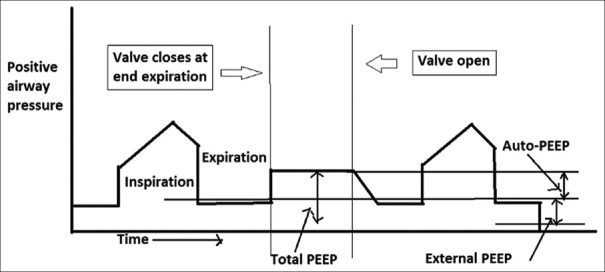
Expiratory hold manoeuvre to estimate auto-positive end-expiratory pressure
Dynamic auto-positive end-expiratory pressure
It is measured by simultaneous recording of airflow and airway pressure at end-expiration. In spontaneously breathing patients, auto-PEEP is determined by simultaneously recording oesophageal pressure and airflow tracings. It is measured at end-expiration as the negative deflection of oesophageal pressure to the point of zero flow. It is less than the static auto-PEEP because it reflects the end-expiratory pressure of the lung units with short time constants and rapid expiration while units with long time constants are still emptying.
Diagnosis of dynamic hyperinflation
Slow filling of manual ventilator bag
Capnography trace not reaching plateau
Expiratory flow not reaching zero in flow-time/volume graph [Figures 3 and 4]
Measure intrinsic PEEP (PEEPi).[12]
Figure 3.
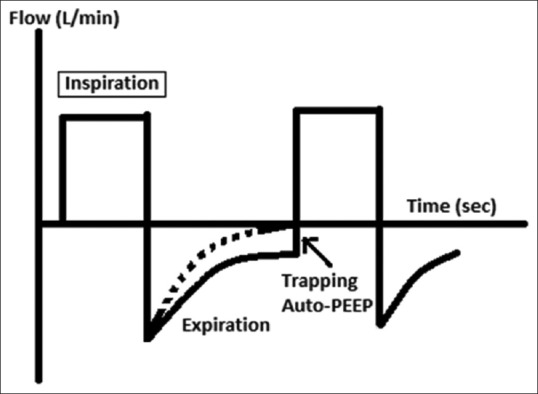
Generation of auto-positive end-expiratory pressure
Figure 4.
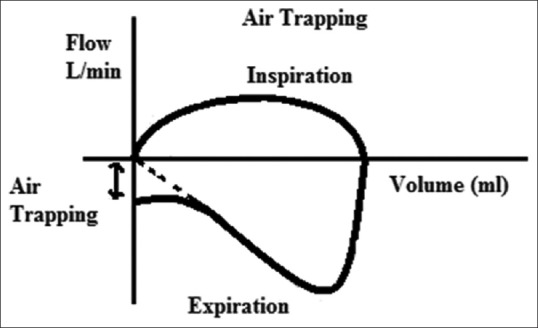
Air trapping in flow-volume loop
Management Allowing more time for exhalation
Reduce the respiratory rate (RR) or I: E ratio (typically to 1:3–1:5) to allow more time for exhalation and reduce breath stacking. However, this will result in low minute ventilation causing hypercapnia, hypoxia or acidosis. This leads to increased pulmonary vascular resistance and worsened haemodynamic instability. If this is a concern, a higher inspiratory flow rate with high peak pressures can be utilised, but this places the patient at increased risk of barotrauma.
Application of positive end-expiratory pressure
The use of external PEEP in ventilated patients with COPD has theoretical benefits by keeping small airways open during late exhalation, so potentially reducing PEEPi or auto PEEP. Additionally, it has been seen that if external PEEP is kept below PEEPi, no significant increase in alveolar pressure and cardiovascular compromise occurs.[13]
Treatment of bronchospasm
Gas flow in small airways may be severely compromised by bronchospasm, which commonly occurs at induction of anaesthesia or during airway instrumentation. It should be treated promptly either by inhaled bronchodilators or by deepening anaesthesia with propofol or increased concentrations of inhalation anaesthetics.
