Abstract
The introduction of laparoscopy has provided trauma surgeons with a valuable diagnostic and, at times, therapeutic option. The minimally invasive nature of laparoscopic surgery, combined with potentially quicker postoperative recovery, simplified wound care, as well as a growing number of viable intraoperative therapeutic modalities, presents an attractive alternative for many traumatologists when managing hemodynamically stable patients with selected penetrating and blunt traumatic abdominal injuries. At the same time, laparoscopy has its own unique complication profile. This article provides an overview of potential complications associated with diagnostic and therapeutic laparoscopy in trauma, focusing on practical aspects of identification and management of laparoscopy-related adverse events.
Keywords: Complications, diagnostic laparoscopy, review, trauma surgery, therapeutic laparoscopy
INTRODUCTION
Historically, penetrating abdominal traumatic injuries mandated laparotomy to minimize the risk of missed injuries or diagnostic delays.[1] However, more than 25% of patients undergoing traditional exploratory celiotomy had “negative laparotomies” while being at risk of multiple potential postoperative complications, including ileus, wound infection, bowel obstruction, cardiovascular morbidity, and even mortality.[2] Laparoscopic surgery became popular in the 1980s, eventually finding its way into diagnostic and therapeutic “trauma laparoscopy” (TL) applications. Introduction of TL brought the promise of reducing the morbidity associated with negative laparotomies and to providing minimally invasive options for management of selected injuries in hemodynamically stable patients.[3] Despite its minimally invasive character, TL carries a number of specific risks and associated complications.[Table 1] This article focuses on morbidity associated with both diagnostic and therapeutic laparoscopy in trauma, with a goal of providing a high-level overview of major complications described in the literature.
Table 1.
Overview of potential complications of laparoscopy in trauma

INDICATIONS AND CONTRAINDICATIONS
Patients selected for diagnostic laparoscopy should not have indications for immediate laparotomy, such as hemodynamic instability, diffuse peritonitis, evisceration, or evidence of end-organ injury (e. g., hematuria, sanguineous nasogastric tube output, etc.).[3,4] Additional contraindication to TL is the high-risk patient who is unable to tolerate abdominal insufflation.[5,6] Conditions such as shock, “frozen abdomen”,[7] increased intracranial pressure, severe myopia, and/or retinal detachment also preclude patients as candidates for TL. Patients with uncorrected coagulopathy, hypovolemia, congestive heart failure or other conditions that may compromise cardiopulmonary status during laparoscopy likewise pose a relative contraindication.[5,6] Although clinical information regarding many of the above factors may be difficult to elicit during the initial trauma evaluation, it may prove highly useful if available prior to the operative intervention. Surgical judgment should ultimately be used to weigh the risks and benefits of TL. As with most invasive procedures, TL has been associated with complications including physiologic changes during peritoneal insufflation, access-related complications, subcutaneous emphysema (SE), pneumothroax, gas embolism, port site hernias, as well as injuries to diaphragm, blood vessels, omentum, viscera, and bladder.[8,9,10,11]
PATIENT SELECTION AND INDICATIONS FOR TRAUMA LAPAROSCOPY
Injured patients selected for laparoscopic management must be hemodynamically stable and should not have any indications for immediate laparotomy, as discussed in previous sections of this review.[3,4] Low-energy penetrating injuries (e. g., stab wounds, low-velocity tangential gunshot wounds (GSWs)) constitute the most commonly accepted mechanisms for the initial consideration of TL. Studies have also examined the use of laparoscopy in hemodynamically stable patients with high-velocity penetrating injuries, including tangential GSWs.[3,4,12] In terms of relevant anatomy, anterior abdominal penetrating injuries are said to be located in the area bordered by the costal margins superiorly, the inguinal ligaments inferiorly, and the anterior axillary lines laterally. The thoracoabdominal region is defined as the area bordered by the nipple line across the anterior and posterior chest, the costal margin inferiorly, and the spine and sternum medially. Finally, bilateral flanks constitute the areas between the hypochondrium and the iliac region, beyond the lateral-most aspect of the thoracoabdominal boundaries outlined above.
TL as a management strategy in hemodynamically stable patients with penetrating injuries is commonly utilized in the setting of left thoracoabdominal trauma, the possibility of full thickness abdominal wall penetration, suspected hollow viscus injury, and scenarios involving high probability of diaphragm injury.[13] In blunt trauma, the suspicion of significant intra-abdominal injury in a hemodynamically stable patient can also be considered an indication for diagnostic and/or therapeutic laparoscopy in carefully selected cases (e. g., suspected diaphragm or hollow viscus injury).
TL has reduced the number of unnecessary laparotomies.[4,14,15] During TL, the abdomen is evaluated for peritoneal penetration, hollow viscus and/or solid organ injury, as well as for diaphragm injury. Patient position may be adjusted depending on the need for visualization of specific abdominal structures (e. g., diaphragm is easier to visualize in reverse Trendelenburg positioning). The stomach, small bowel, colon and rectum are inspected for hollow viscous injury, contusions, bleeding/contusion, and mesenteric injury. Small bowel should be inspected from the ligament of Treitz to the ileocecal valve using specialized bowel graspers. Here, short segments (approximately 5–10 cm at a tune) are “lifted” and examined in detail, including visual inspection of the accessible mesentery and bowel. The entire laparoscopically “accessible” colon should then be evaluated from cecum to rectum. The inframesocolic space, supramesocolic space, liver, gallbladder, Morison's pouch, and spleen should be evaluated as well.[16] Finally, the gastrocolic ligament can be entered to examine the lesser sac and to better visualize the posterior stomach and pancreas. Laparoscopic visualization of surgically correctable intra-abdominal injuries, significant hemoperitoneum, the presence of bile or intestinal contents should all prompt the surgeon to immediately deploy appropriate therapeutic surgical options.
