Abstract
Pulmonary complications are a major cause of morbidity and mortality in the post-operative period after thoracotomy. The type of complications and the severity of complications depend on the type of thoracic surgery that has been performed as well as on the patient's pre-operative medical status. Risk stratification can help in predicting the possibility of the post-operative complications. Certain airway complications are more prone to develop with thoracic surgery. Vocal cord injuries, bronchopleural fistulae, pulmonary emboli and post-thoracic surgery non-cardiogenic pulmonary oedema are some of the unique complications that occur in this subset of patients. The major pulmonary complications such as atelectasis, bronchospasm and pneumonia can lead to respiratory failure. This review was compiled after a search for search terms within ‘post-operative pulmonary complications after thoracic surgery and thoracotomy’ on search engines including PubMed and standard text references on the subject from 2000 to 2015.
Keywords: Complications, post-operative, pulmonary, thoracotomy
INTRODUCTION
Pulmonary complications are a major cause of morbidity and mortality during the post-operative period after thoracic surgery.[1] The incidence of post-operative pulmonary complications has been reported to vary between 5% and 80%.[2] The incidence varies between hospitals. Lower rates of complications have been reported in hospitals with a high volume of patients. Higher rates have been reported in hospitals with a lower volume.[3] Patients undergoing thoracic surgery are usually high-risk patients. They are most often elderly, have concurrent medical comorbidities and have poor physical status either due to the malignancy, malnutrition and the pre-existing primary disease. Most of these patients are smokers, have occupational exposure and are therefore at even greater risk of developing pulmonary complications. Part of their problem is due to their poor baseline pulmonary function. Pulmonary complications may manifest in the operating room itself or in the post-anaesthesia care unit (PACU), intensive care unit (ICU) and also in the surgical ward. Thoracic surgeries are usually of longer duration; they frequently have significant blood loss and fluid shifts. The surgeries per se as well as the anaesthetic techniques run the risk of damaging intrathoracic structures such as the lungs, airways and the peripheral nervous systems. Both general anaesthesia and thoracic surgery produce changes in the respiratory system. Together with underlying conditions, they can be responsible for post-operative pulmonary complications.[4]
The major cause of perioperative morbidity and mortality in the thoracic surgical population is respiratory complications. The major respiratory complications are atelectasis, pneumonia and respiratory failure. These occur in 15–20% of the patients and also account for the majority of the expected 3–4% mortality.[5] The thoracic surgical population is different from other adult surgical populations. In non-thoracic surgery, cardiac and vascular complications are the leading cause of early perioperative morbidity and mortality. In the thoracic patient population, cardiac complications such as arrhythmia, ischaemia, etc., occur in 10–15% of the cases.[6]
IDENTIFICATION OF THE HIGH-RISK PATIENT
Pre-thoracotomy assessment involves all the usual aspects of anaesthetic assessment. It would include past medical history, allergies, medications, upper airway evaluation, etc. All patients undergoing pulmonary resections should have a pre-operative assessment of their respiratory function taking into account three areas-lung mechanical function, pulmonary parenchymal function, as well as cardiopulmonary reserve. These in fact constitute the ‘three-legged-stool’ of respiratory assessment.[7] Individualised strategy is essential. Surgery for pulmonary malignancies needs specific assessment, taking into account the ‘four M's’-mass effects, metabolic effects, metastases and medications.[7] Certain perioperative interventions such as cessation of smoking and lung physiotherapy have been shown to decrease the incidence of respiratory complications after thoracic surgery in high-risk patients.
Lateral thoracotomy by itself, even without surgical manipulation can produce changes in lung physiology such as reduction of forced vital capacity (FVC) and functional residual capacity (FRC). It has been reported that FVC and FRC can decrease to < 60% of their pre-operative values on the first post-operative day itself. The return back to normal can take up to 2 weeks. This decline, especially of the FRC causes physiologic shunting leading to hypoxaemia.[8] Many patients require post-operative ventilation. Low serum albumin concentrations, high American Society of Anesthesiologists physical status scores, history of smoking and prolonged surgery are predictors of likelihood of post-operative pneumonia.[9]
Shapiro et al. developed a system for risk stratification and risk classification following thoracic and abdominal procedures.[10] They incorporated abnormalities of blood gases as well as that of spirometry variables, taking into account medical comorbidities and the possible course of post-operative ambulation and care. Points were generated and the total value indicated the possible degree of risk. From this, the post-operative care was planned. The recommendations were summed up as follows [Table 1]:
Table 1.