PREDICTORS OF POST-OPERATIVE VENTILATION IN PATIENTS OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE OR BRONCHIAL ASTHMA
Predicting post-operative pulmonary complications (PPCs) remains a challenge for most of the researchers. Although many studies have attempted to predict PPCs, they were not specifically for COPD patients. Patients with COPD are unambiguously at an increased risk for PPCs. A recent review estimated the incidence of unadjusted PPCs as 18.2% in COPD patients undergoing surgery.[14] Increasing severity of COPD confers greater risk, from 10% with mild-to-moderate disease to 23% in patients of the severe disease.[15]
Evidence shows that history and physical examination are poor predictors of airway obstruction and its severity. However, the presence of history of >55-pack-year smoking, wheezing on auscultation and patient self-reported wheezing can be considered predictive of airflow obstruction, defined as post-bronchodilator forced expiratory volume 1 (FEV1)/forced vital capacity < 0.70.[2] Spirometry is useful to identify airflow obstruction in symptomatic patients, but its utility in patients without respiratory symptoms is questionable. Smokers with normal spirometry have only a 4% risk of PPC.[16] Symptomatic patients with FEV1 < 60% predicted will benefit from inhaled treatments but evidence does not support treating asymptomatic, regardless of the risk factors and airflow obstruction.[2] However, unlike in pulmonary resection, there is no cut-off value of FEV1 or any other spirometric index to consider these patients unsuitable for surgery.
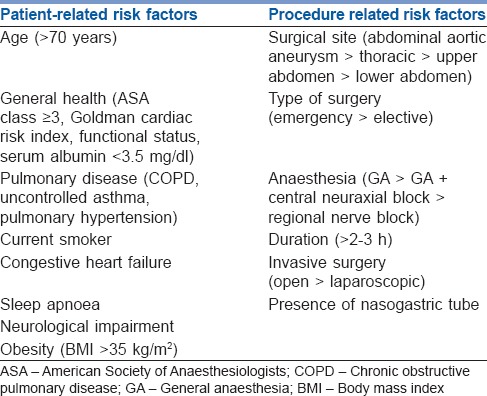
Arterial blood gas (ABG) analyses are not indicated unless the patient's history suggests arterial hypoxaemia or severe enough COPD that one suspects CO2 retention. Then, the ABG should be used in essentially the same manner as one might use pre-operative PFTs, that is, to look for reversible disease or to define the severity of the disease at its baseline. Defining baseline PaO2 and PaCO2 is particularly important if one anticipates post-operatively ventilating a patient who has severe COPD.[17] In general, various independent risk factors [Table 1] and risk indices have been developed that can be used to predict PPCs.[15,18,19,20,21,22]
Table 1.
Independent risk factors for post-operative pulmonary complications
COMMONLY USED RISK INDICES FOR PREDICTING RISKS OF POST-OP PULMONARY COMPLICATIONS IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE AND ASTHMA
-
Score for prediction of post-operative respiratory complications (SPORC).[18] Risk factors and the corresponding scores (in brackets) are American Society of Anesthesiologists score ≥3 (3), emergency procedure, (3) high risk-service, (2), congestive heart failure (2), and chronic pulmonary disease (1).
(Probability of reintubation: 0 points = 0.1%; 1–3 points = 0.4%; 4–6 points = 1.6%; 7–11 points = 6.4%)
-
Respiratory failure risk index:[23]
- Type of surgery
- Emergency
- Albumin (<30 g/L)
- Blood urea nitrogen > 30 mg/dl
- Functional dependency
- COPD
- Age
Probability of respiratory failure (PRF): Class 1 (≤10 points) =0.5%; Class 2 (11–19 points) =2.2%; Class 3 (20–27 points) =5%; Class 4 (28–40 points) =11.6%; Class 5 (>40 points) =30.5%
PRF risk calculator: Available online at http://www.surgicalriskcalculator.com/prf-risk-calculator.[20]
Cardiopulmonary risk index.[24]
MECHANICAL VENTILATORY SUPPORT IN OBSTRUCTIVE PULMONARY DISEASE
Using current evidence, non-invasive positive-pressure ventilation (NPPV) is the first line of treatment for these patients, but invasive positive-pressure ventilation may also be required in patients who have more severe disease. One major cause of the morbidity and mortality arising during mechanical ventilation in these patients is excessive DH with PEEPi, which severely increases the WOB. The main goals of mechanical ventilation are to improve pulmonary gas exchange and to rest compromised respiratory muscles sufficiently to recover from the fatigued state.
