Abstract
A survey was conducted to ascertain practice of antimicrobial stewardship programme (AMSP) in India for 2013. A total of 20 health care institutions (HCI) responded to a detailed questionnaire. All the institutions contacted were tertiary care HCI, of which 12 were funded by government (GHCI) and 8 were corporate/private HCI (PHCI). Further, all catered to both rural and urban populations and were spread across the country. Written documents were available with 40 per cent for AMSP, 75 per cent for hospital infection control (HIC) and HIC guidelines and 65 per cent for antimicrobial agents (AMA) prescription guidelines. Records were maintained for health care associated infections (HCAI) by 60 per cent HCI. Antimicrobial resistance (AMR) data were being analysed by 80 per cent HCI. AMA usage data were analysed by only 25 per cent HCI and AMA prescription audit and feedback by 30 per cent. PHCI performed better than GHCI across all fields of AMSP. The main contributory factor was possibly the much higher level of accreditation of PHCI hospitals and their diagnostic laboratories. The absence of infectious diseases physicians and clinical pharmacists is worrying and demands careful attention.
Keywords: Antimicrobial resistance, antimicrobial stewardship programme, hospital infection control, infectious diseases physicians
Development of antimicrobial resistance (AMR) in myriad groups of bacteria, fungi, viruses and parasites is a complex global health challenge1, largely driven by man in human health care, animal farming, veterinary medicine, agriculture, pisciculture, etc. Our responsibility in human health care becomes paramount as development and the discovery of newer antimicrobial agents (AMA) and newer classes of AMA is rapidly drying up, even though the use/abuse is increasing all over2,3. One of the best methods to prolong the shelf-life of existing and newer future AMA is antimicrobial stewardship programme (AMSP)4,5. The Indian Council of Medical Research (ICMR), New Delhi, India, has launched the Anti Microbial Resistance Surveillance and Research Network (AMRSN) across the country in 2013 with an avowed purpose of rationalizing AMSP in India. This initiative was in line with the recommendations of Chennai Declaration which coincided with the global initiatives to combat antimicrobial resistance6.
Hospital based programmes dedicated to improving antibiotic use, commonly referred to as AMSP have been found helpful in improving the quality of patient care and safety through increased infection cure rates, reducing treatment failures, and increasing the frequency of correct prescribing for therapy and prophylaxis7. Under the Antibiotic Stewardship, Prevention of Infection and Control (ASPIC) programme of ICMR 15 microbiologists, four pharmacologists and one physician were trained in 20128.
Implementation of an effective AMSP requires a multidisciplinary approach involving a variety of experts. It is recommended that the core team should include a clinical pharmacist and a physician trained in infectious diseases, a clinical microbiologist an informatics specialist, a hospital epidemiologist, and an infection control specialist. In a survey of hospitals with stewardship programmes. clinical pharmacists and infectious diseases physicians are the most common element of the team7.
The ICMR set up a Steering Committee for guiding AMSP in the country in 2013. Treatment guidelines for important clinical infections and hospital infection control (HIC) guidelines are being prepared. On the recommendation of the AMSP Steering Committee, a survey was carried out on existing AMSP practices in the country to gauge ground realities and plug the loop holes and strengthen AMSP. Here we report the result of the first survey carried out under the AMSP programme.
The survey
An invitation was extended to 26 health care institutes (HCIs) across the country which were either regional centres of AMRSRN or part of AMSP Steering Committee representing different regions of the country, from both government and private sectors to reply to a questionnaire on AMSP practices being followed by them for the period from January 1, 2013 to December 31, 2013. The filled in questionnaires were received from 20 hospitals.
The questionnaire carried the following information:
-
(i)
General information: Location, population type seeking care, sponsor, level of health care, bed strength [including intensive care units (ICUs)], specialties and super specialty services offered (including transplant, oncology programmes, etc.), accreditations of hospitals and diagnostic laboratories.
-
(ii)
AMSP, HIC: Written documents, teams, frequency of meetings, communication system of minutes of meetings, HIC guidelines & audit of compliance, record of health care associated infections (HCAI), investigation of outbreaks of HCAI.
-
(iii)
AMR data analysis: Frequency, as out patient department (OPD), in patient department (IPD), ICU, community acquired infections (CAI), HCAI, as per site of infection, as per pathogen, antimicrobial susceptibility testing (AMST) guidelines, communication system.
-
(iv)
AMA usage data analysis: Frequency, AMA, units.
-
(v)
AMSP strategies: Formulary restriction, empirical prescription approval, automatic stop, controlling authority.