TL has been especially helpful in ruling out of diaphragmatic injuries without the need for formal celiotomy.[4,14] In selected cases, diaphragm repairs can also be carried out laparoscopically.[12]. It has been proposed that TL may be most beneficial in the setting of penetrating left thoracoabdominal trauma in a hemodynamically stable patient.[13] In a study by Ivatury et al.,[17] TL was >88% accurate in diagnosing diaphragm injuries and 100% sensitive in identifying hemoperitoneum. The authors concluded that TL is useful in identifying hemoperitoneum, solid organ injuries, retroperitoneal hematomas and diaphragm injuries.[17]
Murray et al.,[12] completed a prospective study of 110 hemodynamically stable patients with left-sided thoracoabdominal penetrating injuries, of whom 24% were found to have diaphragm injuries and >95% had one or more associated injuries. Most cases were converted to laparotomy for definitive surgical repair; however, four patients underwent laparoscopic diaphragm repair.[12] Five patients without diaphragm injury underwent laparotomy secondary to adhesions, inadequate visualization, and hemoperitoneum; in this group, no additional injuries were identified. Of note, 62% patients in that study with diaphragm injuries had normal initial chest imaging.[12] Friese et al.,[14] prospectively studied 34 hemodynamically stable patients with thoracoabdominal injuries. All 34 underwent initial TL, followed by laparotomy or thoracotomy. In that series, TL identified seven out of eight diaphragm injuries, with the single missed injury occurring in a setting of GSW with a concurrent splenic injury and hemoperitoneum leading to inadequate diaphragm visualization.[14]
Sosa et al.,[4] prospectively studied laparoscopy in 121 hemodynamically stable patients with abdominal GSWs and no overt peritoneal penetration. Sixty-five percent of these patients had negative TLs thereby avoiding unnecessary laparotomies.[4] In another study, Zantut et al.,[3] studied 510 patients who underwent TL for penetrating abdominal trauma, including 316 stab wounds and 194 gunshot wounds. Criteria for conversion to laparotomy included injuries requiring surgical repair, GSWs that penetrated the peritoneum, significant hemoperitoneum, and high suspicion for bowel injury.[3] Of the 510 patients, 54% did not have peritoneal penetration or other indication for laparotomy. Twenty-nine of those patients underwent therapeutic laparoscopies with repairs, 16 of those patients had laparoscopic diaphragm repairs, with all having uneventful and uncomplicated recoveries.[3] Complications secondary to laparoscopy occurred in 10 patients including trocar injuries and pneumothoraces. Of the 46% of patients who underwent laparotomy, 25% were not therapeutic, but were completed to rule out bowel injury. Therapeutic laparotomies were completed in 75% of patients, of whom 15 had unsuspected bowel injuries and four had unsuspected vascular injuries. The authors concluded that TL (a) may decrease unnecessary laparotomies with careful patient selection; (b) is accurate in determining peritoneal penetration; and (c) helps identify diaphragmatic, splenic, and hepatic injuries. They also concluded that if suspected bowel perforation cannot be ruled out, laparotomy should be performed.[3]
Kawahara et al.,[16] studied 75 hemodynamically stable patients undergoing TL for tangential penetrating abdominal trauma. Indications for conversion to laparotomy included small bowel injury or lesions involving the so-called “blind spots”, such as the retroperitoneum, segments VI and VII of the liver, or the posterior spleen. There were 37 non-therapeutic TLs and 17 therapeutic TLs, indicating that over 73% of potential laparotomies may have been avoided.[16]
EQUIPMENT MALFUNCTION AND FAILURE
Although infrequent, laparoscopic equipment malfunction or failure can produce unacceptable delays in critical situations that involve potentially life-threatening injury. Examples of potentially correctable equipment-related problems may include inadequate supply of insufflation gas, damaged laparoscopic light source/fiberoptic conduit, faulty camera, other instrument malfunction, or any software-related issues (as applicable). If the problem occurs during the setup phase or at the beginning of the case, and can be resolved quickly and definitively, the procedure should continue laparoscopically. If the malfunction cannot be readily corrected, then one should consider proceeding with an open procedure without undue delays.
If the problem occurs in the midst of an operation, a prompt decision regarding potential conversion to an open procedure must be made by the operating surgeon. Such decision should be guided by factors including patient safety, hemodynamic stability, any ongoing critical operative steps, and the estimated time required to remedy the problem at hand. In a stable patient without active bleeding or another immediately life-threatening indication, a short delay may be acceptable. Otherwise, again, a conversion to an open procedure should be performed.
COMPLICATIONS OF GAS INSUFFLATION
Physiologic sequelae of gas insufflation include impaired venous return capnothorax, pneumomediastinum, venous gas embolism (VGE), and SE.[18] These events usually improve with time but may require urgent decompression. Gas insufflation increases intra-abdominal pressure, thereby potentially affecting venous return. Intra-abdominal hypertension, in turn, can lead to hemodynamic changes including bradycardia and hypotension, which may compound pre-operative intra-vascular depletion following additional traumatic injuries.[7,19]
Cardiovascular complications related to gas insufflation include bradyarrhythmias due to peritoneal stretch receptor mediated stimulation of vagal nerves during pneumoperitoneum.[20] Immediate release of pneumoperitoneum usually corrects the problem. Additionally, pneumoperitoneum and reverse Trendelenburg position may impair venous return to the heart and decrease cardiac output. Initial establishment of pneumoperitoneum should occur at a slow rate, with the patient in a supine/flat position. If the patient becomes hypotensive or hemodynamically unstable during any part of the procedure, pneumoperitoneum should be released and patient returned to a neutral/flat position or even placed in Trendelenburg position. In rare cases, the laparoscopic approach may need to be aborted, depending on the operative stage of the procedure.
ACCESS-RELATED COMPLICATIONS
The majority of access-related complications occur at the time of abdominal entry.[21] There are varied access techniques for peritoneal entry such as the open (Hasson) method, the Veress needle (VN) technique, direct trocar insertion (DTI), and hybrid forms of entry.[22,23] All of these techniques can be associated with specific complications, including injury to abdominal wall or intra-abdominal vessels, visceral injury, solid organ (e. g., liver or spleen) injury, bladder injury, and failed peritoneal entry.[10,24,25,26,27] Although rare, the sequelae of such complications include hemorrhage, peritonitis, multiple system organ failure, and death.[10,24,25,26,27,28] Risk factors for access-related complications include obesity, thin body habitus, and anticoagulation.[21,27] While the Hasson, VN, and DTI techniques are the most common methods of entry, other devices and techniques do exist such as optical trocars, optical VN insertion, radial expanding access devices, and endoscopic threaded ports.[29,30,31] An example of a large trocar-associated abdominal wall hematoma can be seen in Figure 1.
Figure 1.