Strategies to improve pulmonary gas exchange
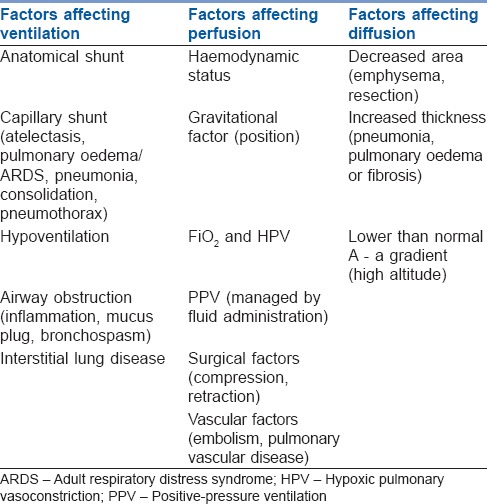
The hypoxaemia of obstructive air diseases is basically due to one of the three general causes: Shunt, ventilation/perfusion abnormalities (V/Q mismatching) and diffusion defects [Table 2]. In general, individuals with acute exacerbations of COPD have a greater degree of ventilation defect (causing hypercapnia) than chronic patients who mainly develop perfusion defect (causing hypoxia). Nonetheless, hypoxic vasoconstriction and collateral ventilation in chronic patients decrease the expected V/Q mismatch.[25] So, managing the cause is of prime importance in the treatment of hypoxaemia of COPD. Moreover, evidence shows beneficial effects of controlled breathing techniques such as active expiration, slow and deep breathing, pursed-lips breathing, relaxation therapy, specific body positions and inspiratory muscle training. Diaphragmatic breathing has not been shown to be beneficial.[26]
Table 2.
Factors affecting pulmonary gas exchange
Strategies to rest compromised respiratory muscles and reduce the work of breathing
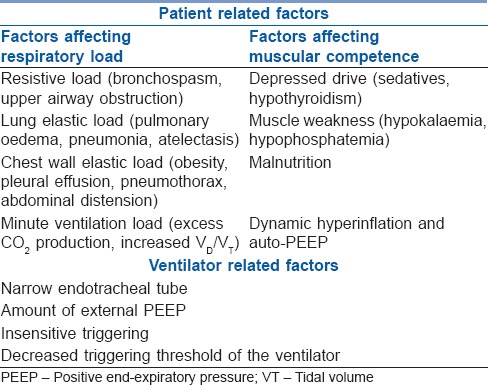
In patients with COPD and asthma, a state of high respiratory drive and poor mechanical advantage cause inspiratory muscle fatigue that can be improved by decreasing respiratory load, increasing muscular competence and providing mechanical ventilatory support [Table 3].
Table 3.
Factors affecting respiratory muscle efficiency
ROLE OF NON-INVASIVE POSITIVE-PRESSURE VENTILATION IN TREATING OBSTRUCTIVE PULMONARY DISEASE PATIENTS
NPPV has been accepted widely as the ventilatory mode of the first choice in treating obstructive airway disease patients with respiratory failure. It provide a significant reduction in endotracheal intubation and thereby its complications (e.g., ventilator-associated pneumonia, tracheal and laryngeal complications) if considered early in the course of the disease.[27,28,29,30,31]
Mechanism of action of non-invasive positive-pressure ventilation
Expiratory positive airway pressure (EPAP) applied offsets PEEPi resulting from expiratory airflow obstruction [Figure 5]. Inspiratory positive airway pressure (IPAP) augments tidal volume for any given respiratory effort leading to less mechanical disadvantage, decreased RR, decreased WOB and improvements in ventilation (generally reduced PaCO2).[32]
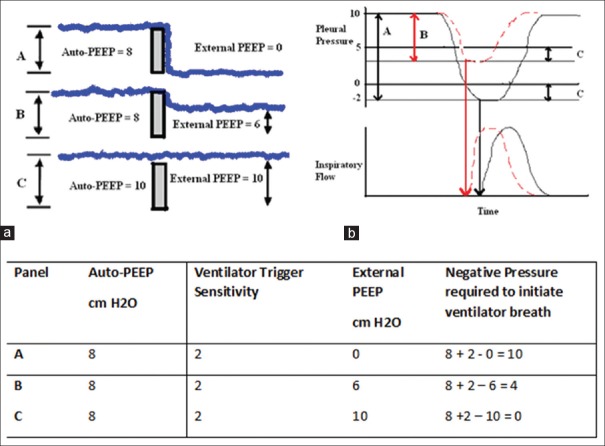
Figure 5.
