Abstract
The Diagnostic and Statistical Manual for Mental Disorders, Fifth Edition (DSM-5), recommends the World Health Organization Disability Assessment Schedule (WHODAS) 2.0 for routine clinical use. We tested the utility of the 12-item WHODAS 2.0 in prodromal Huntington disease. Using data from 726 participants and 630 companions over a 3-year follow-up, linear mixed models were fitted to test (1) baseline and longitudinal differences by progression group; (2) participant and companion differences within each group; and (3) sensitivity of the 12-item WHODAS in comparison to the 36-item WHODAS and the Total Functional Capacity (TFC) score from the Unified Huntington's Disease Rating Scale. Participants showed baseline group differences whereas companions showed baseline and longitudinal group differences. Companions reported worse functional decline over time than participants as the disease progresses. The 12-item WHODAS detected longitudinal change better than the 36-item WHODAS and the TFC in the medium progression group. Results suggest the 12-item WHODAS 2.0 can detect baseline and longitudinal differences in prodromal HD and may be useful in HD clinical trials.
Introduction
The Diagnostic and Statistical Manual for Mental Disorders, Fifth Edition (DSM-5), recommends the World Health Organization (WHO) Disability Assessment Schedule (WHODAS) 2.0 as a disability measure for routine clinical use.1 There are two versions of the WHODAS 2.0—a full version with 36 items and a short version with 12 items. Previously, we reported that the total score of the 36-item WHODAS 2.0 can detect longitudinal changes in daily function in prodromal (before motor diagnosis) HD, and is better at detecting changes in an earlier stage of HD than the Total Functional Capacity (TFC) from the Unified Huntington's Disease Rating Scale.2 The current study investigates the performance of the global score of the 12-item version in a large observational study of people with prodromal HD. The aims of the current study are (i) to test disease progression group differences in baseline values and longitudinal change in a separate analysis of participant and companion ratings on the 12-item WHODAS 2.0; (ii) to compare longitudinal participant and companion ratings on the 12-item version; and (iii) to assess the relative sensitivity of the 12-item version in comparison with the full version and the TFC, in terms of detecting baseline and longitudinal differences.
Materials and methods
Participants
A total of 726 participants and 630 companions were administered the WHODAS 2.0 (starting in 2009) in the Neurobiological Predictors of HD (PREDICT-HD) study3 with up to 3.11 years of follow-up. On the basis of the HD gene-expansion test results, individuals were classified as cases, if the cytosine-adenine-guanine (CAG) expansion ≥36 or controls, if CAG<36. Cases were classified into three progression groups based on their CAG-Age Product (CAP) capturing different disease progression levels at baseline.4 For the low, medium, and high CAP groups, the estimated times to motor diagnosis were >12.8, 7.6–12.8, and <7.6 years, respectively. Four groups were defined in this analysis: control, low, medium, and high. For more details, please refer to the study by Downing et al.2
Measures
The full WHODAS includes 36 items in six domains: understanding and communicating; getting around; self-care; getting along with others; activities at home, work, and school; and participation in society. Each item has five response categories (1=none; 2=mild; 3=moderate; 4=severe; 5=extreme/cannot do). The 12-item WHODAS includes two items from each domain, with a total score computed as the sum of the 12 items.1 To compare the TFC and WHODAS, the TFC was scaled as TFC loss so that higher scores indicate worse function for all outcomes.
Statistical analysis
Analysis 1: To examine group differences in baseline status and longitudinal change, participant and companion ratings were analyzed separately using linear mixed effects regression (LMER).5 Three nested models were fitted for each outcome and compared by Akaike's information criterion. Analysis 2: To test whether longitudinal changes of participant and companion ratings were equal within each group, participant and companion data were analyzed simultaneously. Sixteen possible candidate models were fitted and a model averaging method6 was applied to find reliable slope discrepancies. Fitted curves were drawn using model-averaged parameters over all models. Analysis 3: To evaluate the sensitivity of the 12-item WHODAS, comparison was made to the TFC and the 36-item WHODAS. For each scaled outcome, the model with baseline and longitudinal group effects was fitted and effect sizes were compared among the three outcomes. In all analyses, models were adjusted for gender, years of education, and age at entry.
Results
Progression group differences (Aim 1)
Table 1 shows the best model for each outcome, and the estimated baseline and longitudinal differences between controls and each progression group. Participants showed baseline group differences only, whereas companions showed both baseline and longitudinal group differences. Compared with controls, participants in the medium and high progression groups reported significantly worse functioning at baseline, and companions of those in the high group reported significantly worse functioning at baseline. In contrast, only companions of those in the medium and high groups reported significantly worse functional decline over time.