Calculation of the possible risk of pulmonary complications of thoracic procedures
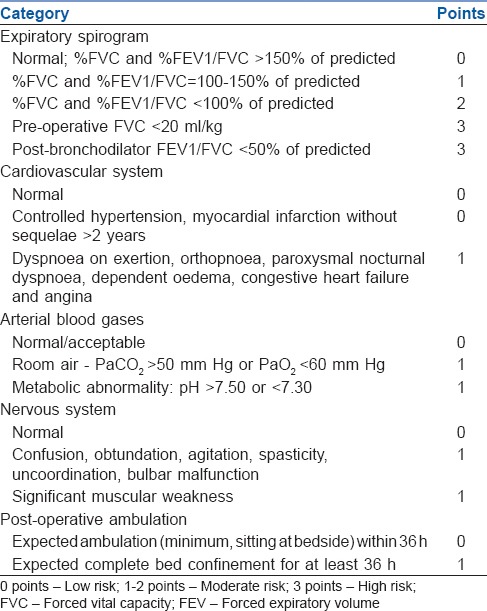
Low-risk patients would possibly not even need oxygen therapy after discharge from the recovery room
Moderate risk patients would require observation either in the ICU or lower monitoring area
High-risk patients would need monitoring in the ICU and possibly interventions.
A fair idea needs to be obtained whether the non-operative lung is diseased or healthy. Whether the pathology for which the surgery is being undertaken is obstructive or restrictive due to a chronic infective process or malignancy should be ascertained. Nutritional status, serum albumin levels and use of pre-surgery radiotherapy that influences perioperative management should also be looked for.
POST-THORACOTOMY COMPLICATIONS
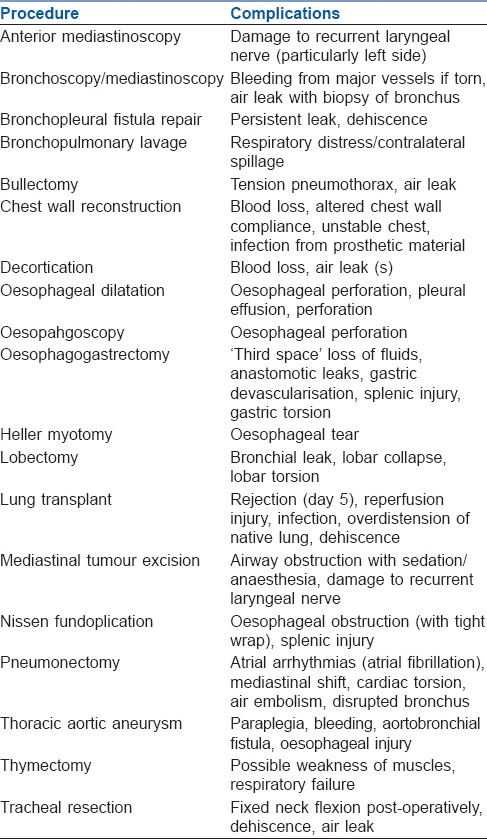
Post-thoracotomy complications can be divided into general complications [Table 2] and complications specific to certain procedures [Table 3].[11]
Table 2.
Complications of thoracic surgery

Table 3.
Complications of specific thoracic procedures
Complications can be categorised as primarily affecting the airway, parenchyma, pleura and chest wall or cardiovascular system.
Airway injuries
The complications to the airway following thoracic surgery could be either due to the anaesthetic management or the surgical technique. The likelihood of airway trauma is more with a double lumen tube (DLT) than the conventional endotracheal tube (ETT). The larger diameter of the DLT along with its more invasive positioning is possibly responsible for injuries. The likely injuries sustained due to anaesthetic techniques such as dental injuries, sore throat, vocal cord injury, laryngeal trauma, bronchial erythema and oedema and tracheobronchial rupture can become apparent in the immediate post-operative period. There is greater risk of vocal cord injuries if the intubating conditions are poor, more proximal type of surgery, use of larger size DLT.[12] The overall incidence of bronchial injury was 25%, and vocal cord injury was 30% in those undergoing pulmonary resections.[13]
Airway bleeding and secretions
The upper and lower airways of patients undergoing lung resection surgery are prone to have accumulation of blood and secretions. It is often difficult to remove these by suctioning through the DLT. Paediatric sized bronchoscopes may be needed to pass through the narrow lumens of the DLT. In extreme scenarios when the suctioning of blood and secretions becomes absolutely imperative, a larger sized DLT may be needed to replace the smaller one.[12]
Vocal cord injuries
The incidences of vocal cord palsies are as high as 1–6% after mediastinoscopy and 5–22% after oesophagectomies.[14] The recurrent laryngeal nerve is likely to be damaged intraoperatively from traction in the anterior mediastinum or by a direct injury on the nerve.[14] It is commoner to encounter unilateral vocal cord injuries, and they are usually benign resulting in voice changes without features of airway obstruction. Bilateral vocal cord injury is fortunately rare. Bilateral vocal cord injury has the potential to lead to dyspnoea, stridor and aspiration, all of which may be life-threatening. Prompt recognition of this situation in the post-operative period is vital. A hoarse voice is common after extubation. However, the presence of stridor and respiratory distress should ring the alarm bells. In the immediate post-operative period, the safest way to deal this emergency setting would be by prompt intubation. Vocal cords are difficult to examine after the trachea has been intubated.[12] If the patient is clinically stable and the symptoms are mild, it would be reasonable to treat this condition with oxygen by mask, steroids and racemic adrenaline. Flexible fibreoptic laryngoscopy may be subsequently done to examine the vocal cords and establish the diagnosis. When one encounters stridor in a patient who has undergone lung surgery using a DLT, bilateral vocal cord paralysis should be considered as an aetiology, and a prompt fibreoptic laryngoscopic examination should be done.[15] There has been a theoretical interest with the use of heliox (70% helium and 30% oxygen) to treat this condition. Effects of heliox are apparent instantly and most of the clinical work has been in the paediatric population.[12]
AIR LEAK, PNEUMOTHORAX AND BRONCHOPLEURAL FISTULA
The presence of bubbles in the water seal chamber of the drainage system would suggest the presence of leaks in the post-operative period. A chest tube that is in proper position with the presence of air leaks indicates that air is passing from the pleural space to the drainage system. For example, if the chest tube was placed for the purpose of treating a pneumothorax, the presence of air leak denotes that the pneumothorax is being properly evacuated and that the leak would resolve only after the potential space is nearly obliterated. Any surgery, which invades the pleural space, creates a pneumothorax.