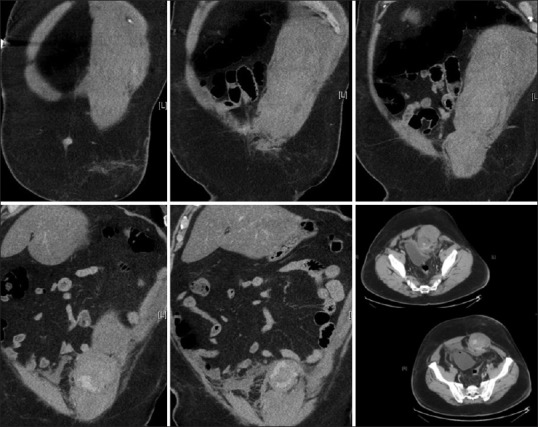
Large left-sided anterior abdominal wall hemorrhage with associated large intramuscular hematoma. This complication was attributed to a forceful placement of a left lower quadrant trocar
LAPAROSCOPY FOLLOWING PREVIOUS ABDOMINAL PROCEDURES
Despite the presence of adhesions, prior abdominal surgery does not appear to affect the rate of major complications such as bowel and vascular injuries during laparoscopic port placement.[32,33] Having said that, port placement may be more difficult in such patients.[34] A comparison of laparoscopic procedures between 368 individuals without prior abdominal surgery to 109 patients with previous abdominal operations revealed that previous surgery was associated with a failure to achieve pneumoperitoneum in 13/109 (12%), subcostal insertion of ports in 17/109 (16%), and omental emphysema in 12/109 (11%) patients while none of these events occurred in those without previous operations.[35]
DIAPHRAGM INJURY
Diaphragm injury during TL is rare. In a series of 1,850 patients undergoing laparoscopic procedures, seven (0.4%) sustained a diaphragmatic injury.[36] One injury occurred during trocar insertion, another during liver retraction, and five resulted from electrocautery.[36] In another study of patients undergoing renal procedures, there were 10/1,765 (0.6%) incidents of diaphragm injury, including two cases related to trocar insertion and the rest attributed to mobilization of adjacent structures such as the liver, spleen, or ascending colon.[37] Overt diaphragmatic injuries can be identified by direct visualization, while less conspicuous injuries may be suggested by the presence of diaphragmatic billowing under decreased pneumoperitoneum (floppy diaphragm sign).[38] Diminished breath sounds, decreased arterial oxygen saturation, increased airway pressure, and elevated end-tidal CO2 may also be suggestive of diaphragmatic injury with concomitant pneumothorax.[36,38]
Thoracostomy tube placement is indicated if hemodynamic instability ensues or a large pneumothroax (>20% hemithorax volume) is evident.[39,40] However, most patients are hemodynamically stable and a chest tube is usually not warranted.[38,41] Such patients may safely tolerate a laparoscopic repair with primary closure or mesh reconstruction of the defect following the evacuation of the pleural air (via forced expiration or using a suction catheter).[36,42,43] The repair is usually performed under lower pneumoperitoneum levels (10 mm Hg or less) and can be safely deferred to the end of the case, as long as the patient remains hemodynamically stable.[36,37] Injuries involving the esophageal hiatus, those anterior to the esophageal hiatus, and those extending toward the pericardium have higher conversion rates. Chest tube is not usually necessary following repair because any residual capnothorax will likely resolve on its own.[38]
SUBCUTANEOUS EMPHYSEMA
Clinically significant SE occurs in approximately 0.3–3.0% of cases.[10,44] Risks factors include older age, higher insufflation pressures, high flow rates of gas, low body mass index (<25), greater than three ports, operative time >3 hours, poor trocar placement, peritoneal insufflation, and failure of anesthesia equipment such as one-way valves in breathing circuits.[45] SE should be suspected if end-tidal carbon dioxide (CO2) increases >25% above baseline, increases >30 minutes after abdominal insufflation, or CO2 levels rise to >50 mm Hg.[11,46] However, this can be corrected by the use of mechanical ventilation.[47] SE may be associated with pneumomediastinum or pneumothrorax by the subcutaneous dissection of CO2 through the pre-fascial planes. Conversely, pneumomediastinum or pneumothorax may also lead to SE. Notably, SE in the neck and upper airway may lead to a potentially life-threatening airway obstruction. Nonetheless, the presence of higher end-tidal CO2 should not negate prompt extubation, provided that all other appropriate criteria are met. SE typically resolves in hours to days. Lowering the intra-abdominal pressure from 12 mmHg to 10 mmHg can substantially reduce the occurrence of SE while maintaining sufficient operating conditions.[46]
PNEUMOTHORAX
Pneumothorax associated with laparoscopy can be unilateral or bilateral. The resultant decrease in lung compliance may cause hypercarbia, hypoxemia, and hemodynamic instability, potentially requiring urgent intervention.[48,49,50] The mechanism of a CO2 -related pneumothorax during laparoscopy is from direct perforation of the diaphragm, congenital diaphragmatic defects, tearing of the diaphragm from high insufflation pressures, ruptured bullae, and diffusion of gas into the retroperitoneum that tracks into the pleural space.[48,51] The diagnosis of CO2 -related pneumothorax includes the elements of: (a) sudden increase in end-tidal CO2; (b) decrease in lung compliance; (c) floppy/bulging diaphragm; and (d) increase in peak inspiratory pressures.[48,49] The treatment is controversial, and may not always require needle decompression or chest tube insertion. CO2 induced pneumothorax may resolve on its own within several hours, whereas rupture of pre-existing bullae will not resolve spontaneously. Pneumothoraces associated with intraoperative use of CO2 may necessitate conversion to an open procedure and placement of a thoracostomy tube. Proper use of laparoscopic instruments, limiting insufflation pressures to as close to 10 mm Hg as possible, and keeping vigilance on airway pressures and end-tidal CO2 will help limit the occurrence of a pneumothorax during laparoscopy, and facilitate prompt intervention if necessary. An example of post-TL pneumothorax is shown in Figure 2.
Figure 2.
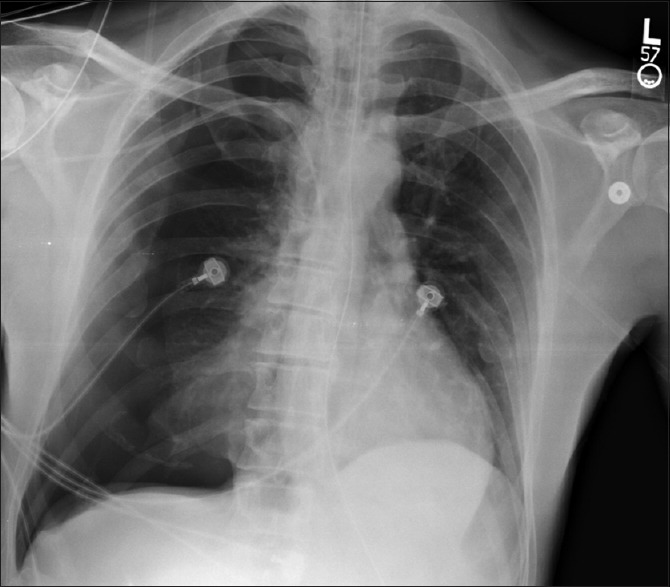
An example of right-sided tension pneumothorax following non-therapeutic diagnostic laparoscopy for a stab wound to right upper quadrant. Although this finding may also be due to pre-existing small pneumothorax exacerbated by positive-pressure ventilation and/or pneumoperitoneum intraoperatively, the pneumothorax was not seen on earlier imaging obtained after an uneventful placement of the right subclavian central venous catheter, prior to the laparoscopic procedure
GAS EMBOLISM
A potentially life-threatening complication of laparoscopy is VGE, which can occur rapidly or gradually through an incremental accretion of gas within the circulatory system.[18] Clinically apparent VGE is very rare, and it has been estimated that one clinically significant event occurs for each 63,845 laparoscopic cases.[52] However, utilizing trans-esophageal echocardiography, gas emboli were identified intraoperatively in nearly 70% of elective laparoscopic cholecystectomy cases, although no physiological effects were noted.[52,53] Infusion of gas can be fatal at approximately 200-300 mL in an adult.[54] The gas embolizes and accumulates in the pulmonary outflow tract of the right heart, effectively interrupting cardiac output. Most often, this occurs during initial insufflation.[55]
Clinical signs range from a characteristic “mill wheel murmur”, cardiac arrhythmias, elevated central venous pressure, a decreased end-tidal CO2, hypercapnia and hypoxemia to fulminant cardiovascular collapse.[54,55] Clinically significant embolisms can be identified by capnographic changes, but echocardiography, especially trans-esophageal echocardiography remains the most sensitive test for VGE in the heart.[53,56] If VGE is suspected, the procedure should be immediately halted, pneumoperitoneum should be released, and the abdominal cavity flushed with irrigation fluid to halt the direct intake of gas into susceptible vessels.[18] The patient should be placed in either the left lateral decubitus position with the head sharply down or the Trendelenburg position to move air away from the cardiac outflow tract.[18,52] A central line should be inserted and air aspirated to remove the mechanical obstruction.[18,52,53,55] Inotropes and aggressive fluid resuscitation may be necessary to support cardiac output. Room air, xenon, helium, nitrogen and CO2 have all been used for insufflation, but CO2 is the most commonly utilized gas as it is inert, non-combustible and highly soluble.[52,57] This solubility confers a lower risk of embolism, as CO2 has an ability to dissolve into the blood before reaching a point of saturation.