-
(vi)
AMA prescription guidelines: ICU, HCAI, CAI, infections post-transplant, infections in oncology and immunocompromised.
-
(vii)
AMA usage audit and feedback: Frequency, audit team, prescription appropriateness.
-
(viii)
AMSP implementation outcome studies: AMA usage, appropriateness of AMA usage, AMR, HCAI incidence, clinical markers, trends in AMSP outcomes.
-
(ix)
Usage of computer assisted programmes in AMSP.
-
(x)
AMSP implementation leading to perception of loss of prescription autonomy.
Once complete information was received, the data were analysed. The information was anonymous and confidential.
Outcome
Completed questionnaires were received from 20 HCI [12 government, i.e.(GHCI), and 8 private, i.e. (PHCI)] from various States and Union Territories such as Jammu and Kashmir, National Capital Territory of Delhi, Haryana, Uttar Pradesh, West Bengal, Chandigarh, Maharashtra, Andhra Pradesh, Karnataka, Tamil Nadu and Puducherry. All participating institutions were tertiary care HCI catering to both rural and urban populations. In addition, all offered most specialities and super-speciality training, including programmes in transplantations and oncology. The authorized bed strengths varied between 182 to 3000 (ICU 11-200), with an average bed occupancy of 65-100 per cent. Accreditation was certified for nine hospitals and seven laboratories, the majority being in PHCI (7 & 5) rather than GHCI (2 &2), respectively.
AMSP written documents were available with eight of 20 (40%) HCI (GHCI 2, PHCI 6) though AMSP teams were formed in 50 per cent HCI (Table I). Infectious disease (ID) physicians were part of these teams in 30 per cent HCI (GHCI nil, PHCI 3). Physicians, surgeons, paediatricians, clinical microbiologists and hospital administrators were well represented on these teams. Clinical pharmacists were available in 50 per cent of teams (GHCI 1, PHCI 4). One GHCI had clinical pharmacologist. Other clinicians were included as per local requirements. Only one AMSP team had a hospital epidemiologist and two hospitals had information technology (IT) personnel as members of AMSP teams - all in PHCI. All teams had regular meetings, though at varied frequencies.
Table I.
antimicrobial stewardship programme (AMSP) and hospital infection control (HIC) features in government and private health care institutions (GHCI and PHCI).
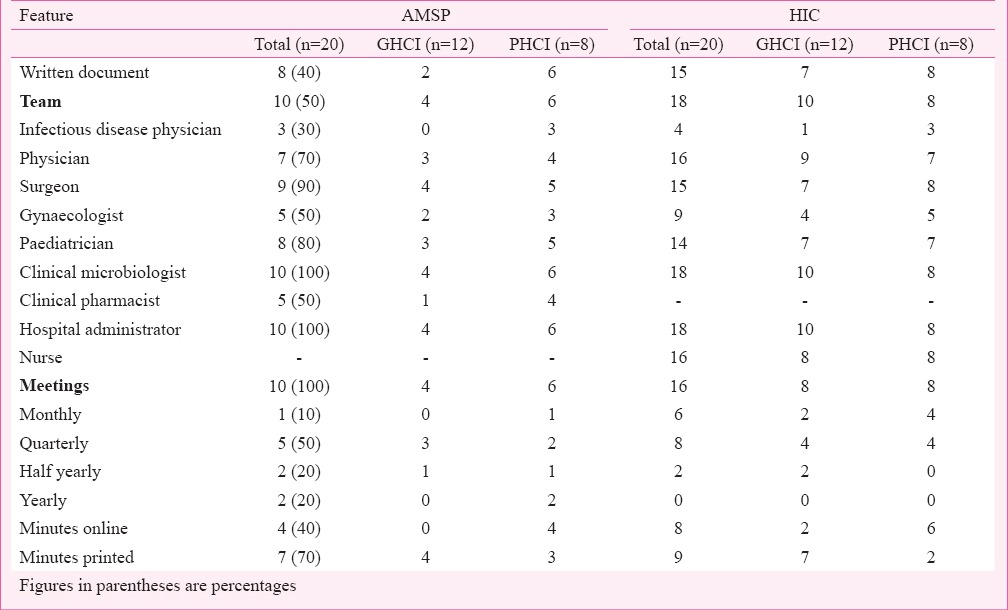
Hospital infection control (HIC) was better addressed than AMSP in the HCI. Written documents were available in 15 of 26 (75%) HCIs (GHCI 7, PHCI 8) and HIC teams in 18 (90%) (GHCI 10, PHCI 8). ID physicians were available in four (22.2%) (GHCI 1, PHCI 3). Physicians, surgeons, paediatricians, clinical microbiologists, hospital administrators and staff nurses were well represented. Additionally, other specialists and infrastructure officials were included, albeit in small numbers. Regular meetings were held at most HCIs, though at variable frequencies (Table I). Reports were available online in 50 per cent HCIs.
