Abstract
The protein tribbles-1, encoded by the gene TRIB1, is increasingly recognized as a major regulator of multiple cellular and physiological processes in humans. Recent human genetic studies, as well as molecular biological approaches, have implicated this intriguing protein in the aetiology of multiple human diseases, including myeloid leukaemia, Crohn's disease, non-alcoholic fatty liver disease (NAFLD), dyslipidaemia and coronary artery disease (CAD). Genome-wide association studies (GWAS) have repeatedly identified variants at the genomic TRIB1 locus as being significantly associated with multiple plasma lipid traits and cardiovascular disease (CVD) in humans. The involvement of TRIB1 in hepatic lipid metabolism has been validated through viral-mediated hepatic overexpression of the gene in mice; increasing levels of TRIB1 decreased plasma lipids in a dose-dependent manner. Additional studies have implicated TRIB1 in the regulation of hepatic lipogenesis and NAFLD. The exact mechanisms of TRIB1 regulation of both plasma lipids and hepatic lipogenesis remain undetermined, although multiple signalling pathways and transcription factors have been implicated in tribbles-1 function. Recent reports have been aimed at developing TRIB1-based lipid therapeutics. In summary, tribbles-1 is an important modulator of human energy metabolism and metabolic syndromes and worthy of future studies aimed at investigating its potential as a therapeutic target.
Keywords: cardiovascular disease, GWAS, lipid metabolism, lipoproteins, Trib1, Tribbles
Introduction
The tribbles family of proteins are being recognized as modulators of many fundamental signalling pathways, biological processes and disease pathologies [1,2]. As the term suggests, this family of pseudokinase proteins is characterized by a distinct lack of kinase activity [3]. However, the past 15 years of research have revealed a myriad of other active functions for these as yet poorly understood proteins. Beyond participating in the regulation of fundamental cellular processes, such as cell cycle progression and proliferation, tribbles proteins are increasingly recognized as potential therapeutic targets. A great deal of prior research has centred on the role of tribbles-1 (TRIB1) in the development and progression of leukaemia [4–6], but more recently unbiased human genetic studies have ignited interest in a role for tribbles-1 in human lipoprotein metabolism and cardiovascular disease (CVD) pathogenesis [9,12,13].
Genetic associations of TRIB1 locus with plasma lipids, liver transaminases and coronary artery disease
CVD is the leading cause of death in the developed world [7]. Dyslipidaemia, in particular high plasma levels of lipoproteins containing apolipoprotein B (apoB) as well as high circulating triglyceride (TG) levels, are the most important risk factors for atherosclerotic CVD [8]. This remains the case despite the widespread success of lipid-lowering therapies such as statins and thus there remains a need for novel therapeutics that might further treat dyslipidaemia and CVD in humans. Genome-wide association studies (GWAS) provide an unbiased approach that can potentially identify such novel biological pathways involved in regulation of plasma lipids that might serve as potential therapeutic targets and in recent years much effort has been spent on GWAS to identify loci in the genome associated with plasma lipids and CVD.
Early GWAS of plasma lipid levels in smaller cohorts of humans (N ≅ 10000) identified a handful of novel genomic loci not previously known to play any role in lipid metabolism. One of these loci exhibiting a significant association with plasma TG levels was the 8q24 locus, with the lead single nucleotide polymorphism (SNP) in these studies falling into a linkage-disequilibrium block that contains the gene TRIB1 [9]. Subsequent studies replicated this finding [10,11], including a landmark GWAS performed by Global Lipids Genetics Consortium (GLGC), which in 2010 published a GWAS analysis for plasma lipid traits and coronary artery disease (CAD) in > 100000 subjects, yielding a total of 95 independent loci associated with at least one major lipid trait, more than two-thirds of which are associated with low-density lipoprotein (LDL)-C and/or TG [12]. This study increased the number of novel plasma lipid loci to 59 and of these novel associations only the TRIB1 locus was associated with all five traits examined: total cholesterol (TC), LDL-C, high-density lipoprotein (HDL)-C, TG and CAD [12]. The most recent GLGC GWAS has identified 157 loci as significantly associated with plasma lipids in humans and TRIB1 remains one of only four loci associated with all plasma lipid traits examined [13].
Independently, the TRIB1 locus has been shown by GWAS to be in association with levels of circulating alanine transaminase (ALT) in humans [14]. High circulating ALT levels can be suggestive of hepatocellular damage [15] and may be a surrogate marker for fatty liver [16]. The authors of the GWAS study specifically tested in ∼10000 individuals the association of SNPs in the TRIB1 region with liver abnormalities identified by computed tomography (CT) scanning that are indicative of hepatic steatosis. Although the TRIB1 locus did show strong associations with hepatic structural abnormalities, this association did not reach statistical significance after correcting for multiple testing [14]. More recently, researchers in Japan tested the association of three SNPs in the TRIB1 genomic region with ultrasonographic non-alcoholic fatty liver disease (NAFLD) in ∼5000 Japanese females and saw significant associations between the SNPs and NAFLD [17]. Contrary to this finding, a larger GWAS study aimed at identifying genomic loci associated with NAFLD as ascertained by CT scanning did not find the TRIB1 locus to be one of the significantly associated genes [18]. These disparate results, however, may be in part due to the difficulty in ascertaining hepatic fat content via non-invasive techniques in large numbers of patients. More highly powered NAFLD GWAS studies are likely to definitively determine the association of TRIB1 with NAFLD in humans, but currently the evidence strongly suggests that this association does exist.