VASCULAR INJURY
Hemorrhagic complications during laparoscopy are infrequent but can be potentially devastating. Even more importantly, active hemorrhage may predispose the surgeon to perform emergency hemostatic or life-saving maneuvers that may increase the risk of injury to other organs and surrounding structures. Vascular injuries occur in about 0.3% of laparoscopic cases,[58] with one out of 328 cases in a major series developing intra-operative hemorrhage.[59] Specific sources of hemorrhage are discussed below.
Hemorrhage at the port site has been reported in approximately 0.7% of laparoscopic cases and is usually related to the insertion of secondary trocars.[9] Safe entry into the abdomen should be either in the midline or away from the epigastric artery to avoid injury.[60] After initial entry, injury to the epigastric vessel may occur during subsequent port placement, despite proper trans-illumination and direct visualization.[25] Bleeding may not be apparent initially as the trocar itself may provide local tamponade of the abdominal wall. Therefore, visualization after both insertion and removal of the trocar is essential.[61] If hemorrhage occurs, hemostasis can be achieved by a combination of direct pressure/tamponade, electrocautery, or directed suture ligation.[9]
Intra-abdominal hemorrhage attributed to major vessel injury is associated with mortality of approximately 15%.[25] Mesenteric, omental, and retroperitoneal vessels are all susceptible to injury.[61] The common iliac vein is the most frequently injured major vessel, followed by greater omental vessels, the inferior vena cava, and aorta.[62] Rates of major vascular injuries can range from 0.05–0.50%, with vast majority occurring during the initial steps of accessing the peritoneal cavity.[63] In a review of 14,243 laparoscopic cases, major bleeding occurred in 12 procedures with 6/12 (50%) requiring blood transfusion and all cases necessitated an open laparotomy for suture or prosthetic repair of the injured vessel.[61] In a series of 361 reoperative laparoscopic surgery, 15/361 cases were complicated by omental bleeding.[64] An expanding omental hematoma was noted in 6/15 cases while active bleeding was present in the remainder.[64] In each scenario, the omental hemorrhage was successfully controlled laparoscopically using electrocautery.[64] Management of retroperitoneal hematomas from laparoscopy-related injuries follows the same algorithm as penetrating abdominal trauma. Zone I (midline retroperitoneum) hematomas should always be explored and injuries repaired.[65] Zone II (bilateral upper lateral retroperitonum) and Zone III (pelvic retroperitoneum) hematomas can be managed without exploration if the hematoma is nonexpanding and the patient is hemodynamically stable. Computed tomography scans can be obtained to follow the injury postoperatively, and endovascular embolization may be an option if hemorrhage continues in a stable patient.
OMENTAL INJURY
Omental injuries occur in as many as 1.6% of laparoscopic cases,[9,34] and usually result from loss of insufflation, suboptimal neuromuscular blockade, adhesions, and/or accessory trocars. Omental injuries may occur at any stage of the procedure (e. g., abdominal access, insufflation, and closure). The rate of omental injury with the VN is higher than that compared to DTI.[66] Excessive movement of a VN upon insertion can enlarge a small puncture injury.[31] Following trocar placement and insufflation, the trocar may slip into the omentum and result in omental emphysema. Omental emphysema resolves spontaneously upon desufflation and removal of the insufflation needle or trocar from the omentum.[67] Finally, omental herniation can occur during port removal and result in omental incorporation into the fascial closure.[68] As such, it may be recommended to deflate the peritoneal cavity prior to removing laparoscopic ports. Most omental injuries do not require open repair. Minimal bleeding can be controlled via laparoscopic fulguration or suture ligation.[67] Stable hematomas can be observed, while expanding hematomas and major bleeding are rare but may necessitate exploration via laparotomy.[67] An example of omental injury during TL procedure is shown in Figure 3.
Figure 3.
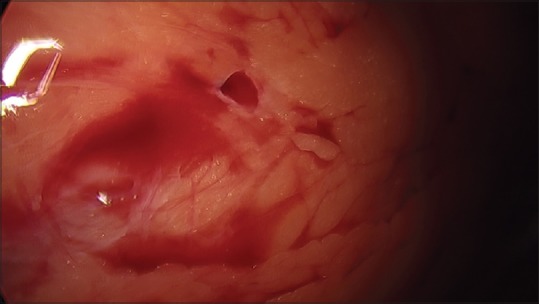
Intraoperative photograph showing a puncture injury with active bleeding and hematoma of the omentum. The injury was caused by a Veress needle excursion in excess of the distance necessary to gain access to the peritoneum
VISCERAL INJURY
During laparoscopic access, the small bowel is injured relatively frequently, with an incidence of up to 0.5%.[27,63,69] In a prospective study of 25,764 laparoscopic procedures, there were 29 gastrointestinal injuries resulting in 26 laparotomies.[21] Of importance, up to half of bowel injuries go undiagnosed during surgery and lead to significant subsequent morbidity/mortality. A high index of suspicion is required to diagnose bowel injury intra- and post-operatively. Visceral injuries are commonly attributed to trocar insertion, direct mechanical instrument injury and electrocautery. A meta-analysis of 28 studies of open versus VN entry showed no significant difference in the rate of visceral injury.[23] However, prior abdominal surgery and increased body-mass index were associated with increased rates of injury for both techniques.[23] Abdominal entry in a quadrant away from prior surgery reduces the risk of injury due to abdominal wall adhesions. While entry technique does not appear to significantly affect injury rate, trocar-associated morbidity rate from visceral injuries is significantly lower for blunt compared to bladed trocars (0.2% versus 0.7%, respectively).[70]
The treatment of direct mechanical injuries varies based on the extent of injury. A VN puncture can usually be repaired by a seromuscular “Lemberting” suture. If a full thickness injury involving > 50% of the lumen is present, a segmental bowel resection should be performed. The exact magnitude of electrocautery-related injuries is difficult to determine because the external appearance of serosal burns may be misleading, and the resultant injury may evolve via lateral spread over a period of approximately 48 hours. Consequently, Wu et al.,[71] recommend surgical bowel excision of up to 5 cm on each aspect of a full thickness electrocautery perforation so as to reduce the risk of secondary operations resulting from inadequate resection (and thus delayed perforation) in the setting of thermal injury. Early recognition and repair of iatrogenic visceral injury may reduce the associated morbidity.
BLADDER INJURIES
Bladder injury during laparoscopic surgery most often occurs upon insertion of a suprapubic trocar. Injuries related to VN and trocar placement are infrequent, ranging between 0.03% and 0.20% cases. Patients with prior abdominal surgeries and adhesions may be at increased risk of such injuries.[72] A series of 14,234 patients undergoing laparoscopic surgery noted only one case of bladder injury.[72] These findings were generally consistent with a different study of 2,650 laparoscopic surgeries where only one case of bladder injury was reported.[73]
Although cosmetically appealing, placement of the trocar in the suprapubic hairline may be too low and create an unnecessary risk of injury to the bladder. The risk of bladder injury can be minimized by pre-operative urinary catheter insertion. Although minor bladder injuries can be managed with immediate suture repair and post-injury urinary catheter drainage, more extensive injuries require formal bladder repair, up to and including a laparotomy.[26,72,73,74,75,76] It is important to maintain a high index of suspicion for bladder injury in patients who exhibit signs of unexplained abdominal pain after a laparoscopic procedure.