Hospital infection control (HIC) guidelines (Table II) were available in 75 per cent (n=15) of HCI (GHCI 7, PHCI 8). House keeping, Laundry and Catering guidelines were available in 13 (65%) HCIs. Hand sanitation guidelines were available in 15 (75%) and guidelines on personal protective clothing, Urine catheterisation, Central and peripheral venous lines, Operation theatre, Mechanical ventilation, Endoscopes and ICU in 60-70 per cent and in lower percentages of 40-55 per cent for bedside minor procedures, lumbar puncture, dialysis and phlebotomy. GHCI showed lower scores when compared with PHCI.
Table II.
Availability of hospital infection control (HIC) and prescription guidelines in health care institutions (HCIs).
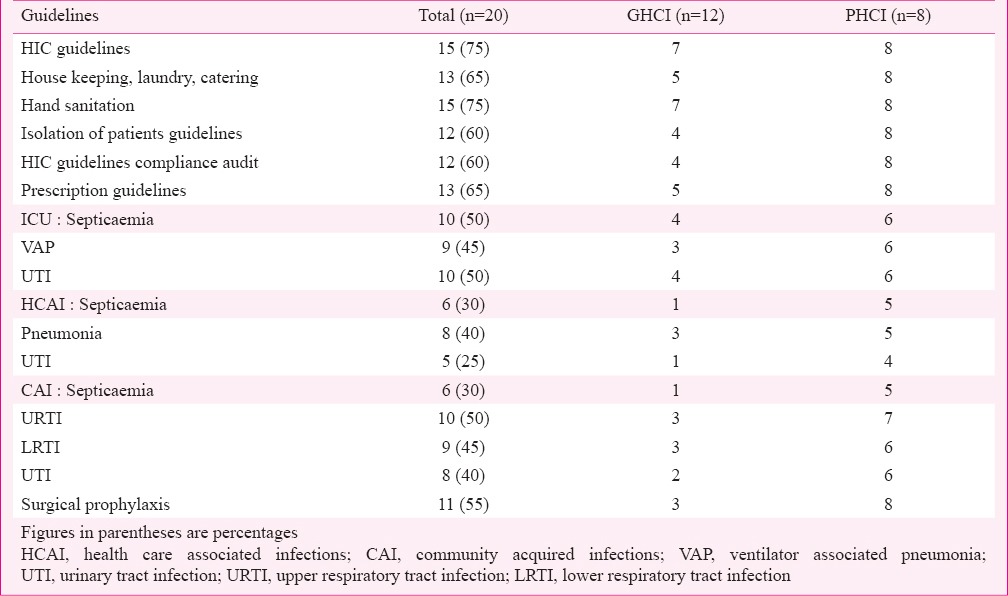
Guidelines were available in 12 HCI (60%) for isolation of patients (GHCI 4, PHCI 8). In decreasing order of availability, these pertained to methicillin resistant Staphylococcus aureus (MRSA), respiratory infections, carbapenem resistant Enterobacteriaceae (CRE), vancomycin resistant Enterococci (VRE), carbapenem resistant non-fermentative Gram-negative bacilli (CRNFGNB) and ESBL Enterobacteriaceae. Emphasis on isolation of patients was more prevalent in PHCI than in GHCI. Some level of audit of implementation of HIC guidelines was seen in 60 per cent of HCI (GHCI 4, PHCI 8).