A surgery like lobectomy entails cutting the lung parenchyma. This can cause air leaks as fistulas develop between distal airways and the pleural space. Once the lung tissue heals, it gets apposed to the parietal pleura and the air leak is resolved. Patients with chronic obstructive pulmonary disease (COPD) have respiratory blebs, which might rupture causing air leaks. Another cause of air leak is inadequate seal at the skin site from where the chest tubes exits. Air tracks back to the pleural space from that site that then communicates with the drainage system.
Small air leaks in the immediate post-operative period are hardly problematic. However, a large air leak, especially in a patient who has undergone pneumonectomy may indicate rupture of a bronchopleural stump and creation of a bronchopleural fistula (BPF). This leads to significant mediastinal shift to the other side and subcutaneous emphysema. Retrospective studies have shown an incidence of 1.9% of the BPF formations in patients undergoing pneumonectomy.[16]
A BPF is often associated with respiratory and haemodynamic instability. In such a scenario, intubation with either a DLT or a single lumen ETT placed into one, the main stem bronchus is necessary. The margin of safety on the left side is obviously more. Spontaneous ventilation is always desirable, but if positive pressure ventilation is deemed necessary, low inspiratory pressures should be aimed for.[12]
MEDIASTINAL EMPHYSEMA
This is a situation when air accumulates in the mediastinal space. This may be a consequence of alveolar rupture, oesophageal rupture or after thoracic surgery. Patients may present with dyspnoea and subcutaneous emphysema. Chest X-ray and computed tomography (CT) scan help in diagnosis. Most often, it does not need any emergency treatment, but a chest tube needs to be inserted when there is evidence of pneumothorax. Being a benign condition, it can be treated expectantly. The patients need to be observed for 24 h.[17] However, if it is due to an oesophageal rupture, surgical repair or stenting is required.[12]
DEEP VENOUS THROMBOSIS AND PULMONARY EMBOLISM
Deep venous thrombosis (DVT) and pulmonary embolism (PE) are potentially fatal complications after thoracic surgery. The incidence of venous thromboembolic events were 12 (1.7%) of 690 patients undergoing thoracic surgery for malignant lung disease in a recent review. Nine of these patients had PE.[18] Prolonged surgical times and malignancy add to the risk. DVT and PE can be difficult to detect. PE can very often remain subclinical. A high index of suspicion is needed to detect these entities. Initially, Chest X-rays may be normal. However, there are certain described features in a plain film such as Fleishner sign: Enlarged pulmonary artery; hampton hump: Peripheral wedge of airspace opacity and implies lung infarction; Westermark's sign: Regional oligaemia and pleural effusion.[19] Pulmonary angiography is often referred to as the investigation of choice to detect PE. Currently CT angiogram, which is more easily performed, is the more commonly used diagnostic technique to establish the diagnosis of PE. Scans reveal filling defects within the pulmonary vasculature. Ventilation/perfusion (V/Q) scans are less and less employed as results are difficult to interpret. A V/Q scan is suggestive when it shows two or more unmatched segmental perfusion defects according to the PIOPED criteria.[20] Electrocardiograms (ECG's) may not suggest anything initially, and it is only with a large PE that ECG changes are observed. The classic finding described in texts is SI QIII TIII pattern, that is, deep S wave in lead I, Q wave in III, inverted T wave in III. This is neither sensitive nor specific for PE and is found in only 20% of patients with PE.[21] In an established PE with haemodynamic compromise, echocardiography may reveal right ventricular dilatation and strain.