(a) Waterfall phenomenon-negative pressure required to trigger the ventilator breath is reduced on application of external positive end-expiratory pressure, (b) effect of applied positive end-expiratory pressure on triggering-extrinsic positive end-expiratory pressure of 5 cm H2O reduces the work of breathing from level A to level B by offsetting the auto-positive end-expiratory pressure in this chronic obstructive pulmonary disease patient with trigger sensitivity of 2 cm H2O
Indications for non-invasive positive-pressure ventilation
Patients with pH between 7.30 and 7.25
-
Non-responders to medical therapy having PaO2 <50 mmHg, PaCO2 > 80–90 mmHg, pH ≤7.2, with following:
- Sick but not moribund
- Able to protect airway
- Conscious and cooperative
- Haemodynamically stable
- No excessive respiratory secretions
- Few co-morbidities
Patients who have declined intubation
As a weaning facilitator
Domiciliary NPPV for patients with recurrent admissions.
Technique of non-invasive positive-pressure ventilation[33,34]
Initial settings
Pressure support (PS) mode at 10 cm H2O of IPAP and 5 cm H2O EPAP
Pressures <8 cm/4 cm H2O (IPAP/EPAP) not advised as this may be inadequate
Adjust (IPAP and/or EPAP) to achieve tidal volume of 5–7 ml/kg.
Adjustment on the basis of arterial blood gas analysis
Increase IPAP by 2 cm H2O if persistent hypercapnia
Increase IPAP and EPAP by 2 cm H2O if persistent hypoxaemia
Maximal IPAP limited to 20–25 cm H2O (avoids gastric distension, improves patient comfort)
Maximal EPAP limited to 10–15 cm H2O. It can be increased by looking at the number of missed breaths
FiO2 to be adjusted to lowest level with an acceptable pulse oximetry value
Backup RR 12–16 breaths/min
If the patient is not able to trigger, have large leaks that lead to auto-cycling with PS, the patient may be switched over to pressure controlled mode. Proportional assist ventilation (PAV) can also been used with promising results.
Predictors of successful trial of non-invasive ventilation (1–2 h)
Decrease in PaCO2 >8 mmHg
Improvement in pH >0.06
Correction of respiratory acidosis.
Predictors of failure
-
Severity of illness
- Acidosis (pH <7.25)
- Hypercapnia (>80 and pH <7.25)
- Acute physiology and chronic health evaluation II score higher than 20
-
- Neurologic score/Kellye–Matthay score >4 (stuporous, arousal only after vigorous stimulation, inconsistently follows commands)
- Encephalopathy score >3 (major confusion, daytime sleepiness or agitation)
- Glasgow Coma Scale score <8
Failure of improvement with 12–24 h of non-invasive ventilation (NIV)
Indications for invasive mechanical ventilation
Major criteria (any 1 of the following)[36,37]
Respiratory arrest
Loss of consciousness
Psychomotor agitation requiring sedation
Haemodynamic instability with systolic blood pressure (BP) <70 or > 180 mmHg
Heart rate <50 beats/min with loss of alertness
Gasping for air.
Minor Criteria (any 2 of the following)
RR >35 breath/min
Worsening acidaemia or pH <7.25
PaO2 <40 mmHg or PaO2/FiO2 <200 mmHg despite oxygen
Decreasing level of consciousness.
SECURING AIRWAY
These patients should be intubated (based on the severity of respiratory distress rather than any absolute value of PaCO2 or RR) followed by 24 h of full ventilatory support to rest the fatigued respiratory muscles. Controlled modes should be used as briefly as possible to avoid disuse atrophy of respiratory muscles and unnecessary prolongation of the period of mechanical ventilation.
Anaesthesia can be provided using ketamine, propofol or fentanyl with midazolam. Before induction, fluid status has to be optimised in these patients as haemodynamic collapse can occur due to increased DH and PEEPi. If a patient becomes hypotensive after intubation that is not responding to fluid, ventilator can be disconnected and if the BP improves, a manual squeeze of the thoracic cage can be performed to reduce DH which can be appreciated on SpO2 tracings as huge respiratory swings.[38]
VENTILATION IN A PASSIVE PATIENT
Ventilation should be adjusted based on the degree of DH and Auto-PEEP and not PaCO2. There are only three factors that determine auto-PEEP: (1) Minute ventilation, (2) I: E ratio, (3) expiratory time constants. Of the three factors, minute ventilation is the most important factor which causes DH. Hence, when ventilating patients with COPD, a smaller VT, slow RR, high peak flow should be used with an aim to target normal pH and not PaCO2 (permissive hypercapnia).
