Abstract
Improvements in healthcare and nutrition have generated remarkable increases in life expectancy worldwide. This is one of the greatest achievements of the modern world yet it also presents a grave challenge: as more people survive into later life, more also experience the diseases of old age, including type 2 diabetes (T2D), cardiovascular disease (CVD) and cancer. Developing new ways to improve health in the elderly is therefore a top priority for biomedical research. Although our understanding of the molecular basis of these morbidities has advanced rapidly, effective novel treatments are still lacking. Alternative drug development strategies are now being explored, such as the repurposing of existing drugs used to treat other diseases. This can save a considerable amount of time and money since the pharmacokinetics, pharmacodynamics and safety profiles of these drugs are already established, effectively enabling preclinical studies to be bypassed. Metformin is one such drug currently being investigated for novel applications. The present review provides a thorough and detailed account of our current understanding of the molecular pharmacology and signalling mechanisms underlying biguanide–protein interactions. It also focuses on the key role of the microbiota in regulating age-associated morbidities and a potential role for metformin to modulate its function. Research in this area holds the key to solving many of the mysteries of our current understanding of drug action and concerted effects to provide sustained and long-life health.
Keywords: aging, biguanides, cancer, cardiovascular disease, metformin, microbiota, phenformin, type 2 diabetes
INTRODUCTION
The biguanide metformin is the most commonly prescribed drug for type 2 diabetes (T2D) taken by an estimated 150 million individuals worldwide. It is derived from the plant Galega officinalis (French lilac), which has a history as a herbal remedy that can be traced back to medieval Europe when it was used to relieve symptoms now attributed to diabetes [1]. In the late 1800s, G. officinalis was discovered to be rich in guanidine, a compound that was subsequently shown to have hypoglycaemic properties [2]. However, the toxicity of guanidine precluded its clinical use and attention shifted towards safer analogues. The biguanides, consisting of two N-linked guanidines, were synthesized in the 1920s but their therapeutic potential was initially overlooked due to the introduction of insulin therapy in the same decade. It was not until 1957 that metformin was recommended for the treatment of diabetes following the publication of a successful trial by the French physician Jean Sterne [1]. Unlike the pre-existing diabetes therapies, biguanides had the advantage of being able to reduce blood glucose levels without inducing hypoglycaemia. At first, the more potent biguanides phenformin and buformin received the greatest popularity; however, increasing reports of associated lactic acidosis led to these drugs being withdrawn from the markets of most countries during the 1970s [3]. Due to its superior safety profile, metformin eventually became established as the first-line treatment for T2D and it is now featured on the World Health Organization's list of essential medicines [4]. Interestingly, the therapeutic potential of metformin treatment extends far beyond its prescribed use as an anti-diabetic drug. There is a rapidly growing body of literature demonstrating an effective role for metformin in the treatment of multiple diseases including cancer and cardiovascular disease (CVD). Additionally there is evidence to suggest that metformin delays the aging process and modulates the microbiota to promote health. How metformin is capable of achieving such pleiotropic actions is not yet fully understood. In the present study, we will review the molecular mechanisms proposed to explain these diverse effects.
METFORMIN AND TYPE 2 DIABETES
According to the latest global estimates, there are 382 million people who are currently living with diabetes [5]. Increasing urbanization and the accompanying rise in obesity and sedentary behaviour mean that this figure is expected to reach 592 million by 2035, presenting national healthcare systems with an overwhelming challenge. T2D accounts for between 85%–95% of diabetes cases and is characterized by hyperglycaemia resulting from insulin resistance or impaired insulin secretion. It is a disorder with a complex aetiology involving interactions between multiple genetic and environmental factors. Strong predictors include family history, increased body-mass index, high blood pressure, physical inactivity, poor diet and advancing age [6]. In the long-term, T2D can give rise to several disabling and life-threatening complications such as CVD, neuropathy, retinopathy and nephropathy [7]. The onset of these complications can be prevented or significantly delayed by effective management of blood glucose levels, which is achieved through lifestyle modifications and, in many cases, the use of oral anti-hyperglycaemic agents such as metformin.
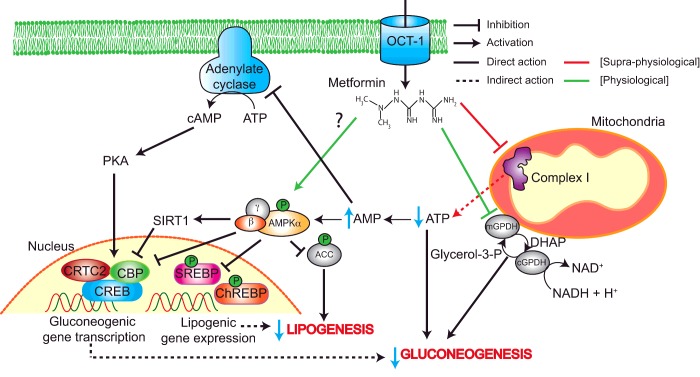
Despite being first introduced as a treatment for T2D in 1957, metformin's mechanism of action is not yet fully understood. The drug's anti-hyperglycaemic effect has been partly attributed to increased hepatic insulin sensitivity and elevated uptake of glucose in peripheral tissues; however, it is now widely accepted that metformin acts predominantly via suppression of hepatic gluconeogenesis. It has been reported that metformin can lower the rate of gluconeogenesis by as much as 36% in diabetic patients [8]. Nevertheless, the molecular pathways responsible for this reduction in glucose production remain a subject of debate. Proposed mechanisms are outlined in Figure 1.
Figure 1. Proposed mechanisms of metformin action in T2D.
Metformin enters the hepatocyte through OCT1 and accumulates in the mitochondria where it inhibits complex I. This leads to a reduction in ATP and concomitant rise in AMP. Elevated AMP levels lead to activation of AMPK, although metformin may also promote AMPK activation in a direct manner. AMPK inhibits gluconeogenic gene transcription by preventing formation of the CREB–CBP–CRTC2 complex, both directly and via SIRT1. Furthermore, AMPK inhibits lipogenesis through ACC, ChREBP and SREBP phosphorylation, which helps to improve insulin sensitivity. Several AMPK-independent mechanisms of metformin action also exist. The reduction in cellular energy status can directly inhibit gluconeogenic flux. Additionally, increased AMP has an inhibitory effect on adenylate cyclase leading to decreased cAMP production. This in turn reduces the activity of PKA and its downstream targets, which include CREB. Metformin also inhibits mGPD. This prevents glycerol from contributing to gluconeogenesis and increases the cytosolic redox state, which in turn makes the conversion of lactate to pyruvate unfavourable thus limiting the use of lactate as a gluconeogenic substrate.
Molecular target of metformin
Due to its unusually hydrophilic nature, metformin is unable to passively diffuse through cell membranes and must rely on members of the organic cation transporter (OCT) family for uptake into hepatocytes. Specifically, OCT1 has been shown to be essential for the therapeutic efficacy of metformin and it has been suggested that genetic polymorphisms in human OCT1 may contribute to variation in clinical response to the drug [9]. Once it has entered the hepatocyte, metformin accumulates within the mitochondrial matrix. It is likely that this uptake results from the positively-charged molecule being driven by the membrane potential of energized mitochondria [10]. Additionally, interactions may take place between metformin's apolar hydrocarbon side chain and the hydrophobic phospholipids of the mitochondrial membrane [11]. It is generally agreed that complex I of the mitochondrial respiratory chain is a key target of metformin. This stems from the work of two independent research groups who reported that metformin selectively inhibits the oxidation of complex I substrates but not complex II or IV substrates [10,12]. Although these findings were first observed in isolated rat hepatocytes, similar results have since been obtained in numerous cell models including primary human hepatocytes [13].
It is not known exactly how metformin inhibits complex I, although it is possible that the metal-binding properties of the drug may be implicated. It has been shown that the elevated pH of the mitochondrial matrix converts metformin into a deprotonated form with a high affinity for copper ions [14]. These copper complexes have the potential to interfere with the sensitive redox reactions of the respiratory chain. This is consistent with the earlier finding that the cellular effects of metformin depend on its ability to bind copper [15]. An alternative mechanism to account for the inhibition of complex I by metformin has recently been described by Bridges et al. [16]. They demonstrated that metformin inhibits ubiquinone reduction in a non-competitive manner, possibly by binding at the interface of the hydrophilic and membrane domains and trapping the enzyme in a deactive-like open-loop conformation. It is worth noting that although the inhibitory effect of metformin is largely confined to complex I, other biguanides have been found to inhibit several respiratory chain enzymes and therefore collectively this class of drugs is best considered non-specific [16,17].
The consequence of complex I inhibition by metformin is a decline in ATP production accompanied by a concomitant increase in ADP and AMP levels. This altered cellular energy charge is detected by the cell's principle energy sensor, AMP-activated protein kinase (AMPK). Whether or not AMPK is a central mediator of metformin action is a controversial issue and both AMPK-dependent and independent models will be outlined in the present study.
AMPK-dependent mechanism
AMPK is a master regulator of cellular energy homoeostasis that is activated by the binding of an ADP or AMP molecule to a site on its regulatory γ-subunit [18,19]. This enables the cell to respond to falling energy status by transforming it from an ATP-consuming anabolic state into an ATP-producing catabolic state.
A key role for AMPK in the mechanism of metformin action was established in 2001 following the publication of an influential study by Zhou et al. [20]. They demonstrated that metformin stimulates AMPK activation in rat primary hepatocytes and used the AMPK inhibitor compound C to show that AMPK is required for the drug's inhibitory effect on glucose production [20]; however, it should be noted that this inhibitor was later found to be non-selective. Nevertheless, these initial findings were supported by a subsequent study by Shaw et al. [21], which found that loss of hepatic liver kinase B1 (LKB1), an upstream activator of AMPK responsible for phosphorylating its catalytic α-subunit, abolished the glucose-lowering effects of metformin in mice fed a high-fat diet.
It was suggested that this LKB1/AMPK pathway, activated by metformin, alters the cell's gluconeogenic programme via inhibition of cAMP response element-binding protein (CREB)-regulated transcription coactivator 2 (CRTC2), a pivotal regulator of gluconeogenic gene expression [21]. In its non-phosphorylated state, CRTC2 is located in the nucleus where it associates with CREB to up-regulate the transcription of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) and its downstream target genes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase. However, AMPK can stimulate the phosphorylation of CRTC2 at Ser171 resulting in its nuclear exclusion [21]. Alternatively, the disabling of CRTC2 may be achieved via AMPK-mediated induction of nicotinamide phosphoribosyltransferase and the accompanying increase in hepatic sirtuin 1 (SIRT1). Deacetylation of CRTC2 by SIRT1 leaves it vulnerable to constitutive photomorphogenesis 1-mediated ubiquitination and degradation, the consequence of which is inhibition of gluconeogenic gene expression [22]. It has also been shown that AMPK triggers the dissociation of the CREB–CREB-binding protein (CBP)–CRTC2 transcription complex via phosphorylation of CBP at Ser436 [23].
Despite showing initial promise, AMPK's status as the major mediator of metformin's action was seriously undermined following the publication of work carried out by Foretz et al. [24]. In the present study, metformin was found to inhibit glucose production normally in transgenic mice lacking hepatic AMPK catalytic subunits or LKB1. It was suggested that the discrepancy between this result and the early findings of Shaw et al. [21] was due to the fact that in the latter study, the direct effect of metformin on hepatic glucose output was not assessed. Instead they investigated the effect of repeated metformin administration on fasting blood glucose levels. Therefore, these results may actually reflect an indirect effect of the AMPK–LKB1 axis on hepatic glucose production, potentially caused by AMPK's suppression of lipogenesis [24]. A well-known target of AMPK is acetyl-CoA carboxylase (ACC); a rate-limiting enzyme required for the generation of malonyl-CoA, which is both a precursor of lipogenesis and an inhibitor of β-oxidation. The inhibition of ACC by AMPK has been shown to regulate metformin-induced improvements in insulin action in mice [25]. Furthermore, AMPK can down-regulate the expression of multiple lipogenic genes by inhibiting the transcriptional activity of sterol regulatory element-binding protein 1 (SREBP-1) [20] and carbohydrate-responsive element-binding protein (ChREBP) [26]. Therefore, even if AMPK is dispensable for the glucose-lowering action of metformin, it could contribute to the therapeutic effects of the drug in the longer term by inducing favourable modifications of lipid metabolism that help to increase insulin sensitivity.