Table 1. Comparison of World Health Organization Disability Assessment Schedule (WHODAS) 12-item scores between the control group and each gene expanded group for the best models.
| Group differences relative to controls | ||||||
|---|---|---|---|---|---|---|
| Baseline differences | Longitudinal differences | |||||
| Measure (best model) | Low est. (SE) | Med est. (SE) | High est. (SE) | Low est. (SE) | Med est. (SE) | High est. (SE) |
| Participant 12-item Total (baseline group effect model) | 0.031 (0.106) | 0.224 (0.092)* | 0.309 (0.087)*** | |||
| Companion 12-item Total (baseline+longitudinal group effect model) | 0.187 (0.106) | 0.099 (0.094) | 0.301 (0.091)*** | −0.057 (0.099) | 0.268 (0.091)** | 0.269 (0.085)** |
Abbreviations: Est., estimate; SE, standard error; Med, medium. Low, Medium, and High groups indicate low, medium, and high estimated probability of motor diagnosis in 5 years, respectively. ***P<0.001, **P<0.01, *P<0.05.
Self- vs informant-rated WHODAS (Aim 2)
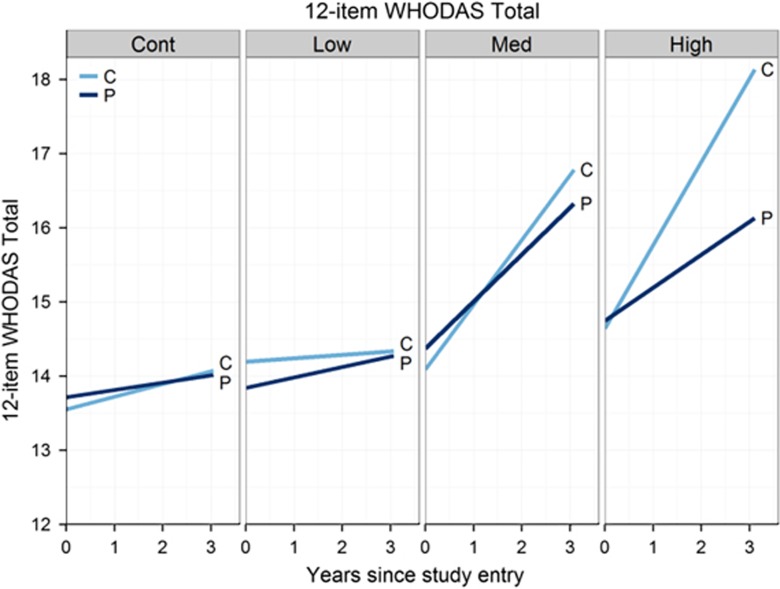
Figure 1 shows the fitted curves for participant and companion outcomes (analyzed simultaneously). The participant (dark blue) and companion (light blue) curves were relatively similar in each group from the control to the medium group, indicating similar rates of change over time in participant and companion ratings. In contrast, there was a divergence between the two curves in the high group, indicating companions reported worse functional decline over time than participants. The divergence in the high group was statistically more reliable than the other groups.
Figure 1.
Fitted linear mixed effects regression curves by group for participant (P) and companion (C) ratings of the 12-item World Health Organization Disability Assessment Schedule (WHODAS) 2.0.
Sensitivity of 12-item WHODAS (Aim 3)
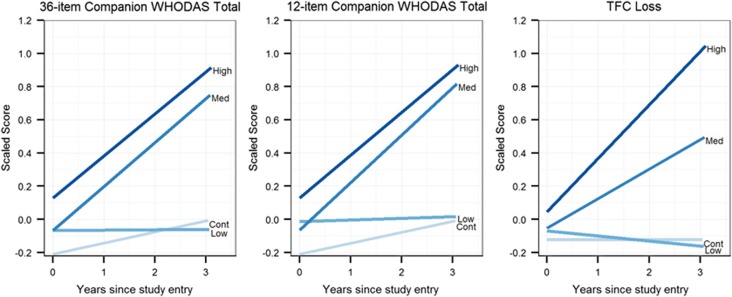
We compared the companion 12-item WHODAS total to the TFC and companion 36-item WHODAS total, focusing on companions because they better detected longitudinal change than participants.2 Figure 2 shows fitted curves for the scaled WHODAS and TFC. At baseline, the 12-item WHODAS (z=3.55) had a larger difference than TFC loss (z=2.33) but a smaller difference than the 36-item WHODAS (z=3.68) in the high group. For longitudinal changes, the 12-item WHODAS (z=2.45) showed a larger difference than both the 36-item WHODAS (z=2.35) and TFC loss (z=1.93) in the medium group. The 12-item WHODAS (z=2.27) showed a smaller difference than the 36-item WHODAS (z=2.33) and TFC loss (z=3.76) in the high group.