Initial settings
Set FiO2 to target SpO2 of 88–92%
Mode-assist control ventilation (preferred)/intermittent mandatory ventilation (IMV ± PS)
VT - 8 ml/kg, RR - 12–14/min, I: E ratio = 1:3 or more depending on expiratory time constant calculation, flow rate-80–100 L/min, peak inspiratory pressure (PIP) of < 40–45 cm H2O and Pplat < 30 cm H2O is acceptable
Adjust the trigger usually by − 1 to 2 cm H2O for pressure and 2 L/min for flow. If trigger is sensitive, respiratory alkalosis may occur while too ‘hard’ a trigger will increase WOB
PEEP setting start at 5 cm H2O, and keep a watch at Pplat or PIP and haemodynamics. Low levels of PEEP improve synchrony and reduce the WOB from level A to level B by off-setting the auto-PEEP in COPD patients [Figure 5]. This beneficial effect of PEEP is the most evident in patients who have flow limitation during tidal expiration and could be probably due to reduction in the lung heterogeneity.[1]
VENTILATION IN SPONTANEOUS PATIENT
PS/PC mode/PAV
PS to generate 8 ml/kg of VT, minimal trigger-flow or pressure, peak flow of 80–100 L/min
PEEP can be added starting at 5 cm H2O in increments of 2 cm H2O
Observe the WOB, RR and missed breaths in flow versus time scalar which show a decrease in RR and no missed breaths
Monitor PIP and Pplat, if there is any increase in these pressures-reduce PEEP. Rarely more than 10 cm H2O PEEP is required
Expiratory sensitivity can be set much above the default setting of 25%
If the patient is still not synchronising, other causes like fever, pain, etc., have to be looked for and in case no other cause is found, sedation can be used.
EXACERBATION OF SEVERE ASTHMA
In addition to standard recommendation for NPPV in all situations, the specific recommendations for patients with acute/severe bronchial asthma are:
Current literature favour relatively small VT (6–10 ml/kg), higher inspiratory flow (80–100 L/min) with PIP < 40–45 cm H2O and Pplat < 25–30 cm H2O, to preserve expiratory time and minimise hyperinflation, barotrauma and hypotension [Figure 6]. The RR should be 8–12 breaths/min to achieve the least possible hyperinflation (auto-PEEP <10 cm H2O) and to maintain pH in an acceptable range, if possible
In contrast to COPD patients, applying PEEP during total ventilatory support of a patient who has DH with fixed airflow obstruction due to severe asthma and without airway collapse may produce potentially dangerous increases in lung volume, airway pressure and intrathoracic pressure, causing circulatory compromise. Although some clinical studies have reported improved airway function (without untoward effects) with continuous positive airway pressure or with NIV and PEEP among patients with acute asthma, the use of PEEP during total ventilatory support of a patient with acute asthma is controversial
Moreover, because the degree of variability in auto-PEEP on a breath-to-breath basis can be high in asthmatic patients receiving mechanical ventilation, the addition of applied PEEP without considering the breath-to-breath variability can lead to lung overdistention. Therefore, PEEP should be used cautiously in asthmatic patients undergoing mechanical ventilation and titrated in real time
Controlled hypoventilation appears to improve the clinical outcome of patients who have status asthmaticus. When reduction of DH is an issue and provided that there is no intracranial hypertension and overt haemodynamic instability, acceptance of moderate acidaemia (pH ≥ 7.2) is reasonable.
Figure 6.