PORT SITE HERNIAS
The incidence of port site hernias varies from 0.74% to 1.7%.[77,78] The umbilical site [Figure 4] is involved in nearly half of all hernias attributable to laparoscopic procedures, and most often includes bowel or omental content.[9,68,77] Risk factors associated with port site hernias include advanced patient age, higher body mass index, pre-existing hernias, trocar type and diameter, infection, longer duration of surgery, and extension of the port site for specimen extraction.[68,77] Hernia rates do not differ significantly between open and closed insertion techniques.[77] An example of a trocar site infection is shown in Figure 5.
Figure 4.
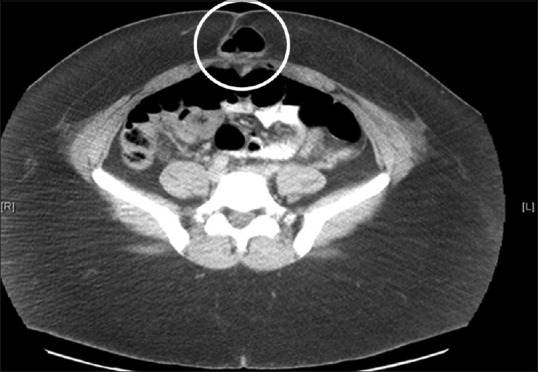
Computed tomographic image showing a ventral incisional hernia (circled) originating at the peri-umbilical trocar site following laparoscopy
Figure 5.
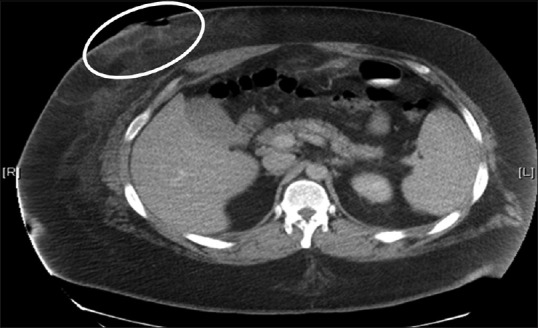
Surgical site infection (circled) at the right upper quadrant trocar site following laparoscopy
Most port site hernias are asymptomatic and the average time to diagnosis is approximately nine months.[77] These hernias may be divided into early and late sub-categories based on the time frame of presentation.[68] Early port site hernias occur within two weeks of the operation through a dehisced fascial closure or persistent fascial defect.[68] Early port site hernias are often Richter hernias, where only the anti-mesenteric portion of a small bowel segment becomes entrapped rather than the entire circumference of the bowel segment. Early port site hernias frequently result in bowel obstructions, therefore urgent intervention is more likely to be necessary for any hernia occurring within 14 days from the initial operation while late hernias can be repaired in an elective fashion.[68] Late port site hernias occur beyond two weeks.[68] While the peritoneum does not line the early hernia space, late hernias do contain a hernia sac of peritoneum thereby, resulting in intermittent symptoms through sliding of hernia contents in and out of the sac.
Once a port site hernia is discovered, it should be repaired to prevent obstruction and strangulation. Computer tomography and ultrasound evaluation are useful modalities for identifying hernias that may not be readily evident on physical examination.[79] Generally, only fascial defects >10 mm require closure, thus many surgeons opt not close defects left by 5 mm ports. Nonetheless, all port sites, regardless of size, are at some degree of risk for hernia formation.[8,80] Additionally, port sites over the liver are also frequently not closed under the assumption that contact with bowel is unlikely due to the liver obstructing the defect orifice; however, this is not a guarantee.
Pneumoperitoneum should be maintained during the closure to ensure that bowel is kept away from the port site and that the site is free of leaks.[68] Prevention of incisional hernias can be accomplished through limited size and number of port incisions, dedicated closure of all incisions >10 mm, removal of accessory ports under direct visualization, evacuation of pneumoperitoneum prior to port removal, and removal of the primary port and camera simultaneously to exclude entrapment of omentum or bowel.[9]
INADEQUATE INJURY VISUALIZATION AND MISSED INJURY
In diagnosing hollow viscus injuries, TL has been known to be less diagnostically reliable, with sensitivity of only 18%, negative predictive value of 83%, and specificity/positive predictive value of 100%.[17] Consequently, the use of TL for diagnosing hollow viscous injury detection remains controversial and is believed to be very dependent on operator skills.[13] Zantut and colleagues recommend that if bowel perforation cannot be confidently ruled out on TL, laparotomy should be performed.[3] In one study, there were seven missed injuries in the TL group, all of which involved hollow viscus.[17] Of additional importance, although the sensitivity of laparoscopy was 97% in the thoracoabdominal area, it was only 43% in the epigastric, flank, and lower quadrants.[17] In another series, out of eight diaphragm injuries, TL missed one (12.5%), which occurred in a patient who sustained a gunshot wound with injury and hemoperitoneum preventing adequate visualization.[14] In a study by Kawahara et al.,[16] the only missed injury on TL was a pancreatic lesion that required a laparotomy. The authors hypothesized that the high rate of missed bowel injury in the past was secondary to using two laparoscopic port sites instead of three, and recommended that a standardized approach to TL not only reduces unnecessary laparotomies but can also more reliably rule out small bowel injury if the above three-port approach is utilized.[16]
RETAINED SURGICAL ITEMS
Although reportedly less frequent in laparoscopic surgery, retained surgical items still occur. Prevention and intraoperative vigilance is critical to preventing these never-events.[81] In addition to the pivotal role of good team coordination and communication,[82] universal knowledge of factors that elevate risk of surgical items retention (intraoperative blood loss >500 mL, longer duration of operation, >1 sub-procedure, lack of surgical counts or incorrect surgical counts, unexpected intraoperative factors, multiple surgical teams) needs to be actively fostered.[83]
CONCLUSIONS
Laparoscopy is a safe and effective modality for diagnostic evaluation and management of penetrating traumatic injuries in select individuals. The widespread acceptance of laparoscopy in traumatic situations can blunt the sequelae associated with negative laparotomies. However, this increases the likelihood that trauma surgeons will encounter complications related to laparoscopy. We have highlighted laparoscopic-related complications including physiologic changes during gas insufflation, access-related complications, SE, pneumothroax, gas embolism, port site hernias, and specific injuries to diaphragm, blood vessels, omentum, viscera, and bladder. Knowledge of each of these entities is crucial to facilitate prevention where possible, expedite prompt diagnostic evaluation, and appropriate management. Maintaining comprehensive and exceptional levels of patient care will be upheld through understanding the risks associated with laparoscopy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Stawicki SP. Trends in nonoperative management of traumatic injuries: A synopsis. OPUS 12 Scientist. 