The prevailing hypothesis is that metformin activates AMPK by increasing ADP/AMP through inhibition of mitochondrial respiration; however, alternative models have been proposed. For example, it has been argued that the metabolic alterations induced by metformin in isolated skeletal muscle cells are not consistent with the interruption of mitochondrial energy supply; instead, they better reflect direct inhibition of the enzyme AMP deaminase [27]. This too would result in increased AMP levels and activation of AMPK. However, the results of the present study have been called into question due to the very high concentrations of metformin used [19]. It has also been claimed that AMPK itself is a direct target of metformin. Specifically, low metformin concentrations can promote the formation of the AMPK αβγ heterotrimeric complex in hepatocytes and when the enzyme is assembled in vitro, leading to increased phosphorylation of the catalytic α-subunit, although potential metformin-binding sites have not been identified [28]. However, this finding contradicts those obtained in previous studies that employed cell-free assays to demonstrate that metformin is not a direct allosteric activator of AMPK [20,29]. Thus, inhibition of the respiratory chain and the accompanying elevation in AMP levels remains the most plausible mechanism to explain the activation of AMPK by metformin.
AMPK-independent mechanisms
With doubts being cast on AMPK's status as the central mediator of metformin action, several AMPK-independent mechanisms have been proposed. One alternative explanation is that the associated change in cellular energy charge directly modulates glucose output. Gluconeogenesis is an energetically demanding process requiring six ATP equivalents for every molecule of glucose synthesized [30]. Since metformin treatment causes ATP levels to fall, hepatocytes must respond by reducing glucose production accordingly. Indeed, Foretz et al. [24] demonstrated that reduction in ATP content and inhibition of glucose production were strongly correlated in mouse primary hepatocytes incubated with metformin, underscoring the close link between hepatic energy status and glucose output. Additionally, metformin-induced changes in cellular energy status may suppress gluconeogenesis via the allosteric inhibition of essential enzymes. For example, AMP is capable of synergizing with fructose 2,6-bisphosphate to inhibit the key gluconeogenic enzyme fructose 1,6-bisphosphatase [31].
The notion that the molecular mechanism of metformin is independent of transcriptional alterations is supported by gene expression studies. Forced expression of PGC-1α, a master co-activator of gluconeogenic genes, does not negate the metformin-induced suppression of glucose production in primary hepatocytes [24]. Moreover, additional studies have found a lack of correlation between gluconeogenic gene expression and hepatic glucose output both in mouse models [32] and in patients with T2D [33]. Taken together, these findings imply that inhibition of gluconeogenic gene expression is not the central determinant of metformin's clinical effects; instead, the changes in cellular energy charge and the associated reduction in gluconeogenic flux may be responsible.
An alternative AMPK-independent mechanism of metformin action has recently been put forward by Miller et al. [34] that involves antagonism of glucagon signalling. The results of a series of in vivo and in vitro experiments on mouse primary hepatocytes have shown that metformin and the related biguanide phenformin block the glucagon-induced activation of adenylate cyclase leading to a reduction in cAMP synthesis [34]. This in turn lowers protein kinase A (PKA) activity, abrogating phosphorylation of critical substrates that enhance gluconeogenesis such as 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 1, CREB-1 and inositol trisphosphate receptor. It has been proposed that the metformin-associated rise in cellular AMP levels is responsible for the inhibition of adenylate cyclase, possibly due to the direct binding AMP's adenine moiety to an inhibitory ‘P-site’ [34].
Although this finding is undoubtedly significant, an important caveat has been raised. If antagonism of glucagon signalling were the major mechanism of metformin action, it would be reasonable to assume that the use of this drug may lead to hypoglycaemic episodes as seen in glucagon receptor knockout mice [35]. However, one of the main advantages of metformin treatment is that hypoglycaemia is rare. This implies that either the suppression of glucagon signalling is incomplete in humans or that compensatory mechanisms are at work [36].
It should be noted that the studies on which these AMPK-independent models are based have received criticism for using concentrations of metformin that are considered higher than the maximally achievable therapeutic concentrations found in diabetic patients [37]. Metformin is administered orally and has a maximum recommended dose of 2.5 g per day in humans. It has been demonstrated that the peak plasma concentration reaches approximately 3 μg/ml (equivalent to 18 μM) in individuals who have taken a single dose of 1.5 g [38]. However, evidence from animal studies show that the concentration of metformin in the hepatic portal vein is considerably higher than that of the systemic plasma [39]. Cao et al. [40] tested the effect of metformin at low concentrations (≤80 μM) in mouse primary hepatocytes. Under these conditions, metformin suppressed gluconeogenic gene expression and hepatic glucose production via AMPK, yet no effect was observed on ATP levels or the AMP–ATP ratio implying that complex I is not inhibited [40]. However, it is unclear how metformin could activate AMPK without inducing a change in cellular energy charge. Furthermore, it has been shown previously that even if changes in the AMP–ATP ratio are undetectable, the activation of AMPK by metformin is still dependent on AMP binding [41].
Although both Foretz et al. [24] and Miller et al. [34] have used high concentrations to provide evidence for an AMPK-independent mechanism of metformin action, it does not necessarily mean that their findings should be dismissed as clinically irrelevant. For in vivo experiments involving rodents, it is common to administer metformin at daily doses between 250–350 mg/kg. These values are obtained using a well-established method for converting doses between species based on normalization to body surface area [42]. According to this formula, a standard therapeutic dose of 20 mg/kg in an adult human (60 kg) is approximately equivalent to a 250 mg/kg dose in a mouse. Moreover, it is known that relatively high concentrations are required in order for the drug to exert its therapeutic effect in diabetic rodents [24,43]. Additionally, metformin has been shown to accumulate at very high concentrations in several tissues. In mice, a dose of 50 mg/kg can lead to concentrations greater than 250 μM in the liver and those within the millimolar range in the small intestine [39]. Thus the use of concentrations that exceed those typically found in the plasma or portal vein can be justifiable for in vitro studies involving primary hepatocytes. Furthermore, it is known that the biological effects of metformin are time-dependent as well as concentration-dependent [20], a property that probably reflects its capacity to accumulate within the mitochondrial matrix. Therefore, it is possible that effects observed at high concentrations may also occur at lower concentrations following more long-term exposure to metformin.
It is also worth mentioning that studies that have used hepatic AMPK knockout models to support an AMPK-independent mechanism of metformin action should be reviewed in light of new evidence that demonstrates the importance of intestinal AMPK in mediating metformin action. Although the liver is considered to be the main site of metformin action, it has recently been demonstrated that activation of intestinal AMPK contributes to the acute glucose-lowering effects of metformin [44]. A study performed on insulin-resistant rats has uncovered the involvement of a gut–brain–liver axis whereby metformin triggers a duodenal AMPK-glucagon-like peptide-1 receptor-PKA-dependent neuronal signalling pathway to reduce hepatic glucose production [44]. The present study alludes to the possibility that the activation of AMPK in organs usually in the presence of elevated concentrations of the drug (in the millimolar range) can regulate from a distance the metabolism of tissues directly responsible for glucose production.
A novel mechanism of metformin action has been recently revealed that is independent of both AMPK activation and cellular energy charge [45]. Specifically, metformin has been found to inhibit the glycerophosphate shuttle enzyme mitochondrial glycerophosphate dehydrogenase (mGPD). This prevents glycerol from being directly used as a gluconeogenic substrate. It also leads to an increase in the cytosolic redox state which makes the conversion of lactate to pyruvate unfavourable and thus limits the contribution of lactate to gluconeogenic flux [45]. In addition to revealing a new therapeutic target, the present study helps to further underscore metformin's status as a non-specific drug that is capable of interfering with multiple molecular targets to bring about the disruption of gluconeogenesis.
METFORMIN AND CARDIOVASCULAR DISEASE
CVD encompasses diseases of the heart and circulation. According to the British Heart Foundation, CVD accounts for more than a quarter of all deaths in the U.K. with an estimated cost per year of £19 billion. The greatest risk factor of CVD is T2D. There is evidence that metformin can offer protection against CVD in diabetic patients. This is especially pertinent given that CVD is the leading cause of mortality within this population [46]. In the landmark UK Prospective Diabetes Study (UKPDS), it was reported that metformin treatment lowered the risk of myocardial infarction by 39% when compared with traditional treatments over a period of 10 years [47]. Subsequent trials have supported a beneficial role for metformin in protecting against the cardiovascular complications of diabetes, including a 10-year follow-up of the original UKPDS trial [48–50]. Although improvements in glucose and lipid metabolism may contribute to some of the positive effects conferred by metformin, numerous alternative mechanisms invoking direct action of the drug on the cardiovascular system have also been discovered.
The results of multiple studies suggest that metformin can protect against atherosclerosis by promoting endothelial integrity and preventing the formation of plaques. For example, it has been reported that the activation of AMPK by metformin limits endothelial cell damage caused by oxidative stress under hyperglycaemic conditions. This occurs via inhibition of the protein kinase C-NAD(P)H oxidase pathway [51]. The resulting reduction in cytosolic reactive oxygen species (ROS) generation halts the initiation of a positive feedback loop involving mitochondrial ROS generation and mitochondrial fission, which in turn prevents the triggering of endothelial apoptosis by the resulting loss of mitochondrial membrane potential [52].
Metformin has also been observed to exhibit anti-thrombotic properties in insulin-resistant models. Specifically, metformin counteracts the stimulatory effect of hyperinsulinaemia on the production of plasminogen activator inhibitor 1 (PAI-1), a negative regulator of fibrinolysis implicated in blood clot formation [53]. It is believed that that this is not mediated by the improvement in insulin sensitivity associated with metformin but rather by the direct inhibition of PAI-1 gene expression [54]. Moreover, metformin treatment has been observed to significantly reduce platelet aggregation in patients with insulin-dependent diabetes [55]. This finding may be explained by the results of a subsequent study in which it was discovered that metformin can enhance Ser1179 phosphorylation of endothelial nitric oxide (NO) synthase (eNOS) in an AMPK-dependent manner leading to an increase in NO bioactivity in the aortas of C57BL/6 mice [56]. NO plays a crucial role in maintaining vascular homoeostasis by carrying out multiple functions, including inhibition of platelet aggregation, and therefore helps to protect against the development of coronary artery disease.
In addition to its endothelial actions, metformin affects cardiomyocyte function and has been associated with improvements in diabetic cardiomyopathy in rodent models. This distinct condition is a major cause of heart failure and is characterized by ventricular dysfunction independent of coronary artery disease and hypertension [57]. It was previously suggested that metformin may correct abnormalities in myocyte relaxation via tyrosine kinase-dependent alterations in calcium handling [58]. More recent studies have linked improvements in diabetic cardiomyopathy to AMPK-mediated up-regulation of autophagy [59,60]. Cardiac autophagy is an important homoeostatic mechanism known to be supressed in diabetic cardiomyopathy. It has been demonstrated that metformin-activated AMPK protects cardiomyocytes by disrupting the Beclin1–Bcl-2 (B-cell lymphoma 2) complex and inducing a switch from apoptotic to autophagic machinery. Importantly, this restoration of autophagy improves cardiac structure and function in diabetic mice [59].