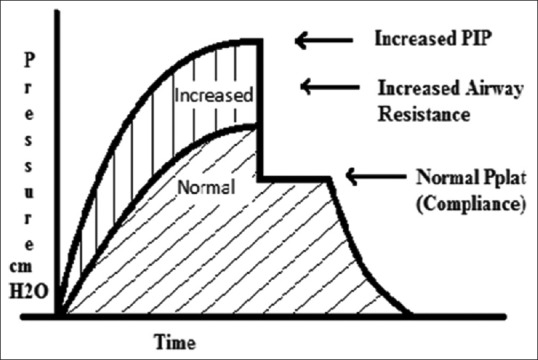
Pressure-time curve indicating increased airway resistance. Peak inspiratory pressure increases whereas Pplat remains same
ROLE OF HELIOX THERAPY IN OBSTRUCTIVE AIRWAY DISEASE
Heliox was introduced in 1934 for the treatment of airway obstruction.[39] As airway turbulence is dependent on density, heliox having a lower density decreases the airway resistance and, therefore, the WOB particularly in situations associated with upper airway obstruction. Moreover, heliox is also found to improve the deposition of aerosolised bronchodilators with a superior particle retention in the lung.[40]
The percentage of oxygen in heliox should be at least 20% to prevent hypoxia, and no more than 40% for heliox to show clinically significant effect.[40] It has been shown to reduce DH by 15% that will probably place the respiratory muscles at a better mechanical advantage and decrease the WOB.[41] Indeed, a significant decline in VCO2 was also noted supporting a reduced WOB leading to small but significant fall in the PaCO2.[42] It also enhances exercise tolerance, at least at constant work rate, and thus can be useful to increase the level of physical training in patients of obstructive airway disease.[43] However, due to presence of conflicting literature, heliox therapy which is costly and cumbersome is not warranted for stable COPD patients at rest with moderate to severe disease, but could be effective as an adjuvant therapy to enhance the efficacy of medical treatment. So, further research to identify the COPD patients potentially able to benefit from this type of therapy is required.[42,43]
WEANING
An aggressive policy toward weaning is justified in COPD patients because an inability to wean is invariably associated with a worse prognosis and prolonged ventilation. It begins when the precipitating factor of the respiratory failure is partially or totally reversed. Marginal respiratory mechanics and continued presence of auto-PEEP make weaning difficult in COPD patients. Hence, factors that increase resistance such as size, secretions, kinking of the tube and the presence of elbow-shaped parts or a heat and moisture exchanger in the circuit have to be optimised to promote early weaning. Furthermore, patients of cor pulmonale may require small dose of inotrope, diuretics and low fluid strategy during weaning.
Weaning can be done with PS mode along with spontaneous breathing trials (SBTs). Sequential weaning (early extubation followed by NPPV) is found to be good alternative in patients showing failed SBTs.[44,45] On the contrary, role of tracheostomy is uncertain, but due to marginal respiratory mechanics, it is also expected to help in weaning.
SUMMARY
Ventilatory support is a lifesaving procedure in acute exacerbation of COPD and asthma. The therapeutic goals are to improve gas exchange, unload ventilatory pump and to relieve respiratory distress. Nowadays, NPPV is regarded as the first line of treatment while invasive ventilation is reserved for life-threatening respiratory failure. However, it can cause considerable increase in morbidity and mortality if not used properly. Therefore, it is necessary to have a good understanding of pathophysiology, mechanics and pattern of flow obstruction and DH to provide the most suitable ventilation to these patients. The ventilatory graphics (flow, pressure and volume) of the most of the modern ventilators becomes a valuable tool in these situations and assist in early diagnosis and management of the patient's condition before it becomes clinically overt.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.García Vicente E, Sandoval Almengor JC, Díaz Caballero LA, Salgado Campo JC. Invasive mechanical ventilation in COPD and asthma. Med Intensiva. 2011;35:288–98. doi: 10.1016/j.medin.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: A clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–91. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 3.Kritek P, Cho A. Approach to the patient with disease of the respiratory system. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 18th ed. USA: McGraw-Hill Companies, Inc; 2012. p. 2058. [Google Scholar]
- 4.Purro A, Appendini L, De Gaetano A, Gudjonsdottir M, Donner CF, Rossi A. Physiologic determinants of ventilator dependence in long-term mechanically ventilated patients. Am J Respir Crit Care Med. 2000;161:1115–23. doi: 10.1164/ajrccm.161.4.9812160. [DOI] [PubMed] [Google Scholar]
- 5.Coussa ML, Guérin C, Eissa NT, Corbeil C, Chassé M, Braidy J, et al. Partitioning of work of breathing in mechanically ventilated COPD patients. J Appl Physiol. 1993;75:1711–9. doi: 10.1152/jappl.1993.75.4.1711. [DOI] [PubMed] [Google Scholar]