The therapeutic options for well-established PE are operative embolectomy, catheter embolectomy, systemic anticoagulation or thrombolytic administration.[22] The size of the PE, its location in the pulmonary vasculature and the degree of haemodynamic alteration decide which mode of therapy to be used.[23]
POST-PNEUMONECTOMY SYNDROME
Wasserman et al., used the term post-pneumonectomy syndrome for the 1st time in 1979.[24] It referred to a rare complication of right pneumonectomy.[25] It was in 1991, however, that Quillin and Shackelford described the first case of the left post-pneumonectomy syndrome.[26] This is characterised by mediastinal shift toward the size of the resected lung along with herniation of the overinflated lung in the same direction. It is an unlikely occurrence after left or right-sided pneumonectomies.[27] Subsequently, there could be compression of the distal trachea and or the main stem bronchus against the vertebral column or the aorta leading to airway compression and obstruction.[28]
Symptoms of this condition may appear months to years after the surgery. They may manifest as progressive dyspnoea and stridor as well as dysphagia and heartburn when oesophageal compression occurs.[27] The diagnosis can be made only when there is a high index of suspicion. Fibreoptic bronchoscopy, pulmonary function tests and a CT scan can be done for confirmation of this condition. One of the treatment modalities that have been described for this condition is mediastinal repositioning with an expandable saline prosthesis.[29,30]
POST-LUNG RESECTION PULMONARY OEDEMA
Zeldin et al. coined the term ‘post-pneumonectomy oedema’ in 1984.[31] The manifestation of pulmonary oedema in the absence of left ventricular failure or infection was ascribed to excessive fluid perfusion volume, which exceeded the drainage capacity of the remaining pulmonary lymphatic tissue. It is an acute, hypoxaemic form of respiratory insufficiency that is secondary to non-cardiogenic pulmonary oedema. The pulmonary artery occlusion pressure (PAOP) is <18 mm Hg. Pulmonary infiltrates appear between 12 h and 5 days following surgery. Pulmonary hypertension and increased pulmonary vascular resistance accompany this.
After lung resection surgery, the appearance of pulmonary oedema is a serious complication. This carries a mortality rate of more than 50%.[32] The incidence of oedema is higher following right-sided pneumonectomy. The symptoms typically occur between the 2nd and 4th post-operative day. Excess fluid administration used to be incriminated for this phenomenon; however, PAOP is often low. This possibly suggests a multifactorial aetiology. A variety of risk factors have been described related to its development. Right pneumonectomy, fluid overload, reduced lymphatic drainage and reduction of the pulmonary capillary bed with oedema due to low outflow are closely associated to its development. The resulting mechanical stress on the capillaries, increased permeability of the alveolocapillary membrane, high intraoperative ventilation pressures, transfusions of fresh frozen plasma and abnormal pre-operative lung function can lead to this condition.[33,34]
Idiopathic post-pneumonectomy pulmonary oedema remains a leading cause of mortality after pneumonectomy. A possible aetiological factor is post-operative hyperinflation of the remaining lung. It has been demonstrated that avoidance of post-pneumonectomy pulmonary oedema can be achieved by solely changing the management of the pneumonectomy space to a balanced drainage system.[35] Lobectomy can also lead to oedema, however, the incidence is low.[32] To diagnose post-lung resection pulmonary oedema, one has to rule out all other causes of pulmonary oedema and congestion such as heart failure, sepsis, pulmonary aspiration, PE and transfusion reaction. Treatment is mainly supportive, but high doses of corticosteroids and pulmonary vasodilators have been used with variable results. As a last resort extracorporeal support has also been used.[36] Inhaled nitric oxide has also been used with occasional success in this condition.[37]
PHRENIC NERVE INJURY
Patients undergoing thoracotomy can have injury to the phrenic surgery during cooling of the heart with ice packs or by direct mechanical trauma.[38] Phrenic nerve injury causes diaphragmatic dysfunction. Patients without significant pulmonary disease usually tolerate a unilateral diaphragmatic paralysis pretty well.[39] Most thoracic surgery patients usually have a preexisting pulmonary condition and impaired pulmonary function. A difficult weaning and extubation process after surgery could raise an alarm bell for possible phrenic nerve injury. Chest X-ray would reveal an elevated hemi-diaphragm, but the confirmation of the diagnosis of this conditioned has to be done by electromyography. Most instances of the paralysis are self-limiting and resolve with time. However, severe cases may need diaphragmatic pacing.[39] Abnormal motions of the diaphragm in the post-operative period may be associated with reduced lung volumes and functions as well as diminished exercise capacity.[40]
ATELECTASIS
Atelectasis is one of the most common post-operative pulmonary complications, particularly after thoracotomy and thoracic surgeries.[41] It manifests as breathlessness and hypoxaemia. The onset of hypoxaemia due to post-operative atelectasis tends to occur after the patient leaves the PACU. Severity increases from the second to fourth post-operative day.[42,43] Post-operative atelectasis develops due to decreased compliance of lung tissue, impaired regional ventilation and retained airway secretions. Post-operative pain may also lead to atelectasis because it interferes with spontaneous deep breathing and coughing.[44]