There is also evidence to suggest that metformin exerts a cardioprotective effect against ischaemia reperfusion injury following myocardial infarction. Administration of metformin during the first 15 min of reperfusion has been shown to reduce myocardial infarct size in hearts isolated from both diabetic and non-diabetic rats [61]. This was attributed to the activation of the protein kinase B (Akt)/phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) signalling pathway by metformin, which prevents the opening of the mitochondrial permeability transition pore (mPTP), a critical trigger of cell death during reperfusion. It has since been shown that AMPK activation also inhibits mPTP opening at reperfusion [62]. Moreover, an AMPK-mediated increase in eNOS activity has also been proposed to explain the positive effect of acute metformin treatment on infarct size [63]. It is interesting to note that a new small-molecule AMPK activator (A-76922) has been shown to act synergistically with metformin to enhance the cardioprotective effects of AMPK activation and therefore may have useful clinical applications during an ischaemia reperfusion episode [64].
The effect of chronic metformin treatment on ischaemia reperfusion injury has also been investigated, although to a lesser extent. One study has shown that administration of metformin over a 4-week period reduced infarct size in isolated rat hearts regardless of diabetes status [65]. The cardioprotection observed in diabetic hearts was associated with improved mitochondrial organization, possibly related to increased AMPK activation and PGC-1α expression. Furthermore, long-term metformin treatment post-myocardial infarction may also have a positive effect. It has been demonstrated that rats treated with metformin for 12 weeks following myocardial infarction exhibited enhanced AMPK activation and improved cardiac remodelling, suggesting that metformin could help attenuate the development of heart failure in such a scenario [66]. Ultimately, the evidence suggests that metformin exerts varied positive effects on the cardiovascular system, particularly in diabetic models. Several randomized control trials in non-diabetic individuals are currently underway and data from these will help to establish whether or not metformin can improve cardiovascular outcomes in a wider section of society.
METFORMIN AND CANCER
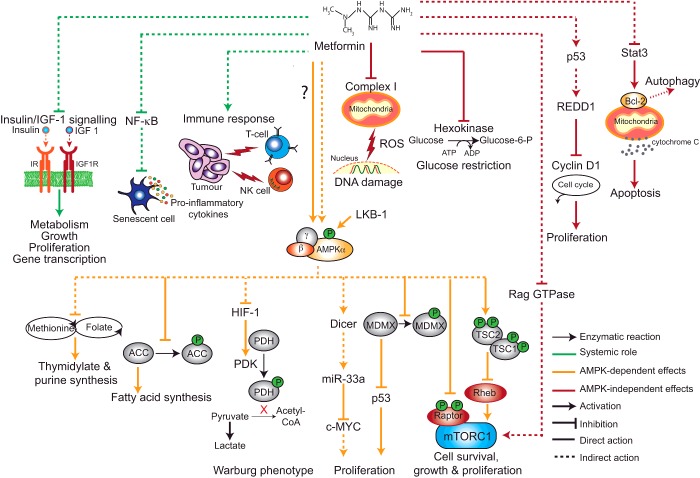
Cancer is a leading cause of morbidity and mortality worldwide. A recent lifetime risk analysis of the British population found that over 50% of adults below the age of 65 will be diagnosed with the disease at some point in their life [67]. Sobering projections like this have stimulated an enormous amount of investment into cancer research and rational drug design. Despite our best efforts, only 5% of oncology drugs entering phase I trials eventually receive approval and it has been argued that so far, targeted therapies have provided only modest survival benefits [68]. Metformin garnered considerable interest within the field of oncology in 2005 following the publication of an epidemiological report highlighting a link between metformin treatment and reduced cancer risk in diabetic patients [69]. This finding has stimulated a great deal of further research and numerous observational studies have supported a protective role for metformin against a variety of cancer types including liver, colorectal, pancreas, stomach and oesophagus cancer in diabetics [70]. However, it is important to note that such studies are prone to bias and confounding factors and contrastingly, meta-analyses of randomized controlled trials do not appear to demonstrate a significant effect of metformin on cancer outcomes [70–72]. Furthermore, it is not clear whether or not any positive results could be extrapolated to non-diabetic individuals. Nevertheless, increasing importance is being placed on the role of altered metabolism in cancer and the ability of metformin to interact with several metabolic pathways suggests that it could be effective at preventing the development and progression of this disease An overview of possible anticancer mechanisms is presented in Figure 2.
Figure 2. Proposed mechanisms to explain the anti-cancer effects of metformin.
At the systemic level metformin may inhibit tumour development by reducing insulin/IGF-1 signalling, preventing the release of pro-inflammatory cytokines through NF-κB and enhancing the immune response [mediated by natural killer cells (NK cells) and cytolytic T-cells (T-cells)] to cancer cells. Metformin also has direct effects in cancer cells, many of which are mediated by AMPK. When activated, AMPK disrupts cancer cell energy metabolism by inhibiting fatty acid synthesis (inhibition of ACC) and suppressing the Warburg phenotype mediated by the action of the hypoxia-induced factor (HIF-1) on the glycolytic enzymes pyruvate kinase (PDK) and pyruvate dehydrogenase (PDH). AMPK may also block folate and methionine metabolism, which is required for nucleotide synthesis. AMPK down-regulates the oncogene c-MYC by inducing the expression of Dicer and up-regulates the tumour suppressor p53 by inhibiting its negative regulator MDMX. Furthermore, AMPK blocks the mTORC1 signalling pathway by inhibiting Raptor and activating TSC2. AMPK independent mechanisms have also been described. Metformin can protect against DNA damage by inhibiting complex I and suppressing ROS production. Metformin also inhibits hexokinase activity and impairs glucose uptake. Metformin can block mTORC1 signalling in the absence of AMPK via inhibition of Rag GTPases. Additionally, metformin reduces levels of the cell cycle regulator cyclin D1 in a p53- and REDD1-dependent manner. Finally, metformin promotes apoptosis by down-regulating the Stat3/Bcl-2 pathway through release of cytochrome C and also promotes autophagy.
It is possible that the systemic effects of metformin could be protective against cancer. Both experimental and epidemiological evidence suggests that insulin and insulin-like growth factor 1 (IGF-1) can promote tumorigenesis by stimulating the proliferation of epithelial cells [73]. Metformin may prevent such neoplastic activity by reducing hyperinsulinaemia and lowering levels of these signalling molecules. Metformin can also modify inflammatory processes known to play a role in cancer progression. For example, it has been reported that metformin blocks the activity of the transcription factor nuclear factor-κB (NF-κB) resulting in decreased secretion of pro-inflammatory cytokines by senescent cells [74]. This mechanism may also contribute to the anti-aging properties of the drug as outlined below. Additionally, metformin has been found to enhance the immune response to cancer cells. A recent study performed in a mouse model demonstrated that metformin protects CD8+ tumour-infiltrating lymphocytes from apoptosis and functional exhaustion [75]. Encouragingly, it has also been shown that metformin was able to improve the efficacy of an experimental anti-cancer vaccine by promoting the survival of memory T-cells [76].
There are several lines of evidence that suggest metformin can exert cancer preventative effects in a cell-autonomous manner. The ability of metformin to inhibit complex I and disrupt oxidative phosphorylation has recently been highlighted as an important requirement for tumorigenesis inhibition [77–79]. Metabolomic analysis of a breast epithelial cell line undergoing neoplastic transformation has revealed that metformin decreases tricarboxylic acid cycle intermediates which implies impaired complex I activity and therefore reinforces the notion that this enzyme is a key target [77]. Crucially, it has been demonstrated that the anti-tumorigenic effect of both metformin and phenformin is reversed in cancer cells that are forced to express yeast NADH dehydrogenase NDI1 which enables complex I function to be bypassed [78,79].
AMPK-dependent mechanism
The activation of the LKB1/AMPK pathway may also significantly contribute to the anti-cancer effects of metformin. The AMPK up-stream activator LKB1 is a known tumour suppressor and mutations in this gene are associated with Peutz–Jeghers syndrome, an inherited cancer-predisposing disorder, as well as several sporadic cancers [80]. Additionally, it has been demonstrated that the protective effect of metformin is attenuated by pharmacological inhibition of AMPK and in AMPK-knockdown models [81–83]. A major consequence of AMPK activation is inhibition of mammalian target of rapamycin (mTOR) signalling, a nutrient-sensitive regulator of protein synthesis, cell growth and proliferation. This is achieved via directly phosphorylation of Ser1345 and Thr1227 on the tumour suppressor protein tuberous sclerosis 2 (TSC2), which forms an mTOR complex 1 (mTORC1)-inhibitory complex with TSC1 [84]. Alternatively, AMPK can prevent mTORC1 activation by phosphorylating its binding partner Raptor [85].
AMPK activation has been associated with suppression of the Warburg effect, a metabolic phenotype adopted by cancer cells characterized by a preference for aerobic glycolysis over oxidative phosphorylation [86]. This transformation is facilitated by hypoxia-inducible factor 1α, a transcription factor that up-regulates glycolytic gene expression partly in response to mTORC1 signalling. A related classic metabolomic hallmark of cancer is increased de novo fatty acid synthesis and elevated levels of the key lipogenic enzyme fatty acid synthase (FAS) has been described in a wide variety of human cancers [87]. It has been demonstrated that the activation of AMPK reduced FAS expression in prostate cancer cells and was able to diminish their viability [88]. Similarly, experimental work conducted in a mouse model of colon carcinoma has shown that metformin down-regulates the expression of FAS and can counteract the stimulatory effect of a high-energy diet on tumour growth [89]. These findings suggest that metformin may exert its anti-cancer effects by inducing alterations in glucose and lipid metabolism that ultimately deprive cancer cells of vital growth substrates.
Recent reports reveal that metformin can act as an anti-folate drug [90,91] and show that metformin impairs the folate cycle similarly to the action of the anti-folate class of chemotherapy drugs. The folate or one-carbon cycle is a major regulator of cell metabolism and an integrator of nutrient status [92]. It assimilates inputs in the form of glucose and amino acids that are processed through chemical reactions and transformed for diverse biological functions. These include cellular biosynthesis, regulation of redox status, regulation of epigenetics through nucleic acid and protein methylation and genome maintenance through the regulation of nucleotides pools. For example, the folate cycle regulates de novo synthesis of nts required for DNA repair and replication and the production of the universal methyl donor S-adenosylmethionine, involved in a number of methylation reactions such as DNA methylation [93]. Chemotherapy drugs, such as methotrexate, aminopterine and azaserine, block folate metabolism and impair de novo synthesis of both thymidylate and purine nts. We have demonstrated that metformin disrupts folate and methionine metabolism in Escherichia coli [90]. Similarly, metformin treatment impairs folate metabolism in several breast cancer cells with concomitant reduction in glutathione and tryptophan metabolites [91]. Thymidine serves as a thymidylate precursor in the pyrimidine salvage pathway and hypoxanthine is an inosine monophosphate precursor in the purine salvage pathway in de novo synthesis. In fact, hypoxanthine and thymidine supplementation rescues the cytotoxic effects of metformin on cancer cells independently of LKB1 but requiring the ataxia telangiectasia mutated kinase (ATM)/APMK axis [91]. In a study by Janzer et al. [77], metformin induced a cancer stem cell-specific depletion of nt triphosphates.
Aside from these metabolic modifications, there is evidence that the activation of AMPK by metformin may help to regulate the cell cycle via interactions with classical oncogenes and tumour suppressors. For example, it has been demonstrated that metformin down-regulates c-MYC in an AMPK-dependent manner in breast cancer cell lines [94]. This is mediated by increased expression of miR-33a, a miRNA that binds to the 3′-UTR region of c-MYC, which in turn results from the up-regulation of the RNAse III enzyme Dicer in response to metformin treatment. The modulation of Dicer by metformin is especially relevant given that low levels of this enzyme have been associated with poor prognosis in ovarian, breast and lung cancer [95–97]. AMPK has also been shown to target p53. One study reported that AMPK initiates cell-cycle arrest by phosphorylating Ser15 of p53 [98], although it has been argued that this event is not sufficient for p53 activation. Findings from a subsequent study have revealed that AMPK phosphorylates the p53 regulator murine double minute X (MDMX) at Ser342 leading to its increased association with 14-3-3 proteins. This suppresses p53 ubiquitylation and enhances p53 stability and activation [99].