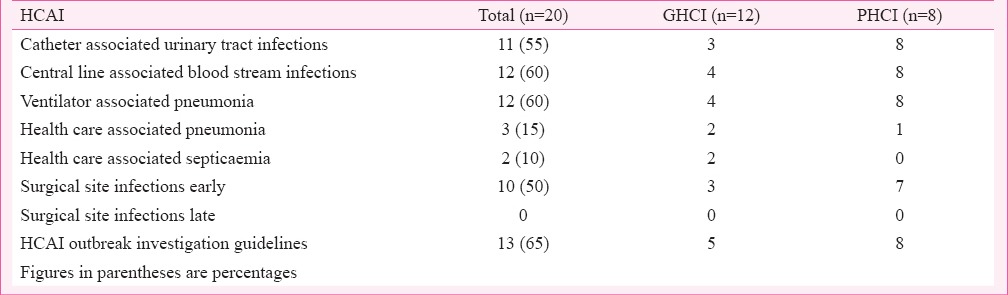
HCAI record (Table III) was being maintained in 12 HCIs (60%) (GHCI 4, PHCI 8), in 12 (60%) each for ventilator associated pneumonia (VAP) and central line associated blood stream infections (CLABSI), 11 (55%) for catheter associated urinary tract infections (CAUTI), 10 (50%) for surgical site-infections (SSI) early (nil for SSI late), in three (15%) for health care associated pneumonia (HCAP) and in two (10%) for health care associated septicaemia (HCAS). Incidence of HCAI was not compared because of paucity of comparable figures and for keeping analysis anonymous. Guidelines for HCAI outbreak investigations were available in 13 (65%) HCI (GHCI 5, PHCI 8). Half of the HCIs reported investigating HCAI outbreaks (n=16) (GHCI 9, PHCI 7). Reports were available online in five PHCI and in printed form in three PHCI and all GHCI.
Table III.
Number of health care institutions (HCIs) - both government and private HCIs maintaining record of health care associated infections (HCAIs).
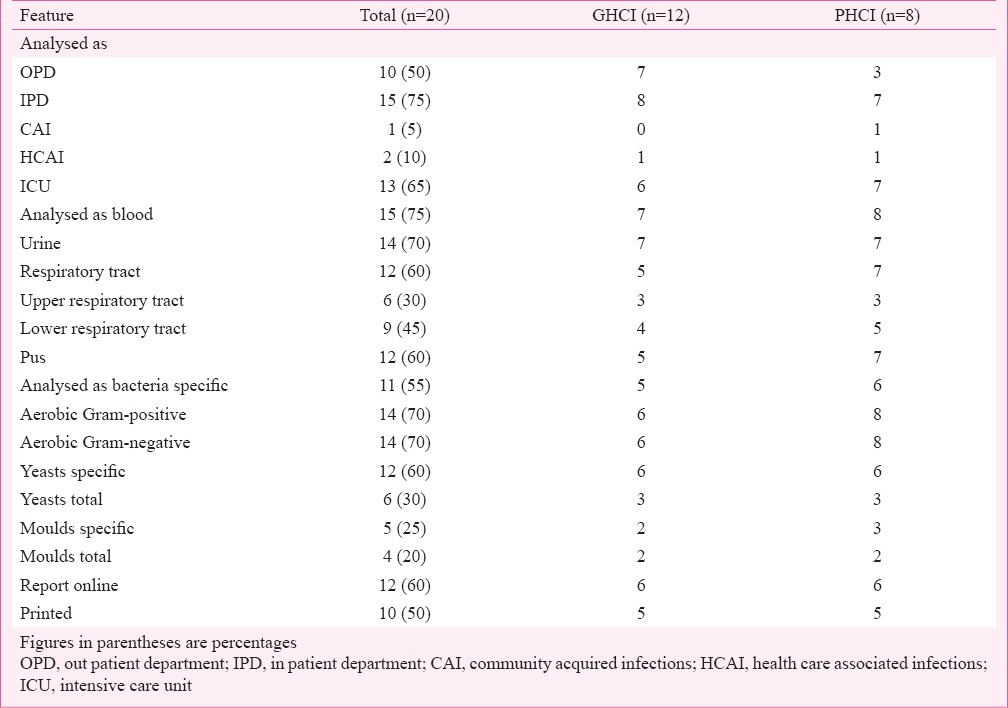
AMR data analysis (Table IV) was regularly performed by 16 of 20 (80%) HCI (GHCI 8, PHCI 8) at varied frequencies (monthly 20%, quarterly 20%, half yearly 35%, yearly 25%). Analysis as CAI was performed by only one PHCI, and as HCAI by one each GHCI and PHCI. Overall figures for PHCI (87.5-100%) were higher than for GHCI (41.7-58.3%). Analysis of specific bacterial data (55%) was less preferred than for aerobic Gram-positive and Gram-negative bacteria (70% each). The PHCI figures were higher than GHCI. Online reporting of data analysis was performed by 12 (60%) HCI and printed in 50 per cent.
Table IV.
Antimicrobial resistance (AMR) data analysis patterns in government and private health care institutions (HCIs).