The initial approach to the management of post-operative atelectasis depends upon the amount of secretions that the patient has. In a patient with marginal or low volume of secretions, continuous positive airway pressure (CPAP) therapy is appropriate whereas those with copious secretions would need chest physiotherapy and suctioning. Closed vigilance is needed during CPAP therapy so that the decision to intubate is not missed. Suctioning and chest physiotherapy are relatively low risk, inexpensive interventions with important potential benefits. They are the mainstay to treat atelectasis in patients with abundant secretions. Current evidence suggests that the role of flexible bronchoscopy is uncertain in this situation. It may have a role in cases that are unresponsive to suctioning and chest physiotherapy.[45,46] N-acetylcysteine has been tried to improve post-operative pulmonary outcomes; however, it has not been found to have any benefit to prevent or improve atelectasis in the post-operative period.[47]
BRONCHOSPASM
Bronchospasm is common during the post-operative period. Manifestations would include dyspnoea, wheezing, chest tightness, tachypnoea, prolonged expiratory time and hypercapnia. The causes of post-operative bronchospasm are aspiration, histamine release caused by medications such as opiates and atracurium or an allergic response to medications. Acute exacerbation of a chronic pulmonary condition such as asthma or COPD causes bronchospasm. Reflex constriction of bronchial smooth muscles is triggered by tracheal stimulation resulting from secretions, suctioning, endotracheal intubation or other surgical stimulation. The first-line pharmacotherapy is short acting inhaled beta-2-agonists. Short-acting inhaled anticholinergic agent, ipratropium bromide, which is also a bronchodilator has an additive effect. Systemic glucocorticoids are added when patients do not improve after one or two doses of the inhaled bronchodilators. Methylxanthines (aminophylline, theophylline) and systemic beta-2-agonists are generally not used for the management of post-operative bronchospasm.[48]
PLEURAL EFFUSIONS
Small pleural effusions are commonly found in the immediate post-operative period. Most effusions were exudates.[49] Most of the effusions usually resolve without specific therapy. They do not require intervention. However, the patient's clinical course and atypical characteristics of either the pleural effusion would warrant diagnostic evaluation of pleural effusions. Post-operative pleural effusions are evaluated in the same way that other pleural effusions are evaluated.[50]
CHEMICAL PNEUMONITIS
The risk for chemical pneumonitis is from the aspiration of acidic gastric contents during the perioperative period. The clinical features of chemical pneumonitis include sudden onset of dyspnoea and tachycardia. These patients may also exhibit fever, bronchospasm, hypoxaemia, cyanosis and/or pink frothy sputum. Infiltrates may appear in one or both lower lobes, usually within the first 24 h.[51] The clinical course of chemical pneumonitis is varied with complete recovery being the usual outcome. However, some patients do develop a secondary bacterial infection (i.e., aspiration pneumonia) or acute respiratory distress syndrome.[52] Witnessed aspiration of gastric secretions is treated by immediately turn the patient's head to the side (i.e., lateral head positioning), and then to suction the patient's oropharynx. If airway reflexes are absent or compromised, endotracheal intubation should be considered.[53] When there is suspicion of an aspiration event, the patient is to be monitored closely for the next 24 to 48 h for the development of aspiration pneumonitis. Supportive treatment for this condition is with supplemental oxygen, non-invasive mechanical ventilation or conventional mechanical ventilation. Prophylactic administration of corticosteroids or antibiotics is not indicated. However, if the clinical scenario does not resolve after 48 h, antibiotic therapy may be considered.[54]
POST-OPERATIVE RESPIRATORY FAILURE
Respiratory failure that requires unplanned reintubation in the post-operative period is associated with very high morbidity.[55] Respiratory failure in the post-operative period leads to a longer hospital stay and an increase in 30-day mortality.[53] The incidence of unanticipated reintubation in the first 72 h after surgery is low (< 1%) but is higher in older patients (up to 3%).[53] Reintubation is associated with an increased likelihood of death (odds ratio 72).[55] The risk of reintubation is greatest within the first 6 h after primary extubation. The main causes that lead to reintubation are pulmonary oedema, atelectasis, pneumonia, airway obstruction impaired brain function and aspiration.
UNIQUE INTRAOPERATIVE PROBLEMS
During on-going thoracic surgeries, certain complications which are unique to the surgical procedure might occur. There could be spillage of pus, blood from the operating side to the non-infected or healthy lungs. This can create difficulty in management of ventilation and oxygenation. Torsion of the remaining part of the lung, sudden massive shifting of the mediastinal contents and injury to major vessels with subsequent massive blood loss are possibilities than occur. Awareness and vigilance along with ability to manage such a problem can prevent a major intraoperative catastrophe.