- 6.Petrof BJ, Legaré M, Goldberg P, Milic-Emili J, Gottfried SB. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141:281–9. doi: 10.1164/ajrccm/141.2.281. [DOI] [PubMed] [Google Scholar]
- 7.Ranieri VM, Giuliani R, Cinnella G, Pesce C, Brienza N, Ippolito EL, et al. Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute ventilatory failure and controlled mechanical ventilation. Am Rev Respir Dis. 1993;147:5–13. doi: 10.1164/ajrccm/147.1.5. [DOI] [PubMed] [Google Scholar]
- 8.Lessard MR, Lofaso F, Brochard L. Expiratory muscle activity increases intrinsic positive end-expiratory pressure independently of dynamic hyperinflation in mechanically ventilated patients. Am J Respir Crit Care Med. 1995;151:562–9. doi: 10.1164/ajrccm.151.2.7842221. [DOI] [PubMed] [Google Scholar]
- 9.Yan S, Kaminski D, Sliwinski P. Reliability of inspiratory capacity for estimating end-expiratory lung volume changes during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:55–9. doi: 10.1164/ajrccm.156.1.9608113. [DOI] [PubMed] [Google Scholar]
- 10.Alvisi V, Romanello A, Badet M, Gaillard S, Philit F, Guérin C. Time course of expiratory flow limitation in COPD patients during acute respiratory failure requiring mechanical ventilation. Chest. 2003;123:1625–32. doi: 10.1378/chest.123.5.1625. [DOI] [PubMed] [Google Scholar]
- 11.Reddy RM, Guntupalli KK. Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2:441–52. [PMC free article] [PubMed] [Google Scholar]
- 12.Lumb A, Biercamp C. Chronic obstructive pulmonary disease and anaesthesia. Contin Educ Anaesth Crit Care Pain. 2014;14:1–5. [Google Scholar]
- 13.Jolliet P, Watremez C, Roeseler J, Ngengiyumva JC, de Kock M, Clerbaux T, et al. Comparative effects of helium-oxygen and external positive end-expiratory pressure on respiratory mechanics, gas exchange, and ventilation-perfusion relationships in mechanically ventilated patients with chronic obstructive pulmonary disease. Intensive Care Med. 2003;29:1442–50. doi: 10.1007/s00134-003-1864-2. [DOI] [PubMed] [Google Scholar]
- 14.Smetana GW, Lawrence VA, Cornell JE American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: Systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–95. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 15.Cook MW, Lisco SJ. Prevention of postoperative pulmonary complications. Int Anesthesiol Clin. 2009;47:65–88. doi: 10.1097/AIA.0b013e3181ba1406. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke K, Lawrence VA, Theroux JF, Tuley MR, Hilsenbeck S. Postoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung disease. Chest. 1993;104:1445–51. doi: 10.1378/chest.104.5.1445. [DOI] [PubMed] [Google Scholar]
- 17.Tamul PC, Ault ML. Respiratory function in anesthesia. In: Barash PG, Cullen BF, Stoelting RK, Cahalan MK, Stock MC, Ortega R, editors. Clinical Anesthesia. 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2013. pp. 281–2. [Google Scholar]
- 18.Brueckmann B, Villa-Uribe JL, Bateman BT, Grosse-Sundrup M, Hess DR, Schlett CL, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology. 2013;118:1276–85. doi: 10.1097/ALN.0b013e318293065c. [DOI] [PubMed] [Google Scholar]
- 19.Yoder MA, Sharma S, Hollingsworth HM. Perioperative Pulmonary Management. Medscape. [Last updated on 2013 Oct 02; Last cited on 2015 Aug 14]. Available from: http://www.emedicine.medscape.com/article/284983 .
- 20.Gupta H, Gupta PK, Fang X, Miller WJ, Cemaj S, Forse RA, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest. 2011;140:1207–15. doi: 10.1378/chest.11-0466. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence VA, Cornell JE, Smetana GW American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: Systematic review for the American College of Physicians. Ann Intern Med. 2006;144:596–608. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 22.Doyle RL. Assessing and modifying the risk of postoperative pulmonary complications. Chest. 1999;115(5 Suppl):77S–81S. doi: 10.1378/chest.115.suppl_2.77s. [DOI] [PubMed] [Google Scholar]
- 23.Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg. 2000;232:242–53. doi: 10.1097/00000658-200008000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein SK, Faling LJ, Daly BD, Celli BR. Predicting complications after pulmonary resection. Preoperative exercise testing vs a multifactorial cardiopulmonary risk index. Chest. 1993;104:694–700. doi: 10.1378/chest.104.3.694. [DOI] [PubMed] [Google Scholar]