Although it is apparent that AMPK activation may protect against cancer via multiple mechanisms, it is also understood that during later stages of development, the activation of AMPK may actually promote the survival of tumour cells. This is because AMPK enables the cells to adapt to metabolic stress imposed by the solid tumour microenvironment. Support for this hypothesis comes from a study demonstrating that the restoration of activated AMPK in LKB1-deficient lung adenocarcinoma cells conferred protection against glucose-starvation-induced cell death [100]. This suggests that the use of AMPK activators such as metformin may be harmful in some situations. Conversely, metformin and other compounds capable of inducing metabolic stress may be particularly effective against tumours lacking a functional LKB1–AMPK axis. Indeed, it has been shown that in a transgenic mouse model of lung cancer, phenformin selectively induces apoptosis in cells bearing LKB1 mutations [101]. This reflects the inability of these cells to respond appropriately to the decline in ATP production associated with complex I inhibition.
AMPK-independent mechanism
AMPK-independent mechanisms to explain the anti-cancer properties of metformin have also been described. For instance, metformin can protect against DNA damage and mutation by inhibiting ROS generation by complex I [102]. Additionally, metformin can activate mTORC1 in the absence of AMPK in a comparable way to amino acid withdrawal. It achieves this by inhibiting Rag GTPases, which are responsible for inducing the translocation of mTORC1 to cellular compartments containing the mTORC1 activator, Ras homologue enriched in brain (Rheb) [103]. There is also evidence to show that the anti-proliferative effect of metformin in a prostate cancer cell line is associated with AMPK-independent inhibition of the critical cell-cycle regulator cyclin D1 [104]. It is thought that this is mediated by a p53-dependent up-regulation of REDD1 (regulated in development and DNA damage response 1) [105]. Furthermore, AMPK activation may be dispensable for the triggering of cell death pathways induced by metformin treatment. A study conducted on oesophageal squamous cell carcinoma cells showed that metformin up-regulates apoptosis and autophagy resulting in reduced tumour growth [106]. This was attributed to inactivation of the Stat3 (signal transducer and activator of transcription 3)/Bcl2 pathway, which was only marginally reverted by AMPK knockdown suggesting a limited contribution. Finally, it has been demonstrated that metformin impairs glucose uptake in a lung cancer cell model via direct allosteric inhibition of hexokinase-II [107]. Deprivation of this energy source leads to mitochondrial depolarization and the subsequent triggering of apoptosis. Similar findings have also been observed in a breast cancer cell line [108].
Anti-cancer polypharmacy therapy with metformin
Although metformin has shown considerable promise as a stand-alone cancer treatment, the effect of metformin in combination with conventional cytotoxic therapies has also been investigated. There is evidence that metformin improves the response of cancer cells to radiotherapy [109]. Metformin has also been found to enhance the sensitivity of multiple cancer cell types to common chemotherapeutic agents including cisplatin, paclitaxel, carboplatin and doxorubicin [110–112]. However, it should be noted that metformin antagonizes the cytotoxic effect of cisplatin in glioma, neuroblastoma, fibrosarcoma and leukaemia cell lines, reportedly via AMPK-independent up-regulation of the Akt survival pathway [113]. Metformin may be particularly effective when used in combination with small molecule kinase inhibitors that suppress glycolysis. In melanoma cells it has been demonstrated that the efficacy of BRAF (v raf murine sarcoma viral oncogene homologue B1) protein kinase inhibitors is limited by the induction of compensatory oxidative phosphorylation [114]. Accordingly, several inhibitors of oxidative phosphorylation were found to enhance the efficacy of these drugs. Although many inhibitors of oxidative phosphorylation are known to be toxic, the favourable safety profile of metformin implies that it may have therapeutic potential [115].
METFORMIN AND AGING
The biological basis underlying the aging process is a central mystery of science and its resolution is critical for developing means to reduce the harmful outcomes of aging. Recent years have seen unprecedented progress towards this goal. Genetic, dietary and pharmacological interventions can improve health and increase lifespan in the laboratory model organisms: the nematode Caenorhabditis elegans, the fruit fly Drosophila melanogaster and the mouse Mus musculus [116,117]. These interventions ameliorate the aging process and extend healthspan by protecting against age-related pathology and loss of function. These studies provide a key proof-of-principle that the aging process can be targeted to achieve wide-ranging protection against aging-associated physiological decline [116,117]. Controlled reduction in food intake without malnutrition [dietary restriction (DR)] has long been known to increase healthspan in diverse animals [118–120] and drugs that mimic DR to achieve its benefits in humans [121]. In this section, we will provide an account for the role of biguanides in aging as potential DR mimetics.
Biguanides and the longevity of C. elegans
Several studies have been performed in C. elegans that support the life and healthspan extending properties of biguanides. An early report shows that administration of buformin at 0.1 mg/ml throughout life (including the larval period) can extend adult mean and maximal lifespan up to 23% and 26% respectively [122]. Similarly, administration of metformin throughout life at a 50 mM dose can also extend median lifespan by 40% without changes in maximum lifespan [123]. Interestingly, lower and higher doses of metformin (10 and 100 mM respectively) do not have lifespan extending properties. Recently, two studies showed that effects of 50 mM metformin are independent of its administration during early developmental time, as positive effects on lifespan are still observed when the drug is administered at a later stage in life [90,124]. Similarly, phenformin over a range of concentrations such as 1.5, 3 and 4.5 mM can extend adult worm mean lifespan by 5%, 21% and 26% respectively. So how do biguanides extend lifespan in C. elegans? Metformin has an intricate and concerted mode-of-action, which potentially accounts for the wide-ranging effects of the drug on host physiology. We have shown that changes in bacterial folate and methionine metabolism induced by metformin are responsible for the life-extending properties of the drug [90]. In parallel, other studies [123,124] as well as ours [90] suggest that metformin acts directly as a metabolic stressor, potentially through inhibition of complex I of the respiratory chain, to activate an oxidative stress and detoxification response involving the metabolic mediators, AMPK and SKN-1 [orthologue of mammalian nuclear factor (erythroid-derived 2)-like 2 (NRF-2)]. In support of this view, administration of antioxidants such as N-acetyl cysteine, which abrogate ROS signalling or mutations in AMPK and SKN-1, impair the development of an adequate detoxifying response and abolish the DR-like state induced by the effects of metformin on bacterial metabolism.
Metformin equally displays healthspan properties. Metformin reduces the accumulation of the molecular damage pigment lipofuscin, promotes youthful mobility into late life, decreases fat accumulation [123], attenuates the morphological decline observed in aging worms [124] and increases survival rate when exposed to long-term anoxia [125].
Biguanides and longevity in insects
Whereas the lifespan extending properties have been well documented for C. elegans, the same does not apply to insects. Administration of 1, 2.5 and 5 mM metformin has no effect on lifespan of male or female Drosophila melanogaster [126]. Intriguingly, higher doses of metformin shortens the lifespan of males (100 mM) and females (25, 50 and 100 mM), despite robust activation of AMPK and reduced lipid stores [126]. This is a striking finding as increased levels of expression and activation of AMPK have been shown to increase lifespan in C. elegans [127] and Drosophila [128]. A well-described feature of pre-diabetes in human patients is infection-induced hyperglycaemia [129]. In a fly model of obesity, metformin administration at 10 mM reduced the systemic infection burden of the fungus Rhizopus, a causing agent of mucormycosis observed in diabetic patients. It led to reduced weight gain, normalized glucose levels due to infection and increased survival rates [130]. Finally, 5 mM metformin had a beneficial effect on age-related changes in fly intestinal stem cells obtained from the midgut, as measured by a decrease in the molecular DNA damage markers gH2AX foci and 8-oxodG [131]. Recently, a study performed in the cricket Acheta domesticus [132], shows that metformin leads to an extension in mean and maximal lifespan of organisms of both sexes, with increases of 43.7% and 23.2% in females and males respectively.
Biguanides and longevity in rodents
Several studies have been performed in rodents suggesting an evolutionarily conserved pro-longevity role for biguanides. Work by Anisimov [133] has greatly contributed to such advancement. Overall, metformin treatment of HER-2/neu, SHR, 129/Sv, C57BL/6 and B6C3F1 mice, over a wide-range of doses led to increases in lifespan ranging from 4% to 38%. However, data suggest that effects of metformin on lifespan are dependent on genotype, gender, biguanide type, dose and duration of the treat-ment. In general, the later the starting of the treatment the fewer lifespan benefits are observed. This is to some extent similar to the results obtained with metformin treatment on C. elegans survival [90,123,124]. For example, treatment of SHR mice at 15 months of age with metformin conferred no longevity benefits in comparison with starting of the treatment at 3 months of age (0% compared with 38% respectively) [133]. Likewise, dose plays an important role in lifespan extension in rodents as observed for C. elegans. Administration of 0.1% of metformin in the diet leads to a lifespan extension of 5.83% compared with–14.4% when 1% of metformin is given to C57BL/6 mice. Reduction in lifespan was caused by drug nephrotoxicity [134]. Gender also influences the effect of metformin on lifespan as biguanide treatment extended the lifespan of 129/Sv female (5%) but not male mice (–13%) [133]. Phenformin administration to C3H/Sn mice also led to a lifespan extension of 21%. Interestingly, the effects of biguanides on the lifespan of rats are not as pronounced as the ones observed for mice. Only buformin and not metformin or phenformin extended the lifespan of rats. Given the wide-ranging effects of metformin in host physiology, which includes alteration of body weight, temperature, normalization of serum glucose, insulin, triglycerides and cholesterol levels, regulation of oestrous function and anti-neoplastic effects, confounders are likely to occur and influence lifespan trials with biguanides and therefore need careful consideration. In a recent study by Martin-Montalvo et al. [134], the calorie restriction mimicking effects of metformin could be the underlying mechanism responsible for the effects the drug on lifespan and healthspan. These include improved fitness, increased insulin sensitivity and reduced low-density lipoprotein and cholesterol levels without a decrease in caloric intake. At the molecular level, metformin alters gene expression similarly to that observed on calorie restriction. Interestingly, metformin increases AMPK activity without concomitant activity changes in the respiratory chain. It activates an antioxidant and anti-inflammatory response leading to reductions in both oxidative damage accumulation and chronic inflammation.
Altogether, the collected data existing from several model organisms show a promising outlook for the potential benefits of using metformin as an anti-aging strategy. However, further investigations are required for its use in humans as its definite anti-aging mechanism is still not fully disclosed.
Metformin and longevity in humans
To date no long-term longitudinal survival/mortality studies have been performed in healthy patients subjected to metformin therapy. A 10-year-long randomized clinical trial of metformin therapy in T2D overweight/obese patients (UKPDS-O) shows long-term beneficial effects on health and survival [50], reducing cardiac and all-cause mortality of patients on metformin compared with usual care. Indeed, the benefits of metformin on host metabolism have been observed even after cessation of treatment, the so-called “legacy effect”. However, the molecular mechanisms by which metformin promotes these long-term positive effects on mortality [50], independent of glycaemic control, remain unknown. Interestingly, another long-term UK Prospective Diabetes Study—UKPDS-S (6.6 years), of metformin plus sulfonylurea versus sulfonylurea in a mixed group of non-overweight and overweight/obese T2D patients showed possible harmful effects of metformin (when combined with sulfonylurea) [47]. In a more recent meta-analysis of randomized clinical trials of metformin therapy in individuals with and without diabetes, which include the two UKPDS studies, Stevens et al. [71] showed no effects on all-cause mortality. Overall, based on the information learned from lower model organisms and reviewed in the present study, in order to ascertain the long-term beneficial effects of metformin on human longevity, new studies targeting healthy younger patients are warranted.