SUMMARY
Post-operative pulmonary complications are a major concern after thoracotomy and thoracic surgery. The coexisting medical diseases that the patient has the poor baseline pulmonary function that exists for which the surgery is needed, the invasiveness of the surgical procedure and the anaesthetic technique may contribute to the type and severity of the post-operative pulmonary problems. A bad post-operative outcome can result from damage to anatomical structures. Most of the common morbidities and mortality arise due to respiratory failure. Anticipation and recognition of the problems can prevent major complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lawrence VA, Cornell JE, Smetana GW. American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: Systematic review for the American College of Physicians. Ann Intern Med. 2006;144:596–608. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 2.Fisher BW, Majumdar SR, McAlister FA. Predicting pulmonary complications after nonthoracic surgery: A systematic review of blinded studies. Am J Med. 2002;112:219–25. doi: 10.1016/s0002-9343(01)01082-8. [DOI] [PubMed] [Google Scholar]
- 3.Dimick JB, Pronovost PJ, Cowan JA, Jr, Lipsett PA, Stanley JC, Upchurch GR., Jr Variation in postoperative complication rates after high-risk surgery in the United States. Surgery. 2003;134:534–40. doi: 10.1016/s0039-6060(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 4.Rock P, Rich PB. Postoperative pulmonary complications. Curr Opin Anaesthesiol. 2003;16:123–31. doi: 10.1097/00001503-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Nakahara K, Ohno K, Hashimoto J, Miyoshi S, Maeda H, Matsumura A, et al. Prediction of postoperative respiratory failure in patients undergoing lung resection for lung cancer. Ann Thorac Surg. 1988;46:549–52. doi: 10.1016/s0003-4975(10)64694-2. [DOI] [PubMed] [Google Scholar]
- 6.Kearney DJ, Lee TH, Reilly JJ, DeCamp MM, Sugarbaker DJ. Assessment of operative risk in patients undergoing lung resection. Importance of predicted pulmonary function. Chest. 1994;105:753–9. doi: 10.1378/chest.105.3.753. [DOI] [PubMed] [Google Scholar]
- 7.Slinger P, Darling G. Preanesthetic assessment for thorcic surgery. In: Slinger P, editor. Principles and Practice of Anesthesia for Thoracic Surgery. New York: Springer; 2011. p. 19. [Google Scholar]
- 8.Nunn JF, Milledge JS, Chen D, Dore C. Respiratory criteria of fitness for surgery and anaesthesia. Anaesthesia. 1988;43:543–51. doi: 10.1111/j.1365-2044.1988.tb06683.x. [DOI] [PubMed] [Google Scholar]
- 9.Garibaldi RA, Britt MR, Coleman ML, Reading JC, Pace NL. Risk factors for postoperative pneumonia. Am J Med. 1981;70:677–80. doi: 10.1016/0002-9343(81)90595-7. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro BA, Harrison RA, Kacmarek RM, Cane RD. Clinical Application of Respiratory Care. 3rd ed. Chicago: Year Book; 1985. [Google Scholar]
- 11.Higgins TL. Postthoracotomy complications. In: Slinger PD, Kaplan JA, editors. Thoracic Anaesthesia. 3rd ed. Philadelphia: Churchill Livingstone; 2013. [Google Scholar]
- 12.Raiten JM, Blank RS. Anesthetic management of post thoracotomy complications. In: Slinger P, editor. Principles and Practice of Anesthesia for Thoracic Surgery. New York: Springer; 2011. p. 603. [Google Scholar]
- 13.Knoll H, Ziegeler S, Schreiber JU, Buchinger H, Bialas P, Semyonov K, et al. Airway injuries after one-lung ventilation: A comparison between double-lumen tube and endobronchial blocker: A randomized, prospective, controlled trial. Anesthesiology. 2006;105:471–7. doi: 10.1097/00000542-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JR, Wadsworth J. Recurrent laryngeal nerve monitoring during mediastinoscopy: Predictors of injury. Ann Thorac Surg. 2007;83:388–91. doi: 10.1016/j.athoracsur.2006.03.124. [DOI] [PubMed] [Google Scholar]
- 15.Jeong DM, Kim GH, Kim JA, Lee SM. Transient bilateral vocal cord paralysis after endotracheal intubation with double-lumen tube – A case report. Korean J Anesthesiol. 2010;59(Suppl):S9–12. doi: 10.4097/kjae.2010.59.S.S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javadpour H, Sidhu P, Luke DA. Bronchopleural fistula after pneumonectomy. Ir J Med Sci. 2003;172:13–5. doi: 10.1007/BF02914778. [DOI] [PubMed] [Google Scholar]