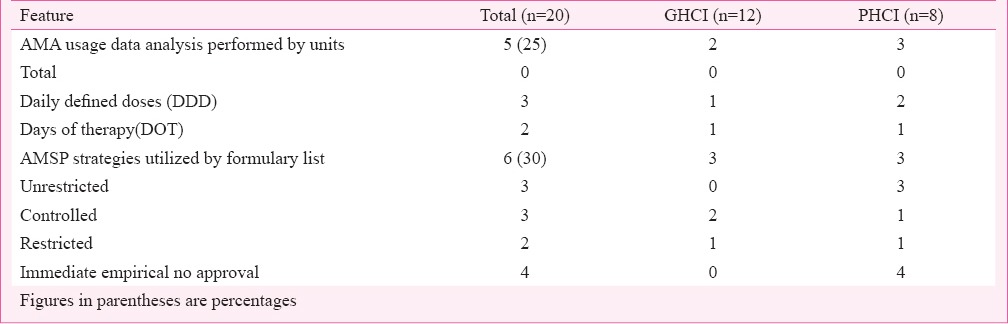
AMA usage data (Table V) were regularly analysed only by five (25%) HCIs. The preferred frequency was monthly by GHCI and annually by PHCI. Analysis was done for both targeted AMA and total AMA. The preferred units for data collection were daily defined doses (DDD) and days of therapy (DOT). AMSP strategies (Table V) in some form were utilized by only six (30%) HCIs. Only some degree of controlled and restricted formularies were in use. Prior or later approvals, automatic stops or cycling were not preferred. The controlling authority was AMSP team.
Table V.
Antimicrobial agents (AMA) usage data and AMSP strategies utilization analysis in health care institutions (HCIs).
AMA prescription guidelines (Table II) were available with 13 (65%) HCIs (GHCI 5, PHCI 8). Guidelines for ICU for septicaemia were available with 50 per cent, and VAP 45 per cent. Guidelines for treatment of HCAI were available in descending order for pneumonia and MRSA (40%), extended spectrum beta lactamase (ESBL) Enterobacteriaceae and carbapenem resistant Enterobacteriaceae (35%), septicaemia and VRE (30%), urinary tract infections (UTI), antibiotic associated diarrhoea and CRNFGNB (25%). Treatment guidelines for CAI in descending order were available for upper respiratory tract infections (URTI) (50%), lower respiratory tract infections (LRTI), diarrhoea and meningitis (45%), UTI (40%), gynaecological infections (35%), septicaemia, tuberculosis and paediatric infections (30%), sexually transmitted infections (20%), infections in transplantation and HIV (15%) and infections in oncology, diabetes mellitus and autoimmune diseases (5%). Figures were higher in PHCI than GHCI.
AMA prescription guidelines for surgical prophylaxis were available in 11 (55%) HCI (GHCI 3, PHCI 8). In descending order, guidelines were for clean contaminated surgery (50%), clean surgery (45%), contaminated and orthopedic surgeries (40%), orthopedic implants (35%), cardiac catheterization (30%), minimal access surgery, cardiac surgery, neurosurgery, gynaecological surgery and joint replacement surgery (25%).
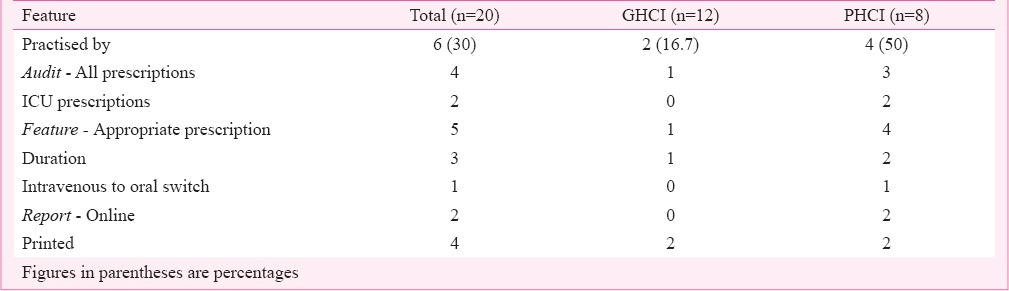
AMA prescription audit and feedback was practiced by only six (30%) HCIs (GHCI 2, PHCI 4), with variable frequencies. Auditors were members of AMSP team. All prescriptions were audited in four HCI and ICU prescriptions in two (Table VI). AMSP implementation outcome was analysed by only seven (35%) HCI (GHCI 2, PHCI 5). Features analysed were total cost of AMA used, reduction in AMA usage, AMR, incidence of HCAI, length of hospital stay, and all cause mortality. None of the HCIs analysed increase of appropriateness of AMA usage, infection related mortality, readmission rates, clinical cure. AMSP implementation outcome trends were analysed by only one HCI. Computer assisted programmes were utilized by only six (30 %) HCIs (GHCI 1, PHCI 5). PHCI had AMA prescription guidelines online in six centres, online AMA prescriptions in three and autoreview of AMA prescriptions in two. Physician AMA prescription autonomy loss was studied by only seven (35%) HCI.