- 25.Cairo JM, editor. Pilbeam's Mechanical Ventilation: Physiological and Clinical Applications. 5th ed. USA: Elsevier Mosby; 2012. Review of abnormal physiological processes; pp. 555–7. [Google Scholar]
- 26.Gosselink R. Controlled breathing and dyspnea in patients with chronic obstructive pulmonary disease (COPD) J Rehabil Res Dev. 2003;40(5 Suppl 2):25–33. doi: 10.1682/jrrd.2003.10.0025. [DOI] [PubMed] [Google Scholar]
- 27.Girou E, Schortgen F, Delclaux C, Brun-Buisson C, Blot F, Lefort Y, et al. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA. 2000;284:2361–7. doi: 10.1001/jama.284.18.2361. [DOI] [PubMed] [Google Scholar]
- 28.Girou E, Brun-Buisson C, Taillé S, Lemaire F, Brochard L. Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA. 2003;290:2985–91. doi: 10.1001/jama.290.22.2985. [DOI] [PubMed] [Google Scholar]
- 29.Evans TW. International Consensus Conference in Intensive Care Medicine: Non-invasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 2001;163:283–91. doi: 10.1164/ajrccm.163.1.ats1000. [DOI] [PubMed] [Google Scholar]
- 30.Keenan SP, Sinuff T, Cook DJ, Hill NS. Which patients with acute exacerbation of chronic obstructive pulmonary disease benefit from noninvasive positive-pressure ventilation? A systematic review of the literature. Ann Intern Med. 2003;138:861–70. doi: 10.7326/0003-4819-138-11-200306030-00007. [DOI] [PubMed] [Google Scholar]
- 31.Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Noninvasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003;326:185. doi: 10.1136/bmj.326.7382.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambrosino N, Strambi S. New strategies to improve exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J. 2004;24:313–22. doi: 10.1183/09031936.04.00002904. [DOI] [PubMed] [Google Scholar]
- 33.Vitacca M, Clini E, Pagani M, Bianchi L, Rossi A, Ambrosino N. Physiologic effects of early administered mask proportional assist ventilation in patients with chronic obstructive pulmonary disease and acute respiratory failure. Crit Care Med. 2000;28:1791–7. doi: 10.1097/00003246-200006000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Hoo GW. Noninvasive Ventilation. Medscape. [Last updated on 2014 Apr 04; Last cited on 2015 Aug 14]. Available from: http://www.emedicine.medscape.com/article/304235 .
- 35.Scala R. Hypercapnic encephalopathy syndrome: A new frontier for non-invasive ventilation? Respir Med. 2011;105:1109–17. doi: 10.1016/j.rmed.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Brochard L, Isabey D, Piquet J, Amaro P, Mancebo J, Messadi AA, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med. 1990;323:1523–30. doi: 10.1056/NEJM199011293232204. [DOI] [PubMed] [Google Scholar]
- 37.Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–22. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 38.Berti JS, Tonon E, Ronchi CF, Berti HW, Stefano LM, Gut AL, et al. Manual hyperinflation combined with expiratory rib cage compression for reduction of length of ICU stay in critically ill patients on mechanical ventilation. J Bras Pneumol. 2012;38:477–86. doi: 10.1590/s1806-37132012000400010. [DOI] [PubMed] [Google Scholar]
- 39.Barach AL. Use of helium as a new therapeutic gas. Proc Soc Exp Biol Med. 1934;32:462–4. [Google Scholar]
- 40.Anderson M, Svartengren M, Bylin G, Philipson K, Camner P. Deposition in asthmatics of particles inhaled in air or in helium-oxygen. Am Rev Respir Dis. 1993;147:524–8. doi: 10.1164/ajrccm/147.3.524. [DOI] [PubMed] [Google Scholar]
- 41.Swidwa DM, Montenegro HD, Goldman MD, Lutchen KR, Saidel GM. Helium-oxygen breathing in severe chronic obstructive pulmonary disease. Chest. 1985;87:790–5. doi: 10.1378/chest.87.6.790. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigo GJ, Pollack CV, Rodrigo C, Rowe BH, Walters EH. Heliox for treatment of exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;(2) doi: 10.1002/14651858.CD003571. Art No: CD003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pecchiari M. Effects of heliox in stable COPD patients at rest and during exercise. Pulm Med. 2012 doi: 10.1155/2012/593985. 593985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prasad SB, Chaudhry D, Khanna R. Role of noninvasive ventilation in weaning from mechanical ventilation in patients of chronic obstructive pulmonary disease: An Indian experience. Indian J Crit Care Med. 2009;13:207–12. doi: 10.4103/0972-5229.60173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glossop AJ, Shephard N, Bryden DC, Mills GH. Non-invasive ventilation for weaning, avoiding reintubation after extubation and in the postoperative period: A meta-analysis. Br J Anaesth. 2012;109:305–14. doi: 10.1093/bja/aes270. [DOI] [PubMed] [Google Scholar]