- 17.Weissberg D, Weissberg D. Spontaneous mediastinal emphysema. Eur J Cardiothorac Surg. 2004;26:885–8. doi: 10.1016/j.ejcts.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 18.Dentali F, Malato A, Ageno W, Imperatori A, Cajozzo M, Rotolo N, et al. Incidence of venous thromboembolism in patients undergoing thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2008;135:705–6. doi: 10.1016/j.jtcvs.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE. Chest radiographic findings in patients with acute pulmonary embolism: Observations from the PIOPED Study. Radiology. 1993;189:133–6. doi: 10.1148/radiology.189.1.8372182. [DOI] [PubMed] [Google Scholar]
- 20.Stein PD, Woodard PK, Weg JG, Wakefield TW, Tapson VF, Sostman HD, et al. Diagnostic pathways in acute pulmonary embolism: Recommendations of the PIOPED II Investigators. Radiology. 2007;242:15–21. doi: 10.1148/radiol.2421060971. [DOI] [PubMed] [Google Scholar]
- 21.Kosuge M, Kimura K, Ishikawa T, Ebina T, Hibi K, Kusama I, et al. Electrocardiographic differentiation between acute pulmonary embolism and acute coronary syndromes on the basis of negative T waves. Am J Cardiol. 2007;99:817–21. doi: 10.1016/j.amjcard.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, Tang AT, Tsang GM. Acute pulmonary thromboembolism complicating pneumonectomy: Successful operative management. Eur J Cardiothorac Surg. 2001;19:223–5. doi: 10.1016/s1010-7940(00)00648-5. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–69. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 24.Wasserman K, Jamplis RW, Lash H, Brown HV, Cleary MG, Lafair J. Post-pneumonectomy syndrome. Surgical correction using silastic implants. Chest. 1979;75:78–81. doi: 10.1378/chest.75.1.78. [DOI] [PubMed] [Google Scholar]
- 25.Mehran RJ, Deslauriers J. Late complications. Postpneumonectomy syndrome. Chest Surg Clin N Am. 1999;9:655–73. x. [PubMed] [Google Scholar]
- 26.Quillin SP, Shackelford GD. Postpneumonectomy syndrome after left lung resection. Radiology. 1991;179:100–2. doi: 10.1148/radiology.179.1.2006258. [DOI] [PubMed] [Google Scholar]
- 27.Scoll C, Hahnloser D, Frauenfelder T, Russi EW, Weder W, Kestenholz PB. The post pneumonectomy syndrome: Successful operative management. Eur J Cardiothorac Surg. 2001;19:223–5. [Google Scholar]
- 28.Shen KR, Wain JC, Wright CD, Grillo HC, Mathisen DJ. Postpneumonectomy syndrome: Surgical management and long-term results. J Thorac Cardiovasc Surg. 2008;135:1210–6. doi: 10.1016/j.jtcvs.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Ng T, Ryder BA, Maziak DE, Shamji FM. Thoracoscopic approach for the treatment of postpneumonectomy syndrome. Ann Thorac Surg. 2009;88:1015–8. doi: 10.1016/j.athoracsur.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Bédard EL, Uy K, Keshavjee S. Postpneumonectomy syndrome: A spectrum of clinical presentations. Ann Thorac Surg. 2007;83:1185–8. doi: 10.1016/j.athoracsur.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Zeldin RA, Normandin D, Landtwing D, Peters RM. Postpneumonectomy pulmonary edema. J Thorac Cardiovasc Surg. 1984;87:359–65. [PubMed] [Google Scholar]
- 32.Slinger P. Post-pneumonectomy pulmonary edema: Is anesthesia to blame? Curr Opin Anaesthesiol. 1999;12:49–54. doi: 10.1097/00001503-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Turnage WS, Lunn JJ. Postpneumonectomy pulmonary edema. A retrospective analysis of associated variables. Chest. 1993;103:1646–50. doi: 10.1378/chest.103.6.1646. [DOI] [PubMed] [Google Scholar]
- 34.van der Werff YD, van der Houwen HK, Heijmans PJ, Duurkens VA, Leusink HA, van Heesewijk HP, et al. Postpneumonectomy pulmonary edema. A retrospective analysis of incidence and possible risk factors. Chest. 1997;111:1278–84. doi: 10.1378/chest.111.5.1278. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez JM, Tan J, Kejriwal N, Ghanim K, Newman MA, Segal A, et al. Idiopathic postpneumonectomy pulmonary edema: Hyperinflation of the remaining lung is a potential etiologic factor, but the condition can be averted by balanced pleural drainage. J Thorac Cardiovasc Surg. 2007;133:1439–47. doi: 10.1016/j.jtcvs.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez JM, Bairstow BM, Tang C, Newman MA. Post-lung resection pulmonary edema: A case for aggressive management. J Cardiothorac Vasc Anesth. 1998;12:199–205. doi: 10.1016/s1053-0770(98)90335-4. [DOI] [PubMed] [Google Scholar]