Figure 2.
Fitted linear mixed effects regression curves by group for scaled companion 36-item World Health Organization Disability Assessment Schedule (WHODAS) 2.0, companion 12-item WHODAS 2.0, and Total Functional Capacity (TFC) loss scores.
Discussion
This is the first study that we know of that has undertaken a longitudinal analysis of the 12-item WHODAS 2.0 in a prodromal HD sample. Both participant and companion ratings showed baseline differences by disease progression group, whereas only companion ratings showed longitudinal change differences by disease progression group. To detect differences in baseline levels and longitudinal change by progression group, the 12-item WHODAS seems to be a useful daily function measure in prodromal HD and may be useful for future clinical trials in HD. Companions reported a faster decline of daily function over time than participants in the high group, suggesting participants might experience declining self-awareness of functional changes owing to frontal-subcortical dysfunction, which occurs in HD.7 This result indicates proxy measures may be more reliable than self-reported ones in later stages of prodromal HD disease progression, and supports the DSM-5 recommendation of using proxy measures when individuals have impaired cognitive function. Our findings with the 12-item WHODAS were consistent with the previous longitudinal analysis of the 36-item WHODAS.2
For individuals with prodromal HD, it has been challenging to find sensitive daily function measures that can detect longitudinal change.8 When we assessed the ability of the 12-item WHODAS 2.0 in comparison with the TFC and the 36-item WHODAS 2.0, the short version of the companion WHODAS detected more reliable functional decline over time than the full version and TFC in the medium group. The 12-item WHODAS was also able to detect longitudinal change in the high group and had similar functional decline over time compared with the 36-item WHODAS. In addition, only 10% of respondents showed longitudinal change for the TFC unlike the 12-item WHODAS, where 68% showed change over time. Therefore, the 12-item WHODAS seems to have better sensitivity in detecting longitudinal change in an earlier stage of prodromal HD than the TFC and the 36-item WHODAS. The current study presents evidence that the 12-item WHODAS 2.0 has similar psychometric properties to the full version. This suggests the importance of selecting reliable items for a reduced measure. In a separate analysis (unpublished), people with gene-expansion showed longitudinal differences relative to controls in three subscales—understanding, getting along, and life activities. Using the largest sample of participants with prodromal HD to date, our findings suggest the short version can be used as a general index of HD disease progression. The 12-item version was better able to detect longitudinal change in daily functioning in the earlier stages of prodromal HD than the TFC and the 36-item WHODAS. The improved detection of functional decline and the briefer administration time of the short version suggest it might be a useful part of a clinical trial battery to test the ability of new treatments and interventions to preserve daily function in prodromal HD. The effect of individual items on the performance of the 12-item WHODAS total is an area of future research.
Acknowledgments
We thank the PREDICT-HD sites, the study participants, the National Research Roster for Huntington Disease Patients and Families, the Huntington's Disease Society of America and the Huntington Study Group. This publication was supported by the National Center for Advancing Translational Sciences, and the National Institutes of Health (NIH), through Grant 2 UL1 TR000442-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This research is supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (5R01NS040068) awarded to Dr Paulsen, CHDI Foundation, Inc (A3917) awarded to Dr Paulsen, Cognitive and Functional Brain Changes in Preclinical Huntington's Disease (HD) (5R01NS054893) awarded to Dr Paulsen, 4D Shape Analysis for Modeling Spatiotemporal Change Trajectories in Huntington's (1U01NS082086), Functional Connectivity in Premanifest Huntington's Disease (1U01NS082083), and Basal Ganglia Shape Analysis and Circuitry in Huntington's Disease (1U01NS082085).
Footnotes
Dr Paulsen and Dr Williams' work has been funded by the National Institutes of Health. Dr Kim, Dr Long, Mr Mills and Dr Downing declare no potential conflicts of interest.