Table VI.
Antimicrobial agent (AMA) prescription audit and feedback data analysis in health care institutions (HCIs).
Discussion & conclusions
Universally, the health care providers are increasingly recognizing the importance of AMSP in HCI as a major contributor to sustaining usefulness of AMA in the treatment of infections. Despite being few in number, compiled evidence from meta-analyses suggests that clinical outcomes are better or at least similar for patients when antimicrobial stewardship is performed9. Other components like discovery and development of newer classes of AMA, restricting use of AMA (at least those used for human infections) in animal farming and agriculture are equally important10,11,12. The major inputs for AMSP are appropriateness of usage of AMA in individual patients, AMR data of infecting pathogens (indicates usefulness of specific AMA for specific pathogen) and control and reduction of incidence of infections, especially HCAI. With a view to understand various nuances in practice of AMSP in India, a detailed questionnaire was prepared, vetted by the AMSP committee of the ICMR and sent to HCIs, both government HCI (GHCI) and private HCI (PHCI), across the country.
A total of 20 HCI responded. These belonged both to GHCI and PHCI, were tertiary care institutes with multiple specialties and were engaged in research and academics of some degree with bed strengths ranging between 182 to 3000. The data collected showed that PHCI had better accreditations in place, possibly out of commercial necessity, but the same was not true for GHCI. It is now universally accepted that accreditation of hospitals and diagnostic laboratories is an important step towards standardization of health care delivery. This will automatically make AMSP practices in GHCI at par with PHCI, in addition to providing many other benefits.
Although the physicians recognize the challenges of antimicrobial resistance, this figures at the bottom of the list of factors influencing their choice of antibiotics, as the antibiotics are chosen for individual benefit13. Having a treatment guidelines document which takes into account the regional data on antimicrobial resistance will thus help physicians in making relevant choices. It was noted that only 50 per cent of HCI had AMSP Teams functioning, and only 40 per cent had written AMSP document to follow, leaving much to the choice of team members. The emphasis should be to reach figures of 100 per cent for both AMSP written documents and AMSP teams formation.
The ID physician is regarded as essential to the efficient functioning of AMSP, diagnosis and treatment of difficult infections, as also control of HCAI4. In our survey, only three PHCI had departments of ID and none were present in GHCI. Other poorly represented member of the team was the clinical pharmacist, having an undisputed role in determining appropriateness of prescriptions, dosing, drug combinations and drug interactions, etc. which can interfere with the efficiency of AMA prescribed as also other drugs4.
The situation concerning HIC was encouraging as written documents were available in 75 per cent HCI and HIC teams were in place in 90 per cent of the participating institutions. The reason for this could be the early recognition of importance of HIC by the medical fraternity. However, the aim should be to achieve figures of 100 per cent for written documents and formation of teams.
Written guidelines were available in 75 per cent HCI. These should include both infrastructure guidelines like house keeping, laundry and catering, as well as for the functioning of health care worker, the most important component being hand sanitation and personal protective clothing. Guidelines on clinical procedures are important and make health care workers more confident of their functioning and help in reducing HCAI.
It is important to perform regular audits of implementation of HIC guideline to keep health care workers attentive and alert to impending risks. The final parameter of efficiency of HIC guideline implementation is the incidence of HCAI, which must be recorded truthfully and accurately to be of any value. In this survey, only 60 per cent of HCI performed some degree of this audit. The HCAI favoured for record were CLABSI, VAP, CAUTI and SSI. It is equally important to have guidelines for investigation of HCAI outbreaks, carry out investigations rapidly and communicate results without loss of time for facilitating corrective actions14.
The AMR data describe both need and outcome of AMSP measures and is fundamental to AMSP1,3,15. The methods, components and ensuing analysis of AMR will determine its usefulness for the purpose of laying down policies of AMSP, especially treatment guidelines. The frequency of analysis should be monthly and also cumulative. It should be communicated to stakeholders at half yearly or yearly intervals, unless monthly data have some significant abnormal deviation which needs to be urgently reported. Regular analysis need to be raised to 100 per cent from the reported 80 per cent in the current survey.