METFORMIN AND THE MICROBIOTA
Living organisms rarely exist in isolation but rather in intimate association with other species, in particular microorganisms [135]. The intestine is home to the majority of the microbes that inhabit the human body and these vast populations of microorganisms collectively designated as the ‘forgotten organ’, the microbiota, is involved in many metabolic processes with important consequences for host physiology and the modulation of metabolic phenotypes [136]. Previous reports provide a link between alterations to the microbiota in the gastrointestinal tract (caused by changes in diet and environmental conditions such as antibiotics) and nutrition-related syndromes such as obesity, T2D, metabolic syndrome, cancer and aging [137,138], suggesting that the gut microbiota could act as a potential biomarker for host health. Therefore, host-targeted drugs, such as metformin, commonly used to treat metabolic diseases associated with microbiota dysfunction may act on the physiology of the bacterial populations to exert their effects on the host (see Figure 3). Does the gut microbiota influence the therapeutic potential of metformin and/or explain its side effects in mammals? To what extent microbiota mediate the effects of metformin on metabolic disease remains an open question. One possibility is that metformin alters the structure and/or function of the microbiota in a way that promotes metabolic health. An increasing number of recent reports support this interpretation and will be discussed in this section.
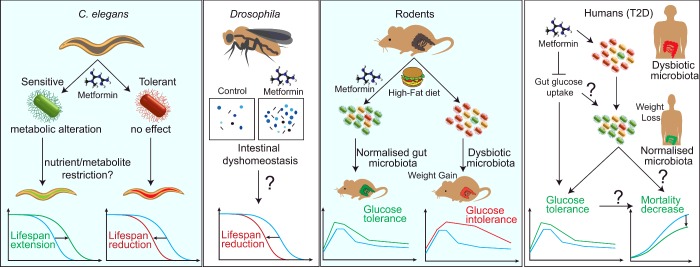
Figure 3. Effects of metformin in gut physiology to regulate T2D and survival in model organisms and humans.
Metformin increases C. elegans lifespan when co-cultured with a sensitive but not a tolerant E. coli strain. In Drosophila, high concentrations of metformin lead to intestinal dyshomoeostasis and increased concentration of faecal output that correlates with an observed shortening of lifespan. Metformin normalizes the gut microbiota of rodents on a high-fat diet and improves glucose homoeostasis due to effects on the gut microbiota. In T2D diabetic patients, metformin promotes weight loss and improved glucose tolerance either by reducing gut glucose uptake or by direct alterations of the gut microbiota which can ultimately lead to increased survival.
Metformin alters microbial metabolism and regulates C. elegans physiology
The nematode C. elegans establishes a beneficial interaction with E. coli bacteria, a relationship that is similar but far simpler than the vast communities of ‘healthy’ bacteria residing in the human gut. We have demonstrated that metformin exerts DR on C. elegans by altering bacterial folate and methionine metabolism [90], providing for the first time a mechanistic explanation for the effects of metformin on the host through action on its microbiota. In C. elegans, metformin slows down the aging process only if bacteria are present and in a dose-dependent manner, an effect which is proportional to its effects on microbial metabolism and the bacterial strain with which the worms are co-cultured [90]. Remarkably, metformin can also cause folate [139] and vitamin B12 [140] deficiency and increase plasma homocysteine levels [141] in humans.
Metformin disrupts intestinal fluid homoeostasis in flies
The gut of Drosophila melanogaster is recently becoming one of the most well-studied organs in fly research. Similarly to humans, flies show an important conservation in gut functions. Fluid retention, absorption, intestinal peristalsis, intestinal transit, defecation rate and nature of excreta are also subject to complex homoeostatic regulation [142]. Previous studies show that the Drosophila microbiome modulates host development and metabolic homoeostasis via insulin signalling [143] and mTOR signalling [144]. However, the precise role of bacteria in gut physiology remains unclear. Given that metformin leads to gastrointestinal upset when administered to diabetic patients, Slack et al. [126] investigated if metformin alters intestinal physiology in aging flies. Using an assay previously developed to evaluate intestinal physiology through examination of fly excreta [145], these authors showed that 100 mM metformin lead to changes in intestinal homoeostasis causing fluid imbalances resulting in more concentrated faecal deposits in a possible attempt to preserve water. Possibly, at such elevated concentrations, metformin acting as a metabolic stressor can produce acid in excess that is counterbalanced by the gut. In fact, the hindgut of flies has the ability to adjust the pH of intestinal material before excretion in response to both internal (e.g. reproductive) or external (e.g. nutrients) signals [145]. Unexpectedly, metformin treatment in flies did not lead to increased lifespan extension despite activation of AMPK. Whether lifespan extension in flies is also dependent on metformin sensitivity of gut flora remains to be tested.
Metformin action on gut function in rodents
Studies performed in mice show that metformin accumulates in the intestine at very high concentrations [39] and this could possibly explain the effects of metformin on host metabolism. Evidence collected in rodents also suggests that the intestine is a major organ regulating the action of metformin. Data show that metformin not only delays glucose absorption along the intestinal tract [43] but also increases its utilization through non-oxidative anaerobic metabolism [146]. Lactate as a product of non-aerobic metabolism can be delivered to the liver through the portal vein and utilized as a substrate for gluconeogenesis. Therefore, the intestine makes an important contribution to the glucose-lowering effects of metformin providing at the same time a safeguard mechanism against hypoglycaemia [147].
Most of the microbes that co-exist within the host also reside in the intestine and are therefore under selective pressure at high concentrations of metformin. T2D and obesity are characterized by structural and functional changes in the microbial populations in the gut, an inflammatory state and gut barrier disruption, also known as leaky gut. There are many similarities between the role of microbiota and metformin effects on obesity and blood glucose regulation. It is therefore plausible to assume that common mechanisms are at play and metformin has a direct or indirect interaction with functions of the microbiota on the host to accomplish its anti-diabetic effects.
Two recent studies by Lee and Ko [148] and Shin et al. [149] show that metformin treatment of mice on a high-fat diet improves markers of metabolic disorders and provokes an alteration in the structure of faecal microbiota towards that of mice fed a normal chow diet. Using metagenomic analysis, these studies show that metformin causes a profound shift in specific subsets of bacterial taxa. The microbial change in mice treated with metformin was accompanied by an increase in the genus Akkermansia spp. [148,149]. Metformin led to an increase in goblet cells, producers of mucin, a nutrient source for the mucin-degrading bacteria Akkermansia municiphila. In fact, probiotic administration of this bacterial strain or metformin is associated with an improved metabolic profile, reducing metabolic endotoxemia, adipose tissue inflammation and improved glycaemic control insulin resistance [149,150]. Further studies to determine a causal link between metformin treatment and Akkermansia to promote its anti-diabetic effects are therefore warranted. An increase in Lactobacilli populations was also observed in high-fat diet mice treated with metformin. Lactobacillus is one of the many bacteria that can utilize glucose to produce lactate. Notably, effects of metformin in improving glucose homoeostasis in high-fat mice are abolished through a cocktail of broad-spectrum antibiotics [149]. These data suggest that gut microbiota is a target of metformin to produce its effects on host physiology. It is possible that extra lactate production in the intestine by metformin could derive, in addition to its production through non-oxidative metabolism by intestinal cells, from lactate-producing bacteria that utilize glucose. Alternatively, increased lactate and other gluconeogenic precursors, such as pyruvate and alanine by metformin [151], in addition to sustaining gluconeogenesis during fasting and protect against hypoglycaemia, could modify the structure of the microbiota by providing new energy substrates favouring specific microorganisms. Whether changes in the structure of the microbiota result directly from high concentrations of the drug in the intestine or indirectly from metabolites resulting from the host upon metformin treatment remains to be elucidated.
In a study performed in rats, Pyra et al. [152] showed that the novel combination therapy of prebiotic fibres such as oligofructose and metformin improves the metabolic profile of high-fat diet-induced obese rats. The combined action of metformin and oligofructose has an improved effect over each individual treatment alone on body weight, hepatic triglycerides, secretion of glucose-dependent insulinotropic polypeptide and leptin and AMPK activation. Effects of this therapeutic approach on gut microbiota were also evaluated in the present study. As expected, oligofructose treatment increased significantly bifidogenic bacteria such as Bifidobacterium spp. [152].
These studies show that metformin administration to rodents on high-fat diets induces significant modifications in the structure of the microbial populations in the gut. This leads to a new understanding of how metformin could affect metabolism to lower glycaemia through targeting the microbiota. However, further studies are required to fully elucidate what effect this has upon the host.
Metformin action on the human intestine and microbiota
Most anti-diabetic drugs have therapeutic doses in the microgram/milligram range to reduce hyperglycaemia in T2D patients. Interestingly, effective metformin doses can reach up to 2.5 g per day [153]. The intestine is the main tissue for metformin absorption [154] and accumulation [155] in humans and other animals. Absorption time T1/2 is estimated to be between 0.9 and 2.6 h and is affected by food intake which can delay the process by 35 min [156]. Metformin is stable, does not bind to proteins and has a bioavailability of 50%–60%. The drug reaches its maximum concentration in peripheral plasma approximately 2 h after oral ingestion, is eliminated from the plasma rapidly with a T1/2 of 1.7–4.5 h and excreted unchanged either in the urine or in the faeces [157]. Similar to what is observed in rodents, high concentrations of metformin also accumulate in the human intestine [155]. In fact, the concentration of metformin in the jejunum can attain 30–300-fold its concentration in the plasma [38] or other tissues [39]. Intralumenal concentrations in the range of 20 mM after administration of an 850 mg dose of metformin have been observed in the intestine of diabetic patients [158]. Therefore, it is likely that the effects of metformin on the intestinal tissue might be different to some extent in comparison with other tissues and also be responsible for the observed side effects associated with this drug.
The most common side effects associated with metformin administration include gastrointestinal upset and lactic acidosis. However, occurrence of lactic acidosis with metformin is an uncommon event (3–9/100000 patient-years) and very rarely causes death (2–4/100000 patient-years) [159]. On the other hand, disturbances of the gastrointestinal tract appear soon after the treatment is initiated, diminish over long periods of therapy and stop if the treatment is discontinued. These mainly include diarrhoea and other minor disorders such as abdominal pain with cramps and swelling, vomiting, nausea, dyspepsia, dysgeusia, flatulence and constipation. These combined digestive disorders can affect up to 29% of the patients treated with up to 2.5 g metformin/day, leading to discontinuation of metformin therapy in 10% of these patients [160]. Although these gastrointestinal effects are widely observed in patients taking metformin, the molecular mechanisms underlying such responses are still unclear. Only a few reports have addressed this question and some possible mechanisms have been put forward and reviewed by Bouchoucha et al. [161]. These include changes in serotonin metabolism, changes in incretin and glucose metabolism and alteration of bile acid metabolism. However, none fully explains the pathophysiological effects of metformin gastrointestinal side effects.
Recently, new studies suggest that metformin affects microbial metabolism in the gut. This is a new interesting hypothesis that could possibly explain inter-individual effects of metformin in digestive disorders. Karlsson et al. [162] evaluated the structure and function of faecal microbiota of a cohort of 145 70-year-old European women with normal, impaired and diabetic glucose control with metformin. Metagenomic analysis of T2D human patients treated with metformin show increased levels of enterobacteriaceae (e.g. Escherichia, Shigella, Klebsiella and Salmonella) and decreased levels of Clostridium and Eubacterium. Interestingly, there was a significant correlation of E. coli levels and glucagon-like peptide levels. Secretion of glucagon like peptide is reduced in obese and T2D patients [163] and metformin increases its levels in the plasma [164]. According to the authors of the present study, changes of structure and function of the microbiota in T2D patients treated with metformin might be a consequence of the treatment and increased glucose availability in the intestinal lumen.