Contributor Information
the PREDICT-HD Investigators and Coordinators of the Huntington Study Group:
Stephen Cross, Patricia Ryan, Isabella De Soriano, Courtney Shadrick, Amanda Miller, Eric A Epping, Edmond Chiu, Joy Preston, Anita Goh, Stephanie Antonopoulos, Samantha Loi, Phyllis Chua, Angela Komiti, Lynn Raymond, Joji Decolongon, Mannie Fan, Allison Coleman, Christopher A Ross, Mark Varvaris, Maryjane Ong, Nadine Yoritomo, William M Mallonee, Greg Suter, Ali Samii, Emily P Freney, Alma Macaraeg, Randi Jones, Cathy Wood-Siverio, Stewart A Factor, Roger A Barker, Sarah Mason, Natalie Valle Guzman, Elizabeth McCusker, Jane Griffith, Clement Loy, Jillian McMillan, David Gunn, Michael Orth, Sigurd Süβmuth, Katrin Barth, Sonja Trautmann, Daniela Schwenk, Carolin Eschenbach, Kimberly Quaid, Melissa Wesson, Joanne Wojcieszek, Mark Guttman, Alanna Sheinberg, Albie Law, Irita Karmalkar, Susan Perlman, Brian Clemente, Michael D Geschwind, Sharon Sha, Joseph Winer, Gabriela Satris, Tom Warner, Maggie Burrows, Anne Rosser, Kathy Price, Sarah Hunt, Frederick Marshall, Amy Chesire, Mary Wodarski, Charlyne Hickey, Peter Panegyres, Joseph Lee, Maria Tedesco, Brenton Maxwell, Joel Perlmutter, Stacey Barton, Shineeka Smith, Zosia Miedzybrodzka, Daniela Rae, Vivien Vaughan, Mariella D'Alessandro, David Craufurd, Judith Bek, Elizabeth Howard, Pietro Mazzoni, Karen Marder, Paula Wasserman, Rajeev Kumar, Diane Erickson, Christina Reeves, Breanna Nickels, Vicki Wheelock, Lisa Kjer, Amanda Martin, Sarah Farias, Wayne Martin, Oksana Suchowersky, Pamela King, Marguerite Wieler, Satwinder Sran, Anwar Ahmed, Stephen Rao, Christine Reece, Alex Bura, Lyla Mourany, Jane S Paulsen, Eric A Epping, Megan M Smith, Jeffrey D Long, Hans J Johnson, Jeremy H Bockholt, Kelsey Montross, Jean Paul Vonsattell, Carol Moskowitz, Stacie Vik, Deborah Harrington, Tamara Hershey, Holly Westervelt, Megan M Smith, David J Moser, Janet Williams, Nancy Downing, Hans J Johnson, Elizabeth Aylward, Christopher A Ross, Vincent A Magnotta, Stephen Rao, Eric A Epping, David Craufurd, Jeffrey D Long, Ji-In Kim, James A Mills, Ying Zhang, Dawei Liu, Wenjing Lu, Spencer Lourens, Cheryl Erwin, Eric A Epping, Janet Williams, Martha Nance, H. Jeremy Bockholt, and Ryan Wyse
References
- 1Ustun TB, Chatterji S, Kostanjsek N et al: Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ 2010; 88: 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2Downing NR, Kim JI, Williams JK et al: WHODAS 2.0 in prodromal Huntington disease: measures of functioning in neuropsychiatric disease. Eur J Hum Genet 2014; 22: 958–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Paulsen JS, Hayden M, Stout JC et al: Preparing for preventive clinical trials: the Predict-HD study. Arch Neurol 2006; 63: 883–890. [DOI] [PubMed] [Google Scholar]
- 4Zhang Y, Long JD, Mills JA, Warner JH, Lu WJ, Paulsen JS: Indexing disease progression at study entry with individuals at-risk for Huntington disease. Am J Med Genet B Neuropsychiatr Genet 2011; 156B: 751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Verbeke G, Molenberghs G: Linear mixed models for longitudinal data. New York: Springer, 2000. [Google Scholar]
- 6Burnham KP, Anderson DR: Model Selection and Multimodel Inference: a Practical Information-theoretic Approach, 2nd edn. New York: Springer, 2002. [Google Scholar]
- 7Duff K, Paulsen JS, Beglinger LJ et al: "Frontal" behaviors before the diagnosis of Huntington's disease and their relationship to markers of disease progression: evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci 2010; 22: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Paulsen JS, Wang C, Duff K et al: Challenges assessing clinical endpoints in early Huntington disease. Mov Disord 2010; 25: 2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]