Even though it is ideal to analyse AMR data as CAI and HCAI, but almost all prefer to analyse it as OPD, IPD and ICU. The reason for this could simply be logistics and ease of data collection. It will be more meaningful to analyse data as per specific pathogen rather than as Gram-negative, Gram-positive, total yeasts or moulds. Similarly, it would be more prudent to analyse data as per site of infection and additionally as subsites.
The most controversial aspect of AMSP is the implementation strategies4,5,7 to be applied, which were being done by only 30 per cent HCI. Controlled or restricted formularies were in use in a few HCI. AMSP teams must analyse their needs, decide and implement best suited strategies.
AMA prescription guidelines must be easily available to all clinicians, and should be updated, preferably, on yearly basis, in line with AMR data changes. Guidelines on ICU infections were better covered than other HCAI. CAI guidelines also received better coverage. Important guidelines which were not well covered were the management of infections in transplantation, HIV, oncology, diabetes mellitus and autoimmune diseases.
Surgical prophylaxis guidelines were available in only about half of HCIs. The survey results indicated that minimal access surgery, cardiac surgery, neurosurgery, gynaecological surgery and joint replacement surgery were less likely to be covered under the available guidelines. Since inappropriate choice of AMA and duration and timing of AMA are common when given for surgical prophylaxis, this lacunae should be addressed in the guidelines.
AMA prescription audit and feedback is now considered an essential supplement to education4,5. The frequency of audits should be daily and feedbacks on a monthly basis. Ideally, all prescriptions should be audited (possible only with online prescriptions). Important features should include appropriateness, AMA combinations, AMA antimicrobial susceptibility testing (AMST) mismatch, dosing, duration, intravenous to oral switch and drug interactions. This important strategy is yet underutilized in India as in this survey only 30 per cent HCIs were found to utilize this facility of prescription audit and feedback. Carling et al16 analysed results from three years pre-intervention and seven years post-intervention, and documented a reduction in use of third-generation cephalosporins and aztreonam and a stable rate of use of fluoroquinolones and imipenem when ID physician and clinical pharmacist monitored prescription of broad-spectrum antimicrobials and gave feedback to prescribers. This also led to a reduction in Clostridium difficile infections and infections with drug resistant Enterobacteriaceae, when compared to pre-intervention rates and remained stable thereafter. Only 35 per cent of HCI were undertaking AMSP outcome analysis and this must be improved.
Computer assisted programmes were utilized by only 30 per cent of HCI. All HIC and AMA guidelines as well as reports can be put up online for ease and speed of access. Availability of IT personnel is essential for extensive and meaningful utilization of computer assisted programmes in AMSP4. The increasing use of computer assisted programmes in different spheres of hospital functioning offers new opportunities to educate physicians and can be utilized for optimizing antimicrobial use. This can be achieved by introducing online prescription order entry systems in hospitals, providing a link to the institution's guidelines for therapy to promote their wider usage, or for devising customized therapeutic regimens for patients using patient-specific laboratory and microbiology data. One of the cardinal requirements for the success of AMSP is its acceptance and implementation by physicians without a feeling of loss of prescription autonomy4,5.
A major finding of this study was better performance by PHCI than GHCI on almost all aspects of AMSP. One of the obvious factors was higher level of accreditation in PHCI, on account of basic commercial requirement. The very process of accreditation requires extensive preparation of guidelines for almost each and every aspect of their functioning. It is time the government makes accreditation of its HCI mandatory to have this advantage.
AMSP offers significant advantage in health care system. It is also important to strengthen AMSP in our HCI and also continue research in all controversial aspects of AMSP so that better and more effective strategies may be evolved and followed and those found less useful amended or even discarded17,18,19.
The following suggestions can be given based on the outcome of the present survey. (i) For the standardization of health care (including AMSP practices) in the country, the government must make accreditation of all hospitals and their diagnostic laboratories mandatory. (ii) ID physicians must be available in all HCI providing tertiary and secondary health care. (iii) Clinical pharmacists must be available in all tertiary and secondary HCI for better control and use of therapeutics especially AMA. (iv) All HCI must have written documents on AMSP and perform frequent audits to ascertain how well guidelines are being followed. (v) A comprehensive record of HCAI must be kept and trends analyzed regularly. (vi) AMR data must be regularly analyzed specific to acquisition of infection, site of infection and the pathogen. (vii) AMA usage data must be analyzed regularly. (viii) Regular education, easy availability of guidelines and audit of AMA prescription practices and their feedback are essential. (ix) Major stakeholders, physicians and surgeons must be involved in and even be leaders in all aspects of AMSP, guidelines and audits. (x) Continuous research is warranted in all aspects of AMSP to obtain the best programme for local needs.