Other side effects associated with long-term metformin therapy that could possibly be explained by the effects of metformin on microbiota are vitamin B12 and folate deficiency [139] and alterations in bile acid metabolism [165]. Bacteria such as Bifidobacterium are important suppliers of vitamins to their host. In addition, several anaerobic bacteria are involved in the transformation of bile acids in the intestine, whose main function is to facilitate the metabolism of dietary fat and absorption of fat-soluble vitamins [166]. Recently, Napolitano et al. [165] have characterized these interactions in human patients by relating glycaemic control, bile acid metabolism and microbiome alteration. These authors find important changes in the levels of members of the microbiota (Firmicutes/Bacteriodetes), changes in the entero-hepatic levels of bile acids (e.g. cholic acid) and entero-endocrine hormones (glucagon-like peptide-1 and peptide YY). Altogether, these suggest a mechanistic link between bile acid-induced microbial changes and glycaemic control in T2D patients. Interestingly, cholic acid administration to rodents produces similar changes in the relative abundance of Firmicutes and Bacteroidetes [167]. Altogether, new reports support the evidence from earlier studies showing that intravenous administration of metformin while attaining therapeutically effective blood concentrations does not improve blood glucose in stark contrast with oral administration of the drug [168,169]. However, how exactly metformin affects the intestine and the microbiota contained in it is still unclear. Therefore, new studies are required to understand how metformin affects physiology and re-evaluate its mode of action.
CONCLUSION
Metformin has been used as a safe and effective treatment for T2D for over half a century, yet the precise mechanism of action of this drug still remains elusive. The anti-hyperglycaemic properties of metformin are chiefly mediated by the suppression of hepatic gluconeogenesis and it is generally accepted that this is achieved via inhibition of complex I in the mitochondrial respiratory chain. The subsequent reduction in cellular energy status has been shown to directly impede gluconeogenic flux, interfere with glucagon signalling and promote activation of the major metabolic regulator AMPK, although which of these make the greatest contribution to metformin's therapeutic effects is a subject of debate. Moreover, it is becoming increasingly apparent that complex I is not the only molecular target of metformin; for example, it has recently been confirmed that metformin inhibits mGPD. It is highly probable that additional targets will be identified in the near future. Our work on C. elegans serves as a reminder that it is necessary to consider the effect of drugs not only on the individual but also on their microbiota. Very little is currently known about the bacterial targets of metformin and it is possible that the microbiota could regulate some of its effects on host physiology via unknown mechanisms. This undoubtedly warrants further investigation. Currently, metformin is only approved as a treatment for T2D, however in recent years, a vast number of studies have highlighted the therapeutic potential of metformin in the context of other diseases. In particular, metformin has shown promise as a treatment for CVD and cancer. The various mechanisms proposed to account for these beneficial effects have been outlined in the present review, although there are a number of outstanding issues that must still be resolved. Ultimately, a greater understanding of the molecular pathways involved will help to guide novel applications of metformin.
Acknowledgments
We thank Helena Cochemé for critical revision of the manuscript.
Abbreviations
- ACC
acetyl-CoA carboxylase
- Akt
protein kinase B
- AMPK
AMP-activated protein kinase
- Bcl-2
B-cell lymphoma 2
- CBP
CREB-binding protein
- ChREBP
carbohydrate-responsive element-binding protein
- CREB
cAMP response element-binding protein
- CRTC2
CREB-regulated transcription coactivator 2
- CVD
cardiovascular disease
- DR
dietary restriction
- eNOS
endothelial nitric oxide synthase
- FAS
fatty acid synthase
- IGF-1
insulin-like growth factor 1
- LKB1
liver kinase B1
- MDMX
murine double minute X
- mGPD
mitochondrial glycerophosphate dehydrogenase
- mPTP
mitochondrial permeability transition pore
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- OCT1
organic cation transporter 1
- PAI-1
plasminogen activator inhibitor 1
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator 1 α
- PKA
protein kinase A
- REDD1
regulated in development and DNA damage response 1
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- SREBP-1
sterol regulatory element-binding protein 1
- Stat3
signal transducer and activator of transcription 3
- T2D
type 2 diabetes
- TSC
tuberous sclerosis
- UKPDS
UK Prospective Diabetes Study
AUTHOR CONTRIBUTION
Rosina Pryor and Filipe Cabreiro contributed equally to this work.
FUNDING
This work was supported by the Medical Research Council [grant number MR/J003867/1 (to R.P.)]; and the Royal Society/Wellcome Trust (Sir Henry Dale fellowship) [grant number 102531/Z/13/Z (to F.C.)].
References
- 1.Bailey C., Day C. Metformin: its botanical background. Pract. Diabetes Int. 2004;21:115–117. doi: 10.1002/pdi.606. [DOI] [Google Scholar]
- 2.Watanabe C.K. Studies in the metabolic changes induced by administration of guanidine bases. J. Biol. Chem. 1918;33:253–265. I. Influence of injected guanidine hydrochloride upon blood sugar content. [Google Scholar]
- 3.Nattrass M., Alberti M. Biguanides. Diabetologia. 1978;74:71–74. doi: 10.1007/BF01263443. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO Model List of Essential Medicines. Geneva: World Health Organization; 2013. [Google Scholar]
- 5.Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation. Brussels: 2013. International Diabetes Federation IDF Diabetes Atlas. [Google Scholar]
- 7.Nathan D.M. Long-Term Complications of Diabetes-Mellitus. N. Engl. J. Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 8.Hundal R.S., Krssak M., Dufour S., Laurent D., Lebon V., Chandramouli V., Inzucchi S.E., Schumann W.C., Petersen K.F., Landau B.R., et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shu Y., Sheardown S.A., Brown C., Owen R.P., Zhang S., Castro R.A., Ianculescu A.G., Yue L., Lo J.C., Burchard E.G., et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Invest. 2007;117:1422–1431. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen M.R., Doran E., Halestrap A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000;348:607–614. doi: 10.1042/bj3480607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viollet B., Guigas B., Sanz Garcia N., Leclerc J., Foretz M., Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin. Sci. 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Mir M.-Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 13.Stephenne X., Foretz M., Taleux N., van der Zon G.C., Sokal E., Hue L., Viollet B., Guigas B. Metformin activates AMP-activated protein kinase in primary human hepatocytes by decreasing cellular energy status. Diabetologia. 2011;54:3101–3110. doi: 10.1007/s00125-011-2311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Repiscak P., Erhardt S., Rena G., Paterson M.J. Biomolecular mode of action of metformin in relation to its copper binding properties. Biochemistry. 2014;53:787–795. doi: 10.1021/bi401444n. [DOI] [PubMed] [Google Scholar]
- 15.Logie L., Harthill J., Patel K., Bacon S., Hamilton D.L., Macrae K., McDougall G., Wang H.-H., Xue L., Jiang H., et al. Cellular responses to the metal-binding properties of metformin. Diabetes. 2012;61:1423–1433. doi: 10.2337/db11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridges H.R., Jones A.J.Y., Pollak M.N., Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014;462:475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drahota Z., Palenickova E., Endlicher R., Milerova M., Brejchova J., Vosahlikova M., Svoboda P., Kazdova L., Kalous M., Cervinkova Z., et al. Biguanides inhibit complex I, II and IV of rat liver mitochondria and modify their functional properties. Physiol. Res. 2014;63:1–11. doi: 10.33549/physiolres.932600. [DOI] [PubMed] [Google Scholar]
- 18.Carling D., Mayer F.V., Sanders M.J., Gamblin S.J. AMP-activated protein kinase: nature's energy sensor. Nat. Chem. Biol. 2011;7:512–518. doi: 10.1038/nchembio.610. [DOI] [PubMed] [Google Scholar]
- 19.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw R.J., Lamia K.A., Vasquez D., Koo S.H., Bardeesy N., Depinho R.A., Montminy M., Cantley L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caton P.W., Nayuni N.K., Kieswich J., Khan N.Q., Yaqoob M.M., Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J. Endocrinol. 2010;205:97–106. doi: 10.1677/JOE-09-0345. [DOI] [PubMed] [Google Scholar]
- 23.He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M.A., Radovick S., Wondisford F.E. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foretz M., Hebrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fullerton M.D., Galic S., Marcinko K., Sikkema S., Pulinilkunnil T., Chen Z.P., O'Neill H.M., Ford R.J., Palanivel R., O'Brien M., et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi T., Osatomi K., Yamashita H., Kabashima T., Uyeda K. Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J. Biol. Chem. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang J., Parakhia R.A., Ochs R.S. Metformin activates AMP kinase through inhibition of AMP deaminase. J. Biol. Chem. 2011;286:1–11. doi: 10.1074/jbc.M110.121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng S., Cao J., He Q., Xiong L., Chang E., Radovick S., Wondisford F.E., He L. Metformin activates AMP-activated protein kinase by promoting formation of the alphabetagamma heterotrimeric complex. J. Biol. Chem. 2015;290:3793–3802. doi: 10.1074/jbc.M114.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawley S.A., Gadalla A.E., Olsen G.S., Hardie D.G. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 30.Miller R.A., Birnbaum M.J. An energetic tale of AMPK-independent effects of metformin. J. Clin. Invest. 2010;120:2267–2270. doi: 10.1172/JCI43661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGrane M.M., El-Maghrabi M.R., Pilkis S.J. The interaction of fructose 2,6-bisphosphate and AMP with rat hepatic fructose 1,6-bisphosphatase. J. Biol. Chem. 1983;258:10445–10454. [PubMed] [Google Scholar]
- 32.Burgess S.C., He T., Yan Z., Lindner J., Sherry A.D., Malloy C.R., Browning J.D., Magnuson M.A. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5:313–320. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel V.T., Beddow S.A., Iwasaki T., Zhang X.M., Chu X., Still C.D., Gerhard G.S., Shulman G.I. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc. Natl. Acad. Sci. U.S.A. 2009;106:12121–12126. doi: 10.1073/pnas.0812547106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller R.A., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelling R.W., Du X.Q., Dichmann D.S., Romer J., Huang H., Cui L., Obici S., Tang B., Holst J.J., Fledelius C., et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pernicova I., Korbonits M. Metformin–mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 37.He L., Wondisford F.E. Metformin action: concentrations matter. Cell Metab. 2015;21:159–162. doi: 10.1016/j.cmet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Tucker G.T., Casey C., Phillips P.J., Connor H., Ward J.D., Woods H.F. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br. J. Clin. Pharmacol. 1981;12:235–246. doi: 10.1111/j.1365-2125.1981.tb01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilcock C., Bailey C.J. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica. 1994;24:49–57. doi: 10.3109/00498259409043220. [DOI] [PubMed] [Google Scholar]
- 40.Cao J., Meng S., Chang E., Beckwith-Fickas K., Xiong L., Cole R.N., Radovick S., Wondisford F.E., He L. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK) J. Biol. Chem. 2014;289:20435–20446. doi: 10.1074/jbc.M114.567271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawley S.A., Ross F.A., Chevtzoff C., Green K.A., Evans A., Fogarty S., Towler M.C., Brown L.J., Ogunbayo O.A., Evans A.M., et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 43.Wilcock C., Bailey C.J. Sites of metformin-stimulated glucose metabolism. Biochem. Pharmacol. 1990;39:1831–1834. doi: 10.1016/0006-2952(90)90136-9. [DOI] [PubMed] [Google Scholar]