2007;1:19–35. doi: 10.4103/IJCIIS.IJCIIS_7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renz BM, Feliciano DV. Unnecessary laparotomies for trauma: A prospective study of morbidity. J Trauma. 1995;38:350–6. doi: 10.1097/00005373-199503000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Zantut LF, Ivatury RR, Smith RS, Kawahara NT, Porter JM, Fry WR, et al. Diagnostic and therapeutic laparoscopy for penetrating abdominal trauma: A multicenter experience. J Trauma. 1997;42:825–9. doi: 10.1097/00005373-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Sosa JL, Arrillaga A, Puente I, Sleeman D, Ginzburg E, Martin L. Laparoscopy in 121 consecutive patients with abdominal gunshot wounds. J Trauma. 1995;39:501–4. doi: 10.1097/00005373-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Orlando R, 3rd, Crowell KL. Laparoscopy in the critically ill. Surg Endosc. 1997;11:1072–4. doi: 10.1007/s004649900532. [DOI] [PubMed] [Google Scholar]
- 6.Kraut EJ, Anderson JT, Safwat A, Barbosa R, Wolfe BM. Impairment of cardiac performance by laparoscopy in patients receiving positive end-expiratory pressure. Arch Surg. 1999;134:76–80. doi: 10.1001/archsurg.134.1.76. [DOI] [PubMed] [Google Scholar]
- 7.Smith BP, Adams RC, Doraiswamy VA, Nagaraja V, Seamon MJ, Wisler J, et al. Review of abdominal damage control and open abdomens: Focus on gastrointestinal complications. J Gastrointestin Liver Dis. 2010;19:425–35. [PubMed] [Google Scholar]
- 8.Bergemann JL, Hibbert ML, Harkins G, Narvaez J, Asato A. Omental herniation through a 3-mm umbilical trocar site: Unmasking a hidden umbilical hernia. J Laparoendosc Adv Surg Tech A. 2001;11:171–3. doi: 10.1089/10926420152389332. [DOI] [PubMed] [Google Scholar]
- 9.Karthik S, Augustine AJ, Shibumon MM, Pai MV. Analysis of laparoscopic port site complications: A descriptive study. J Minim Access Surg. 2013;9:59–64. doi: 10.4103/0972-9941.110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashizume M, Sugimachi K. Needle and trocar injury during laparoscopic surgery in Japan. Surg Endosc. 1997;11:1198–201. doi: 10.1007/s004649900568. [DOI] [PubMed] [Google Scholar]
- 11.Murdock CM, Wolff AJ, Van Geem T. Risk factors for hypercarbia, subcutaneous emphysema, pneumothorax, and pneumomediastinum during laparoscopy. Obstet Gynecol. 2000;95:704–9. doi: 10.1016/s0029-7844(00)00781-x. [DOI] [PubMed] [Google Scholar]
- 12.Murray JA, Demetriades D, Asensio JA, Cornwell EE, 3rd, Velmahos GC, Belzberg H, et al. Occult injuries to the diaphragm: Prospective evaluation of laparoscopy in penetrating injuries to the left lower chest. J Am Coll Surg. 1998;187:626–30. doi: 10.1016/s1072-7515(98)00246-4. [DOI] [PubMed] [Google Scholar]
- 13.Como JJ, Bokhari F, Chiu WC, Duane TM, Holevar MR, Tandoh MA, et al. Practice management guidelines for selective nonoperative management of penetrating abdominal trauma. J Trauma. 2010;68:721–33. doi: 10.1097/TA.0b013e3181cf7d07. [DOI] [PubMed] [Google Scholar]
- 14.Friese RS, Coln CE, Gentilello LM. Laparoscopy is sufficient to exclude occult diaphragm injury after penetrating abdominal trauma. J Trauma. 2005;58:789–92. doi: 10.1097/01.ta.0000158243.78299.b5. [DOI] [PubMed] [Google Scholar]
- 15.Ertekin C, Onaran Y, Guloglu R, Gunay K, Taviloglu K. The use of laparoscopy as a primary diagnostic and therapeutic method in penetrating wounds of lower thoracal region. Surg Laparosc Endosc. 1998;8:26–9. [PubMed] [Google Scholar]
- 16.Kawahara NT, Alster C, Fujimura I, Poggetti RS, Birolini D. Standard examination system for laparoscopy in penetrating abdominal trauma. J Trauma. 2009;67:589–95. doi: 10.1097/TA.0b013e3181a60593. [DOI] [PubMed] [Google Scholar]
- 17.Ivatury RR, Simon RJ, Stahl WM. A critical evaluation of laparoscopy in penetrating abdominal trauma. J Trauma. 1993;34:822–7. doi: 10.1097/00005373-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Wenham TN, Graham D. Venous gas embolism: An unusual complication of laparoscopic cholecystectomy. J Minim Access Surg. 2009;5:35–6. doi: 10.4103/0972-9941.55105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, et al. Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motew M, Ivankovich AD, Bieniarz J, Albrecht RF, Zahed B, Scommegna A. Cardiovascular effects and acid-base and blood gas changes during laparoscopy. Am J Obstet Gynecol. 1973;115:1002–12. doi: 10.1016/0002-9378(73)90683-2. [DOI] [PubMed] [Google Scholar]
- 21.Jansen FW, Kapiteyn K, Trimbos-Kemper T, Hermans J, Trimbos JB. Complications of laparoscopy: A prospective multicentre observational study. Br J Obstet Gynaecol. 1997;104:595–600. doi: 10.1111/j.1471-0528.1997.tb11539.x. [DOI] [PubMed] [Google Scholar]
- 22.Agresta F, De Simone P, Ciardo LF, Bedin N. Direct trocar insertion vs Veress needle in nonobese patients undergoing laparoscopic procedures: A randomized prospective single-center study. Surg Endosc. 2004;18:1778–81. doi: 10.1007/s00464-004-9010-y. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad G, O’Flynn H, Duffy JM, Phillips K, Watson A. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2012;2:CD006583. doi: 10.1002/14651858.CD006583.pub3. [DOI] [PubMed] [Google Scholar]
- 24.Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001;192:677–83. doi: 10.1016/s1072-7515(01)00913-9. [DOI] [PubMed] [Google Scholar]
- 25.Krishnakumar S, Tambe P. Entry complications in laparoscopic surgery. J Gynecol Endosc Surg. 2009;1:4–11. doi: 10.4103/0974-1216.51902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrzenski A, Ostrzenska KM. Bladder injury during laparoscopic surgery. Obstet Gynecol Surv. 1998;53:175–80. doi: 10.1097/00006254-199803000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Molloy D, Kaloo PD, Cooper M, Nguyen TV. Laparoscopic entry: A literature review and analysis of techniques and complications of primary port entry. Aust N Z J Obstet Gynaecol. 2002;42:246–54. doi: 10.1111/j.0004-8666.2002.00246.x. [DOI] [PubMed] [Google Scholar]
- 28.Mayol J, Garcia-Aguilar J, Ortiz-Oshiro E, De-Diego Carmona JA, Fernandez-Represa JA. Risks of the minimal access approach for laparoscopic surgery: Multivariate analysis of morbidity related to umbilical trocar insertion. World J Surg. 1997;21:529–33. doi: 10.1007/pl00012281. [DOI] [PubMed] [Google Scholar]