- 37.Samano MN, Sancho LM, Beyruti R, Jatene FB. Postpneumonectomy pulmonary edema. J Bras Pneumol. 2005;31:69–75. [Google Scholar]
- 38.Mogayzel PJ, Jr, Colombani PM, Crawford TO, Yang SC. Bilateral diaphragm paralysis following lung transplantation and cardiac surgery in a 17-year-old. J Heart Lung Transplant. 2002;21:710–2. doi: 10.1016/s1053-2498(01)00385-0. [DOI] [PubMed] [Google Scholar]
- 39.Qureshi A. Diaphragm paralysis. Semin Respir Crit Care Med. 2009;30:315–20. doi: 10.1055/s-0029-1222445. [DOI] [PubMed] [Google Scholar]
- 40.Ugalde P, Miro S, Provencher S, Quevillon M, Chau L, Deslauriers DR, et al. Ipsilateral diaphragmatic motion and lung function in long-term pneumonectomy patients. Ann Thorac Surg. 2008;86:1745–51. doi: 10.1016/j.athoracsur.2008.05.081. [DOI] [PubMed] [Google Scholar]
- 41.Xue FS, Li BW, Zhang GS, Liao X, Zhang YM, Liu JH, et al. The influence of surgical sites on early postoperative hypoxemia in adults undergoing elective surgery. Anesth Analg. 1999;88:213–9. doi: 10.1097/00000539-199901000-00040. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg J, Ullstad T, Rasmussen J, Hjørne FP, Poulsen NJ, Goldman MD. Time course of postoperative hypoxaemia. Eur J Surg. 1994;160:137–43. [PubMed] [Google Scholar]
- 43.Powell JF, Menon DK, Jones JG. The effects of hypoxaemia and recommendations for postoperative oxygen therapy. Anaesthesia. 1996;51:769–72. doi: 10.1111/j.1365-2044.1996.tb07893.x. [DOI] [PubMed] [Google Scholar]
- 44.Wahba RM. Airway closure and intraoperative hypoxaemia: Twenty-five years later. Can J Anaesth. 1996;43:1144–9. doi: 10.1007/BF03011842. [DOI] [PubMed] [Google Scholar]
- 45.Marini JJ, Pierson DJ, Hudson LD. Acute lobar atelectasis: A prospective comparison of fiberoptic bronchoscopy and respiratory therapy. Am Rev Respir Dis. 1979;119:971–8. doi: 10.1164/arrd.1979.119.6.971. [DOI] [PubMed] [Google Scholar]
- 46.Feldman NT, Huber GL. Fiberoptic bronchoscopy in the intensive care unit. Int Anesthesiol Clin. 1976;14:31–42. doi: 10.1097/00004311-197614010-00004. [DOI] [PubMed] [Google Scholar]
- 47.Jepsen S, Nielsen PH, Klaerke A, Nielsen ST, Simonsen O. Peroral N-acetylcysteine as prophylaxis against bronchopulmonary complications of pulmonary surgery. Scand J Thorac Cardiovasc Surg. 1989;23:185–8. doi: 10.3109/14017438909105992. [DOI] [PubMed] [Google Scholar]
- 48.Nair P, Milan SJ, Rowe BH. Addition of intravenous aminophylline to inhaled beta (2)-agonists in adults with acute asthma. Cochrane Database Syst Rev. 2012;12:CD002742. doi: 10.1002/14651858.CD002742.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: The diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–13. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 50.Gonlugur U, Gonlugur TE. The distinction between transudates and exudates. J Biomed Sci. 2005;12:985–90. doi: 10.1007/s11373-005-9014-1. [DOI] [PubMed] [Google Scholar]
- 51.Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56–62. doi: 10.1097/00000542-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Kozlow JH, Berenholtz SM, Garrett E, Dorman T, Pronovost PJ. Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999-2000. Crit Care Med. 2003;31:1930–7. doi: 10.1097/01.CCM.0000069738.73602.5F. [DOI] [PubMed] [Google Scholar]
- 53.Ramachandran SK, Nafiu OO, Ghaferi A, Tremper KK, Shanks A, Kheterpal S. Independent predictors and outcomes of unanticipated early postoperative tracheal intubation after nonemergent, noncardiac surgery. Anesthesiology. 2011;115:44–53. doi: 10.1097/ALN.0b013e31821cf6de. [DOI] [PubMed] [Google Scholar]
- 54.Rebuck JA, Rasmussen JR, Olsen KM. Clinical aspiration-related practice patterns in the intensive care unit: A physician survey. Crit Care Med. 2001;29:2239–44. doi: 10.1097/00003246-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Peñuelas O, Abraira V, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–30. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]