- 44.Duca F.A., Cote C.D., Rasmussen B.A., Zadeh-Tahmasebi M., Rutter G.A., Filippi B.M., Lam T.K. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 2015;21:506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madiraju A.K., Erion D.M., Rahimi Y., Zhang X-M, Braddock D.T., Albright R.A., Prigaro B.J., Wood J.L., Bhanot S., MacDonald M.J., et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrish N.J., Wang S.L., Stevens L.K., Fuller J.H., Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S14–S21. doi: 10.1007/PL00002934. [DOI] [PubMed] [Google Scholar]
- 47.UK Prosepctive Diabetes Study Group: Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 48.Johnson J.A., Simpson S.H., Toth E.L., Majumdar S.R. Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with type 2 diabetes. Diabet. Med. 2005;22:497–502. doi: 10.1111/j.1464-5491.2005.01448.x. [DOI] [PubMed] [Google Scholar]
- 49.Kooy A., de Jager J., Lehert P., Bets D., Wulffele M.G., Donker A.J., Stehouwer C.D. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch. Intern. Med. 2009;169:616–625. doi: 10.1001/archinternmed.2009.20. [DOI] [PubMed] [Google Scholar]
- 50.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 51.Batchuluun B., Inoguchi T., Sonoda N., Sasaki S., Inoue T., Fujimura Y., Miura D., Takayanagi R. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis. 2014;232:156–164. doi: 10.1016/j.atherosclerosis.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 52.Bhatt M.P., Lim Y.C., Kim Y.M., Ha K.S. C-peptide activates AMPKalpha and prevents ROS-mediated mitochondrial fission and endothelial apoptosis in diabetes. Diabetes. 2013;62:3851–3862. doi: 10.2337/db13-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anfosso F., Chomiki N., Alessi M.C., Vague P., Juhan-Vague I. Plasminogen activator inhibitor-1 synthesis in the human hepatoma cell line Hep G2. Metformin inhibits the stimulating effect of insulin. J. Clin. Invest. 1993;91:2185–2193. doi: 10.1172/JCI116445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He G., Pedersen S.B., Bruun J.M., Lihn A.S., Richelsen B. Metformin, but not thiazolidinediones, inhibits plasminogen activator inhibitor-1 production in human adipose tissue in vitro. Horm. Metab. Res. 2003;35:18–23. doi: 10.1055/s-2003-38386. [DOI] [PubMed] [Google Scholar]
- 55.Gin H., Freyburger G., Boisseau M., Aubertin J. Study of the effect of metformin on platelet aggregation in insulin-dependent diabetics. Diabetes Res. Clin. Pract. 1989;6:61–67. doi: 10.1016/0168-8227(89)90058-2. [DOI] [PubMed] [Google Scholar]
- 56.Davis B.J., Xie Z., Viollet B., Zou M.H. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 57.Boudina S., Abel E.D. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 58.Ren J., Dominguez L.J., Sowers J.R., Davidoff A.J. Metformin but not glyburide prevents high glucose-induced abnormalities in relaxation and intracellular Ca2+ transients in adult rat ventricular myocytes. Diabetes. 1999;48:2059–2065. doi: 10.2337/diabetes.48.10.2059. [DOI] [PubMed] [Google Scholar]
- 59.He C., Zhu H., Li H., Zou M.H., Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62:1270–1281. doi: 10.2337/db12-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie Z., He C., Zou M.H. AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy. 2011;7:1254–1255. doi: 10.4161/auto.7.10.16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhamra G.S., Hausenloy D.J., Davidson S.M., Carr R.D., Paiva M., Wynne A.M., Mocanu M.M., Yellon D.M. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res. Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 62.Paiva M.A., Rutter-Locher Z., Goncalves L.M., Providencia L.A., Davidson S.M., Yellon D.M., Mocanu M.M. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am. J. Phys. Heart Circ. Physiol. 2011;300:H2123–H2134. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calvert J.W., Gundewar S., Jha S., Greer J.J., Bestermann W.H., Tian R., Lefer D.J. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57:696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]
- 64.Timmermans A.D., Balteau M., Gelinas R., Renguet E., Ginion A., de Meester C., Sakamoto K., Balligand J.L., Bontemps F., Vanoverschelde J.L., et al. A-769662 potentiates the effect of other AMP-activated protein kinase activators on cardiac glucose uptake. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H1619–H1630. doi: 10.1152/ajpheart.00965.2013. [DOI] [PubMed] [Google Scholar]
- 65.Whittington H.J., Hall A.R., McLaughlin C.P., Hausenloy D.J., Yellon D.M., Mocanu M.M. Chronic metformin associated cardioprotection against infarction: not just a glucose lowering phenomenon. Cardiovasc. Drugs Ther. 2013;27:5–16. doi: 10.1007/s10557-012-6425-x. [DOI] [PubMed] [Google Scholar]
- 66.Yin M., van der Horst I.C., van Melle J.P., Qian C., van Gilst W.H., Sillje H.H., de Boer R.A. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H459–H468. doi: 10.1152/ajpheart.00054.2011. [DOI] [PubMed] [Google Scholar]
- 67.Ahmad A.S., Ormiston-Smith N., Sasieni P.D. Trends in the lifetime risk of developing cancer in Great Britain: comparison of risk for those born from 1930 to 1960. Br. J. Cancer. 2015;112:943–947. doi: 10.1038/bjc.2014.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta S.C., Sung B., Prasad S., Webb L.J., Aggarwal B.B. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol. Sci. 2013;34:508–517. doi: 10.1016/j.tips.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Evans J.M., Donnelly L.A., Emslie-Smith A.M., Alessi D.R., Morris A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franciosi M., Lucisano G., Lapice E., Strippoli GFM, Pellegrini F., Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevens R.J., Ali R., Bankhead C.R., Bethel M.A., Cairns B.J., Camisasca R.P., Crowe F.L., Farmer A.J., Harrison S., Hirst J.A., et al. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia. 2012;55:2593–2603. doi: 10.1007/s00125-012-2653-7. [DOI] [PubMed] [Google Scholar]
- 72.Thakkar B., Aronis K.N., Vamvini M.T., Shields K., Mantzoros C.S. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: a meta-analysis using primary data of published studies. Metabolism. 2013;62:922–934. doi: 10.1016/j.metabol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 73.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 74.Moiseeva O., Deschênes-Simard X., St-Germain E., Igelmann S., Huot G., Cadar A.E., Bourdeau V., Pollak M.N., Ferbeyre G. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell. 2013;12:489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- 75.Eikawa S., Nishida M., Mizukami S., Yamazaki C., Nakayama E., Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc. Natl. Acad. Sci. U.S.A. 2015;112:1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pearce E.L., Walsh M.C., Cejas P.J., Harms G.M., Shen H., Wang L-S, Jones R.G., Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janzer A., German N.J., Gonzalez-Herrera K.N., Asara J.M., Haigis M.C., Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc. Natl. Acad. Sci. U.S.A. 2014;111:10574–10579. doi: 10.1073/pnas.1409844111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Birsoy K., Possemato R., Lorbeer F.K., Bayraktar E.C., Thiru P., Yucel B., Wang T., Chen W.W., Clish C.B., Sabatini D.M. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wheaton W.W., Weinberg S.E., Hamanaka R.B., Soberanes S., Sullivan L.B., Anso E., Glasauer A., Dufour E., Mutlu G.M., Budigner G.S., et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. ELife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene. 2007;26:7825–7832. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- 81.Gotlieb W.H., Saumet J., Beauchamp M.C., Gu J., Lau S., Pollak M.N., Bruchim I. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol. Oncol. 2008;110:246–250. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 82.Shi W.Y., Xiao D., Wang L., Dong L.H., Yan Z.X., Shen Z.X., Chen S.J., Chen Y., Zhao W.L. Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis. 2012;3:e275. doi: 10.1038/cddis.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zakikhani M., Dowling R., Fantus I.G., Sonenberg N., Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 84.Inoki K., Zhu T., Guan K.-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 85.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., Turk B.E., Shaw R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faubert B., Boily G., Izreig S., Griss T., Samborska B., Dong Z., Dupuy F., Chambers C., Fuerth B.J., Viollet B., et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Menendez J.A., Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 88.Xiang X., Saha A.K., Wen R., Ruderman N.B., Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem. Biophys. Res. Commun. 321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 89.Algire C., Amrein L., Zakikhani M., Panasci L., Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr. Relat. Cancer. 2010;17:351–360. doi: 10.1677/ERC-09-0252. [DOI] [PubMed] [Google Scholar]
- 90.Cabreiro F., Au C., Leung K.Y., Vergara-Irigaray N., Cocheme H.M., Noori T., Weinkove D., Schuster E., Greene N.D., Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corominas-Faja B., Quirantes-Pine R., Oliveras-Ferraros C., Vazquez-Martin A., Cufi S., Martin-Castillo B., Micol V., Joven J., Segura-Carretero A., Menendez J.A. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging. 2012;4:480–498. doi: 10.18632/aging.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Locasale J.W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ulrich C.M. Folate and cancer prevention: a closer look at a complex picture. Am. J. Clin. Nutr. 2007;86:271–273. doi: 10.1093/ajcn/86.2.271. [DOI] [PubMed] [Google Scholar]
- 94.Blandino G., Valerio M., Cioce M., Mori F., Casadei L., Pulito C., Sacconi A., Biagioni F., Cortese G., Galanti S., et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat. Commun. 2012;3:865. doi: 10.1038/ncomms1859. [DOI] [PubMed] [Google Scholar]
- 95.Grelier G., Voirin N., Ay A.S., Cox D.G., Chabaud S., Treilleux I., Leon-Goddard S., Rimokh R., Mikaelian I., Venoux C., et al. Prognostic value of Dicer expression in human breast cancers and association with the mesenchymal phenotype. Br. J. Cancer. 2009;101:673–683. doi: 10.1038/sj.bjc.6605193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karube Y., Tanaka H., Osada H., Tomida S., Tatematsu Y., Yanagisawa K., Yatabe Y., Takamizawa J., Miyoshi S., Mitsudomi T., et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005;96:111–115. doi: 10.1111/j.1349-7006.2005.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merritt W.M., Lin Y.G., Han L.Y., Kamat A.A., Spannuth W.A., Schmandt R., Urbauer D., Pennacchio L.A., Cheng J.F., Nick A.M., et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N. Engl. J. Med. 2008;359:2641–2650. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones R.G., Plas D.R., Kubek S., Buzzai M., Mu J., Xu Y., Birnbaum M.J., Thompson C.B. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 99.He G., Zhang Y-W, Lee J-H, Zeng S.X., Wang Y.V., Luo Z., Dong X.C., Viollet B., Wahl G.M., Lu H. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Mol. Cell. Biol. 2014;34:148–157. doi: 10.1128/MCB.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeon S.M., Chandel N.S., Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shackelford D.B., Abt E., Gerken L., Vasquez D.S., Seki A., Leblanc M., Wei L., Fishbein M.C., Czernin J., Mischel P.S., et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Algire C., Moiseeva O., Deschênes-Simard X., Amrein L., Petruccelli L., Birman E., Viollet B., Ferbeyre G., Pollak M.N. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev. Res. 2012;5:536–543. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 103.Kalender A., Selvaraj A., Kim S.Y., Gulati P., Brule S., Viollet B., Kemp B.E., Bardeesy N., Dennis P., Schlager J.J., et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ben Sahra I., Laurent K., Loubat a., Giorgetti-Peraldi S., Colosetti P., Auberger P., Tanti J.F., Le Marchand-Brustel Y., Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 105.Ben Sahra I., Regazzetti C., Robert G., Laurent K., Le Marchand-Brustel Y., Auberger P., Tanti J.F., Giorgetti-Peraldi S., Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 106.Feng Y., Ke C., Tang Q., Dong H., Zheng X., Lin W., Ke J., Huang J., Yeung S.-C.J., Zhang H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5:e1088. doi: 10.1038/cddis.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salani B., Marini C., Rio A.D., Ravera S., Massollo M., Orengo A.M., Amaro A., Passalacqua M., Maffioli S., Pfeffer U., et al. Metformin impairs glucose consumption and survival in Calu-1 cells by direct inhibition of hexokinase-II. Sci. Rep. 2013;3:2070. doi: 10.1038/srep02070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marini C., Salani B., Massollo M., Amaro A., Esposito A.I., Orengo A.M., Capitanio S., Emionite L., Riondato M., Bottoni G., et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle. 2013;12:3490–3499. doi: 10.4161/cc.26461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song C.W., Lee H., Dings R.P., Williams B., Powers J., Santos T.D., Choi B.H., Park H.J. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci. Rep. 2012;2:362. doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dong L., Zhou Q., Zhang Z., Zhu Y., Duan T., Feng Y. Metformin sensitizes endometrial cancer cells to chemotherapy by repressing glyoxalase I expression. J. Obstet. Gynaecol. Res. 2012;38:1077–1085. doi: 10.1111/j.1447-0756.2011.01839.x. [DOI] [PubMed] [Google Scholar]