References
- 1.Geneva: WHO; 2014. World Heath Organization (WHO). Antimicrobial resistance: global report on surveillance. [Google Scholar]
- 2.Howard SJ, Catchpole M, Watson J, Davies SC. Antibiotic resistance: global response needed. Lancet Infect Dis. 2013;13:1001–3. doi: 10.1016/S1473-3099(13)70195-6. [DOI] [PubMed] [Google Scholar]
- 3.Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, et al. Antibiotic resistance - the need for global solutions. Lancet Infect Dis. 2013;13:1057–98. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 4.MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18:638–56. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith M, Postelnick M, Scheetz M. Antimicrobial stewardship programs: methods of operation and suggested outcomes. Expert Rev Anti Infect Ther. 2012;10:63–73. doi: 10.1586/eri.11.153. [DOI] [PubMed] [Google Scholar]
- 6.Ghafur A, Mathai D, Muruganathan A, Jayalal JA, Kant R, Chaudhary D, et al. The Chennai Declaration: A roadmap to tackle the challenge of antimicrobial resistance. Indian J Cancer. 2013;50:71–3. doi: 10.4103/0019-509X.104065. [DOI] [PubMed] [Google Scholar]
- 7.Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2014. [accessed on August 19, 2014]. Centres for Disease Control and Prevention (CDC). Core elements of Hospital Antibiotic Stewardship Programs. Available from: http://www.cdc.gov/getsmart/healthcare/pdfs/core-elements.html . [Google Scholar]
- 8.Chandy SJ, Michael JS, Veeraraghavan B, Abraham OC, Bachhav SS, Kshirsagar NA. ICMR programme on Antibiotic Stewardship, Prevention of Infection & Control (ASPIC) Indian J Med Res. 2014;139:226–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Davey P, Brown E, Fenelon L, Finch R, Gould I, Hartman G, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2005;4:CD003543. doi: 10.1002/14651858.CD003543.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Centres for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States, 2013. US Department of Health and Human Services, CDC. 2013. [accessed on August 20, 2014]. Available from: www.cdc.gov/drugresistance/threat-report-2013 .
- 11.Bartlett JG, Gilbert DN, Spellberg B. Seven ways to preserve the miracle of antibiotics. Clin Infect Dis. 2013;56:1445–50. doi: 10.1093/cid/cit070. [DOI] [PubMed] [Google Scholar]
- 12.Ganguly NK, Arora NK, Chandy SJ, Fairoze MN, Gill JPS, Gupta U, et al. Antibiotic Resistance Partnership (GARP) - India Working Group. Rationalising antibiotic use to limit antibiotic resistance in India. Indian J Med Res. 2011;134:281–94. [PMC free article] [PubMed] [Google Scholar]
- 13.Giblin TB, Sinkowitz-Cochran RL, Harris PL, Jacobs S, Liberatore K, Palfreyman MA, et al. Clinicians’ perceptions of the problem of antimicrobial resistance in health care facilities. Arch Intern Med. 2004;164:1662–8. doi: 10.1001/archinte.164.15.1662. [DOI] [PubMed] [Google Scholar]
- 14.Sandor TJ, Goldmann DA. Preventing lethal hospital outbreaks of antibiotic resistant bacteria. N Engl J Med. 2012;367:2168–70. doi: 10.1056/NEJMp1212370. [DOI] [PubMed] [Google Scholar]
- 15.Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–8. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carling P, Fung T, Killion A, Terrin N, Barza M. Favorable impact of a multidisciplinary antibiotic management program conducted during 7 years. Infect Control Hosp Epidemiol. 2003;24:699–706. doi: 10.1086/502278. [DOI] [PubMed] [Google Scholar]
- 17.Hranjec T, Rosenberger LH, Swenson W, Metzqer R, Flohr TR, Politano AD, et al. Aggressive versus conservative initiation of antimicrobial treatment in critically ill surgical patients with suspected intensive-care-unit-acquired infections.: a quasi-experimental, before and after observational cohort study. Lancet Infect Dis. 2012;12:774–80. doi: 10.1016/S1473-3099(12)70151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related blood stream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 19.Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013;26:338–44. doi: 10.1097/QCO.0b013e3283630f04. [DOI] [PubMed] [Google Scholar]