- 29.Mettler L, Schmidt EH, Frank V, Semm K. Optical trocar systems: Laparoscopic entry and its complications (a study of cases in Germany) Gynaecol Endosc. 1999;8:383–9. [Google Scholar]
- 30.Minervini A, Davenport K, Pefanis G, Keeley FX, Jr, Timoney AG. Prospective study comparing the bladeless optical access trocar versus Hasson open trocar for the establishment of pneumoperitoneum in laparoscopic renal procedures. Arch Ital Urol Androl. 2008;80:95–8. [PubMed] [Google Scholar]
- 31.Vilos GA, Ternamian A, Dempster J, Laberge PY. The Society of Obstetricians and Gynaecologists of Canada. Laparoscopic entry: A review of techniques, technologies, and complications. J Obstet Gynaecol Can. 2007;29:433–65. doi: 10.1016/S1701-2163(16)35496-2. [DOI] [PubMed] [Google Scholar]
- 32.Lecuru F, Leonard F, Philippe Jais J, Rizk E, Robin F, Taurelle R. Laparoscopy in patients with prior surgery: Results of the blind approach. JSLS. 2001;5:13–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Li LB, Cai XJ, Mou YP, Wei Q. Reoperation of biliary tract by laparoscopy: Experiences with 39 cases. World J Gastroenterol. 2008;14:3081–4. doi: 10.3748/wjg.14.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tinelli A, Malvasi A, Guido M, Tsin DA, Hudelist G, Stark M, et al. Laparoscopy entry in patients with previous abdominal and pelvic surgery. Surg Innov. 2011;18:201–5. doi: 10.1177/1553350610393989. [DOI] [PubMed] [Google Scholar]
- 35.Rafii A, Camatte S, Lelievre L, Darai E, Lecuru F. Previous abdominal surgery and closed entry for gynaecological laparoscopy: A prospective study. BJOG. 2005;112:100–2. doi: 10.1111/j.1471-0528.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- 36.Aron M, Colombo JR, Jr, Turna B, Stein RJ, Haber GP, Gill IS. Diaphragmatic repair and/or reconstruction during upper abdominal urological laparoscopy. J Urol. 2007;178:2444–50. doi: 10.1016/j.juro.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 37.Del Pizzo JJ, Jacobs SC, Bishoff JT, Kavoussi LR, Jarrett TW. Pleural injury during laparoscopic renal surgery: Early recognition and management. J Urol. 2003;169:41–4. doi: 10.1016/S0022-5347(05)64030-X. [DOI] [PubMed] [Google Scholar]
- 38.Voyles CR, Madden B. The “floppy diaphragm” sign with laparoscopic-associated pneumothorax. JSLS. 1998;2:71–3. [PMC free article] [PubMed] [Google Scholar]
- 39.Castillo OA, Vitagliano G, Moreno M, Diaz MA, Cortes O. Management of diaphragmatic injury during transperitoneal laparoscopic urological procedures. Int Braz J Urol. 2007;33:323–8. doi: 10.1590/s1677-55382007000300004. [DOI] [PubMed] [Google Scholar]
- 40.Kwiatt M, Tarbox A, Seamon MJ, Swaroop M, Cipolla J, Allen C, et al. Thoracostomy tubes: A comprehensive review of complications and related topics. Int J Crit Illn Inj Sci. 2014;4:143–55. doi: 10.4103/2229-5151.134182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Msezane LP, Zorn KC, Gofrit ON, Schade GR, Shalhav AL. Case report: Conservative management of a large capnothorax following laparoscopic renal surgery. J Endourol. 2007;21:1445–7. doi: 10.1089/end.2007.0122. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez CM, Batler RA, Feldman M, Rubenstein JN, Nadler RB, Schoor RA. Repair of a diaphragmatic injury during hand assisted laparoscopic nephrectomy using an onlay patch of polypropylene and polyglactin mesh. J Urol. 2002;167:2512–3. [PubMed] [Google Scholar]
- 43.Potter SR, Kavoussi LR, Jackman SV. Management of diaphragmatic injury during laparoscopic nephrectomy. J Urol. 2001;165:1203–4. [PubMed] [Google Scholar]
- 44.Bonjer HJ, Hazebroek EJ, Kazemier G, Giuffrida MC, Meijer WS, Lange JF. Open versus closed establishment of pneumoperitoneum in laparoscopic surgery. Br J Surg. 1997;84:599–602. [PubMed] [Google Scholar]
- 45.Celik H, Cremins A, Jones KA, Harmanli O. Massive subcutaneous emphysema in robotic sacrocolpopexy. JSLS. 2013;17:245–8. doi: 10.4293/108680813X13654754535151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee DW, Kim MJ, Lee YK, Lee HN. Does intraabdominal pressure affect development of subcutaneous emphysema at gynecologic laparoscopy? J Minim Invasive Gynecol. 2011;18:761–5. doi: 10.1016/j.jmig.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Critchley LA, Ho AM. Surgical emphysema as a cause of severe hypercapnia during laparoscopic surgery. Anaesth Intensive Care. 2010;38:1094–100. doi: 10.1177/0310057X1003800622. [DOI] [PubMed] [Google Scholar]
- 48.Hawasli A. Spontaneous resolution of massive laparoscopy-associated pneumothorax: The case of the bulging diaphragm and review of the literature. J Laparoendosc Adv Surg Tech A. 2002;12:77–82. doi: 10.1089/109264202753486993. [DOI] [PubMed] [Google Scholar]
- 49.Joris JL, Chiche JD, Lamy ML. Pneumothorax during laparoscopic fundoplication: Diagnosis and treatment with positive end-expiratory pressure. Anesth Analg. 1995;81:993–1000. doi: 10.1097/00000539-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 50.Mangar D, Kirchhoff GT, Leal JJ, Laborde R, Fu E. Pneumothorax during Laparoscopic Nissen fundoplication. Can J Anaesth. 1994;41:854–6. doi: 10.1007/BF03011593. [DOI] [PubMed] [Google Scholar]
- 51.Rankin JM, Silbert PL, Yadava OP, Hankey GJ, Stewart-Wynne EG. Mechanism of stroke complicating cardiopulmonary bypass surgery. Aust N Z J Med. 1994;24:154–60. doi: 10.1111/j.1445-5994.1994.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 52.Cobb WS, Fleishman HA, Kercher KW, Matthews BD, Heniford BT. Gas embolism during laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2005;15:387–90. doi: 10.1089/lap.2005.15.387. [DOI] [PubMed] [Google Scholar]
- 53.Webber S, Andrzejowski J, Francis G. Gas embolism in anaesthesia. Br J Anaesth CEPD Rev. 2002;2:53–7. [Google Scholar]
- 54.Gordy S, Rowell S. Vascular air embolism. Int J Crit Illn Inj Sci. 2013;3:73–6. doi: 10.4103/2229-5151.109428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrin M, Fletcher A. Laparoscopic abdominal surgery. Contin Educ Anaesth Crit Care Pain. 2004;4:107–10. [Google Scholar]
- 56.Stawicki SP, Seamon MJ, Kim PK, Meredith DM, Chovanes J, Schwab CW, et al. Transthoracic echocardiography for pulmonary embolism in the ICU: Finding the “right” findings. J Am Coll Surg. 2008;206:42–7. doi: 10.1016/j.jamcollsurg.2007.06.293. [DOI] [PubMed] [Google Scholar]