- 111.Iliopoulos D., Hirsch H.A., Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lengyel E., Litchfield L.M., Mitra A.K., Nieman K.M., Mukherjee A., Zhang Y., Johnson A., Bradaric M., Lee W., Romero I.L. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am. J. Obstet. Gynecol. 2014;212:479.e1–479.e10. doi: 10.1016/j.ajog.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Janjetovic K., Vucicevic L., Misirkic M., Vilimanovich U., Tovilovic G., Zogovic N., Nikolic Z., Jovanovic S., Bumbasirevic V., Trajkovic V., et al. Metformin reduces cisplatin-mediated apoptotic death of cancer cells through AMPK-independent activation of Akt. Eur. J. Pharmacol. 2011;651:41–50. doi: 10.1016/j.ejphar.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 114.Haq R., Shoag J., Andreu-Perez P., Yokoyama S., Edelman H., Rowe G.C., Frederick D.T., Hurley A.D., Nellore A., Kung A.L., et al. Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell. 2013;23:302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pollak M. Targeting oxidative phosphorylation: why, when, and how. Cancer Cell. 2013;23:263–264. doi: 10.1016/j.ccr.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 116.Cabreiro F., Gems D. Treating aging: progress toward dietary restriction mimetics. F1000 Biol. Rep. 2010;2:76. doi: 10.3410/B2-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lepez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The Hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mair W., Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 119.Masoro E.J., Yu B.P., Bertrand H.A. Action of food restriction in delaying the aging process. Proc. Natl. Acad. Sci. U.S.A. 1982;79:4239–4241. doi: 10.1073/pnas.79.13.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Walker G., Houthoofd K., Vanfleteren J.R., Gems D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech. Ageing Dev. 2005;126:929–937. doi: 10.1016/j.mad.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 121.Ingram D.K., Anson R.M., de Cabo R., Mamczarz J., Zhu M., Mattison J., Lane M.A., Roth G.S. Development of calorie restriction mimetics as a prolongevity strategy. Ann. N.Y. Acad. Sci. 2004;1019:412–423. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 122.Bakaev V. Effect of 1-butylbiguanide hydrochloride on the longevity in the nematoda Caenorhabditis elegans. Biogerontology. 2002;3(Suppl. 1):23–24. [Google Scholar]
- 123.Onken B., Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PloS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De Haes W., Frooninckx L., Van Assche R., Smolders A., Depuydt G., Billen J., Braeckman B.P., Schoofs L., Temmerman L. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc. Nal. Acad. Sci. U.S.A. 2014;111:E2501–E2509. doi: 10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.LaRue B.L., Padilla P.A. Environmental and genetic preconditioning for long-term anoxia responses requires AMPK in Caenorhabditis elegans. PLoS One. 2011;6:e16790. doi: 10.1371/journal.pone.0016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Slack C., Foley A., Partridge L. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS One. 2012;7:e47699. doi: 10.1371/journal.pone.0047699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mair W., Morantte I., Rodrigues A.P., Manning G., Montminy M., Shaw R.J., Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ulgherait M., Rana A., Rera M., Graniel J., Walker D.W. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8:1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.MacIntyre E.J., Majumdar S.R., Gamble J.M., Minhas-Sandhu J.K., Marrie T.J., Eurich D.T. Stress hyperglycemia and newly diagnosed diabetes in 2124 patients hospitalized with pneumonia. Am. J. Med. 2012;125:1036.e17–1036.e23. doi: 10.1016/j.amjmed.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 130.Shirazi F., Farmakiotis D., Yan Y., Albert N., Kim-Anh D., Kontoyiannis D.P. Diet modification and metformin have a beneficial effect in a fly model of obesity and mucormycosis. PLoS One. 2014;9:e108635. doi: 10.1371/journal.pone.0108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Na H.J., Park J.S., Pyo J.H., Lee S.H., Jeon H.J., Kim Y.S., Yoo M.A. Mechanism of metformin: inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech. Ageing Dev. 2013;134:381–390. doi: 10.1016/j.mad.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 132.Hans H., Lone A., Aksenov V., Rollo C.D. Impacts of metformin and aspirin on life history features and longevity of crickets: trade-offs versus cost-free life extension? Age (Dordr) 2015;37:31. doi: 10.1007/s11357-015-9769-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anisimov V.N. Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12:3483–3489. doi: 10.4161/cc.26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martin-Montalvo A., Mercken E.M., Mitchell S.J., Palacios H.H., Mote P.L., Scheibye-Knudsen M., Gomes A.P., Ward T.M., Minor R.K., Blouin M.J., et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosenberg E., Zilber-Rosenberg I. Symbiosis and development: the hologenome concept. Birth Defects Res. C Embryo Today. 2011;93:56–66. doi: 10.1002/bdrc.20196. [DOI] [PubMed] [Google Scholar]
- 136.O'Hara A.M., Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cho I., Blaser M.J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O'Connor E.M., Cusack S., Harris H.M., Coakley M., Lakshminarayanan B., O'Sullivan O., et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 139.Sahin M., Tutuncu N.B., Ertugrul D., Tanaci N., Guvener N.D. Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. J. Diabetes Complications. 2007;21:118–123. doi: 10.1016/j.jdiacomp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 140.Kang D., Yun J.S., Ko S.H., Lim T.S., Ahn Y.B., Park Y.M., Ko S.H. Higher prevalence of metformin-induced vitamin B12 deficiency in sulfonylurea combination compared with insulin combination in patients with type 2 diabetes: a cross-sectional study. PLoS One. 2014;9:e109878. doi: 10.1371/journal.pone.0109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wulffele M.G., Kooy A., Lehert P., Bets D., Ogterop J.C., Borger van der Burg B., Donker A.J., Stehouwer C.D. Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J. Intern. Med. 2003;254:455–463. doi: 10.1046/j.1365-2796.2003.01213.x. [DOI] [PubMed] [Google Scholar]
- 142.Lemaitre B., Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Ann. Rev. Genet. 2013;47:377–404. doi: 10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- 143.Shin S.C., Kim S.H., You H., Kim B., Kim A.C., Lee K.A., Yoon J.H., Ryu J.H., Lee W.J. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 144.Storelli G., Defaye A., Erkosar B., Hols P., Royet J., Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 145.Cognigni P., Bailey A.P., Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bailey C.J., Mynett K.J., Page T. Importance of the intestine as a site of metformin-stimulated glucose utilization. Br. J. Pharmacol. 1994;112:671–675. doi: 10.1111/j.1476-5381.1994.tb13128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Radziuk J., Bailey C.J., Wiernsperger N.F., Yudkin J.S. Metformin and its liver targets in the treatment of type 2 diabetes. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2003;3:151–169. doi: 10.2174/1568008033340298. [DOI] [PubMed] [Google Scholar]
- 148.Lee H., Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 2014;80:5935–5943. doi: 10.1128/AEM.01357-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shin N.R., Lee J.C., Lee H.Y., Kim M.S., Whon T.W., Lee M.S., Bae J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 150.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U.S.A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nattrass M., Todd P.G., Hinks L., Lloyd B., Alberti K.G. Comparative effects of phenformin, metformin and glibenclamide on metabolic rhythms in maturity-onset diabetics. Diabetologia. 1977;13:145–152. doi: 10.1007/BF00745143. [DOI] [PubMed] [Google Scholar]
- 152.Pyra K.A., Saha D.C., Reimer R.A. Prebiotic fiber increases hepatic acetyl CoA carboxylase phosphorylation and suppresses glucose-dependent insulinotropic polypeptide secretion more effectively when used with metformin in obese rats. J. Nutr. 2012;142:213–220. doi: 10.3945/jn.111.147132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Scarpello J.H., Howlett H.C. Metformin therapy and clinical uses. Diab. Vasc. Dis. Res. 2008;5:157–167. doi: 10.3132/dvdr.2008.027. [DOI] [PubMed] [Google Scholar]
- 154.Vidon N., Chaussade S., Noel M., Franchisseur C., Huchet B., Bernier J.J. Metformin in the digestive tract. Diabetes Res. Clin. Pract. 1988;4:223–229. doi: 10.1016/S0168-8227(88)80022-6. [DOI] [PubMed] [Google Scholar]
- 155.Bailey C.J., Wilcock C., Scarpello J.H. Metformin and the intestine. Diabetologia. 2008;51:1552–1553. doi: 10.1007/s00125-008-1053-5. [DOI] [PubMed] [Google Scholar]
- 156.Sambol N.C., Chiang J., O'Conner M., Liu C.Y., Lin E.T., Goodman A.M., Benet L.Z., Karam J.H. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J. Clin. Pharmacol. 1996;36:1012–1021. doi: 10.1177/009127009603601105. [DOI] [PubMed] [Google Scholar]
- 157.Pentikainen P.J., Neuvonen P.J., Penttila A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur. J. Clin. Pharmacol. 1979;16:195–202. doi: 10.1007/BF00562061. [DOI] [PubMed] [Google Scholar]
- 158.Proctor W.R., Bourdet D.L., Thakker D.R. Mechanisms underlying saturable intestinal absorption of metformin. Drug Metab. Dispos. 2008;36:1650–1658. doi: 10.1124/dmd.107.020180. [DOI] [PubMed] [Google Scholar]
- 159.Howlett H.C., Bailey C.J. A risk-benefit assessment of metformin in type 2 diabetes mellitus. Drug Saf. 1999;20:489–503. doi: 10.2165/00002018-199920060-00003. [DOI] [PubMed] [Google Scholar]
- 160.Scarpello J.H. Optimal dosing strategies for maximising the clinical response to metformin in type 2 diabetes. Br. J. Diabetes Vasc. Dis. 2001;1:28–36. doi: 10.1177/14746514010010010501. [DOI] [Google Scholar]
- 161.Bouchoucha M., Uzzan B., Cohen R. Metformin and digestive disorders. Diabetes Metabol. 2011;37:90–96. doi: 10.1016/j.diabet.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 162.Karlsson F.H., Tremaroli V., Nookaew I., Bergstrom G., Behre C.J., Fagerberg B., Nielsen J., Backhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 163.Vilsboll T., Krarup T., Deacon C.F., Madsbad S., Holst J.J. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–613. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 164.Maida A., Lamont B.J., Cao X., Drucker D.J. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-alpha in mice. Diabetologia. 2011;54:339–349. doi: 10.1007/s00125-010-1937-z. [DOI] [PubMed] [Google Scholar]
- 165.Napolitano A., Miller S., Nicholls A.W., Baker D., Van Horn S., Thomas E., Rajpal D., Spivak A., Brown J.R., Nunez D.J. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS One. 2014;9:e100778. doi: 10.1371/journal.pone.0100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 167.Islam K.B., Fukiya S., Hagio M., Fujii N., Ishizuka S., Ooka T., Ogura Y., Hayashi T., Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 168.Bonora E., Cigolini M., Bosello O., Zancanaro C., Capretti L., Zavaroni I., Coscelli C., Butturini U. Lack of effect of intravenous metformin on plasma concentrations of glucose, insulin, C-peptide, glucagon and growth hormone in non-diabetic subjects. Curr. Med. Res. Opin. 1984;9:47–51. doi: 10.1185/03007998409109558. [DOI] [PubMed] [Google Scholar]
- 169.Sum C.F., Webster J.M., Johnson A.B., Catalano C., Cooper B.G., Taylor R. The effect of intravenous metformin on glucose metabolism during hyperglycaemia in type 2 diabetes. Diabet. Med. 1992;9:61–65. doi: 10.1111/j.1464-5491.1992.tb01716.x. [DOI] [PubMed] [Google Scholar]