- 57.Ikechebelu JI, Obi RA, Udigwe GO, Joe-Ikechebelu NN. Comparison of carbon dioxide and room air pneumoperitoneum for day-case diagnostic laparoscopy. J Obstet Gynaecol. 2005;25:172–3. doi: 10.1080/01443610500051528. [DOI] [PubMed] [Google Scholar]
- 58.Jansen FW, Kolkman W, Bakkum EA, de Kroon CD, Trimbos-Kemper TC, Trimbos JB. Complications of laparoscopy: An inquiry about closed- versus open-entry technique. Am J Obstet Gynecol. 2004;190:634–8. doi: 10.1016/j.ajog.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 59.Vallancien G, Cathelineau X, Baumert H, Doublet JD, Guillonneau B. Complications of transperitoneal laparoscopic surgery in urology: Review of 1,311 procedures at a single center. J Urol. 2002;168:23–6. [PubMed] [Google Scholar]
- 60.Saber AA, Meslemani AM, Davis R, Pimentel R. Safety zones for anterior abdominal wall entry during laparoscopy: A CT scan mapping of epigastric vessels. Ann Surg. 2004;239:182–5. doi: 10.1097/01.sla.0000109151.53296.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schafer M, Lauper M, Krahenbuhl L. A nation's experience of bleeding complications during laparoscopy. Am J Surg. 2000;180:73–7. doi: 10.1016/s0002-9610(00)00416-5. [DOI] [PubMed] [Google Scholar]
- 62.Pemberton RJ, Tolley DA, van Velthoven RF. Prevention and management of complications in urological laparoscopic port site placement. Eur Urol. 2006;50:958–68. doi: 10.1016/j.eururo.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 63.Wind J, Cremers JE, van Berge Henegouwen MI, Gouma DJ, Jansen FW, Bemelman WA. Medical liability insurance claims on entry-related complications in laparoscopy. Surg Endosc. 2007;21:2094–9. doi: 10.1007/s00464-007-9315-8. [DOI] [PubMed] [Google Scholar]
- 64.Brill AI, Nezhat F, Nezhat CH, Nezhat C. The incidence of adhesions after prior laparotomy: A laparoscopic appraisal. Obstet Gynecol. 1995;85:269–72. doi: 10.1016/0029-7844(94)00352-E. [DOI] [PubMed] [Google Scholar]
- 65.Feliciano DV. Management of traumatic retroperitoneal hematoma. Ann Surg. 1990;211:109–23. doi: 10.1097/00000658-199002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borgatta L, Gruss L, Barad D, Kaali SG. Direct trocar insertion vs. Verres needle use for laparoscopic sterilization. J Reprod Med. 1990;35:891–4. [PubMed] [Google Scholar]
- 67.Shirk GJ, Johns A, Redwine DB. Complications of laparoscopic surgery: How to avoid them and how to repair them. J Minim Invasive Gynecol. 2006;13:352–9. doi: 10.1016/j.jmig.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Tonouchi H, Ohmori Y, Kobayashi M, Kusunoki M. Trocar site hernia. Arch Surg. 2004;139:1248–56. doi: 10.1001/archsurg.139.11.1248. [DOI] [PubMed] [Google Scholar]
- 69.Rabl C, Palazzo F, Aoki H, Campos GM. Initial laparoscopic access using an optical trocar without pneumoperitoneum is safe and effective in the morbidly obese. Surg Innov. 2008;15:126–31. doi: 10.1177/1553350608317354. [DOI] [PubMed] [Google Scholar]
- 70.Antoniou SA, Antoniou GA, Koch OO, Pointner R, Granderath FA. Blunt versus bladed trocars in laparoscopic surgery: A systematic review and meta-analysis of randomized trials. Surg Endosc. 2013;27:2312–20. doi: 10.1007/s00464-013-2793-y. [DOI] [PubMed] [Google Scholar]
- 71.Wu MP, Ou CS, Chen SL, Yen EY, Rowbotham R. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67–73. doi: 10.1016/s0002-9610(99)00267-6. [DOI] [PubMed] [Google Scholar]
- 72.Schafer M, Lauper M, Krahenbuhl L. Trocar and Veress needle injuries during laparoscopy. Surg Endosc. 2001;15:275–80. doi: 10.1007/s004640000337. [DOI] [PubMed] [Google Scholar]
- 73.Orlando R, Palatini P, Lirussi F. Needle and trocar injuries in diagnostic laparoscopy under local anesthesia: What is the true incidence of these complications? J Laparoendosc Adv Surg Tech A. 2003;13:181–4. doi: 10.1089/109264203766207708. [DOI] [PubMed] [Google Scholar]
- 74.Kocot A, Gerharz EW, Riedmiller H. Urological complications of laparoscopic inguinal hernia repair: A case series. Hernia. 2011;15:583–6. doi: 10.1007/s10029-010-0696-6. [DOI] [PubMed] [Google Scholar]
- 75.Levy BF, De Guara J, Willson PD, Soon Y, Kent A, Rockall TA. Bladder injuries in emergency/expedited laparoscopic surgery in the absence of previous surgery: A case series. Ann R Coll Surg Engl. 2012;94:e118–20. doi: 10.1308/003588412X13171221502149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer A, Blanc P, Balique JG, Kitamura M, Juan RT, Delacoste F, et al. Laparoscopic totally extraperitoneal inguinal hernia repair: Twenty-seven serious complications after 4565 consecutive operations. Rev Col Bras Cir. 2013;40:32–6. doi: 10.1590/s0100-69912013000100006. [DOI] [PubMed] [Google Scholar]
- 77.Bunting DM. Port-site hernia following laparoscopic cholecystectomy. JSLS. 2010;14:490–7. doi: 10.4293/108680810X12924466007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Owens M, Barry M, Janjua AZ, Winter DC. A systematic review of laparoscopic port site hernias in gastrointestinal surgery. Surgeon. 2011;9:218–24. doi: 10.1016/j.surge.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 79.Bevan KE, Venkatasubramaniam A, Mohamed F, Moran BJ, Cecil TD. Respect for the laparoscopic port site: Lessons in diagnosis, management, and prevention of port-site hernias following laparoscopic colorectal surgery. J Laparoendosc Adv Surg Tech A. 2010;20:451–4. doi: 10.1089/lap.2009.0419. [DOI] [PubMed] [Google Scholar]
- 80.Hussain A, Mahmood H, Singhal T, Balakrishnan S, Nicholls J, El-Hasani S. Long-term study of port-site incisional hernia after laparoscopic procedures. JSLS. 2009;13:346–9. [PMC free article] [PubMed] [Google Scholar]
- 81.Stawicki SP, Moffatt-Bruce SD, Ahmed HM, Anderson HL, 3rd, Balija TM, Bernescu I, et al. Retained surgical items: A problem yet to be solved. J Am Coll Surg. 2013;216:15–22. doi: 10.1016/j.jamcollsurg.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 82.Stawicki SP, Cook CH, Anderson HL, 3rd, Chowayou L, Cipolla J, Ahmed HM, et al. OPUS 12 Foundation Multicenter Trials Group. Natural history of retained surgical items supports the need for team training, early recognition, and prompt retrieval. Am J Surg. 2014;208:65–72. doi: 10.1016/j.amjsurg.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 83.Moffatt-Bruce SD, Cook CH, Steinberg SM, Stawicki SP. Risk factors for retained surgical items: A meta-analysis and proposed risk stratification system. J Surg Res. 2014;190:429–36. doi: 10.1016/j.jss.2014.05.044. [DOI] [PubMed] [Google Scholar]