The association of TRIB1 with CAD was definitively demonstrated in a separate GWAS performed by the CARDIoGRAMplusC4D consortium in ∼200000 individuals aimed at identifying novel CAD loci [19]. Since the larger GWAS are mainly carried out in humans of European descent, targeted studies have shown that the associations of TRIB1 with plasma lipids replicate in both African American, as well as Indian populations [20,21].
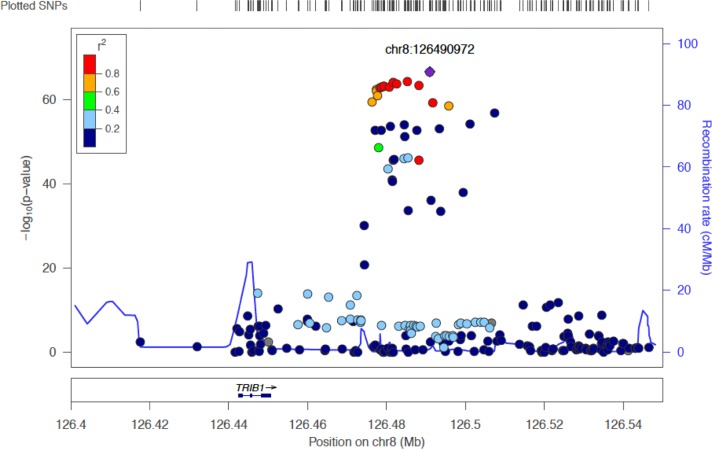
The preponderance of associations in multiple populations combined with the magnitude of these associations clearly indicates that the genomic region containing TRIB1 plays a critical role in human lipid metabolism. However, one shortcoming of these studies is that the causal variants in this locus have yet to be identified. Indeed, as part of the 2010 GLGC report, genome-wide significant SNPs were assayed for expression quantitative trait locus (eQTL) association with hepatic transcript levels of nearby genes and no eQTL was identified between TRIB1 and nearby SNPs despite the GWAS signal lying ∼40 kb downstream of the transcript in the same linkage disequilibrium block as TRIB1 and with no other gene within 100 kb (Figure 1). A recent report from Douvris et al. [22] found an eQTL between one SNP in the GWAS region and TRIB1 transcript levels in whole blood from 160 patients. The authors also found that SNPs in the GWAS region affect the transcription of a long non-coding RNA that they dubbed TRIBAL and suggested that this may, in part, underlie some of the genetic association in the region. Clearly, this region requires further conditional analyses, fine-mapping and more powerful investigations of SNP–transcript interactions utilizing strategies such as RNA-seq and allele-specific expression, before determining exactly how many genetic signals are present in the region and what the downstream functional effects of the SNPs in these regions are.
Figure 1. Association of TRIB1 region with plasma TG levels.
The 8q24 interval is depicted showing the location of the TRIB1 gene and the downstream SNPs identified as having significant associations with plasma TGs in humans. The left y-axis measures the P-value of the SNPs and the colour of each SNPs indicate its r2-value relative to the lead SNP (rs2954029). Association data to TGs is from the 2013 GLGC study [13] and the figure was generated using LocusZoom [44].
In vivo validation of tribbles-1 as a regulator of plasma lipid metabolism
Despite the next closest gene to the 8q24 GWAS signal being >100 kb away, it cannot be simply assumed that TRIB1 is the causal gene involved in modulating plasma lipid metabolism. Burkhardt et al. [23] utilized an adeno-associated virus (AAV) system to express tribbles-1 in vivo via hepatic overexpression of murine Trib1 in adult C57B/6 mice. The authors cloned the Trib1 coding sequence in front of the thyroxin-binding globulin (TBG) liver-specific promoter and established stable liver-specific overexpression using AAV serotype 8, known for its high liver specificity and affinity [24]. This overexpression system is ideal for testing the involvement of genes identified by GWAS in hepatic lipid metabolism [25].
The authors observed that overexpression of Trib1 in the livers of wild–type (WT) mice resulted in reduced levels of plasma cholesterol and TG in a dose-dependent manner. The decreases in cholesterol and TG were present in all lipoprotein fractions examined and FPLC revealed a significant reduction in the very low-density lipoprotein (VLDL)–TG peak, suggestive of a VLDL-specific mechanism of regulation. The authors repeated the Trib1 overexpression in various mouse models of lipid metabolism, including the LDL receptor (LDLR)-deficient hyperlipidaemic model and the Ldlr KO (knockout)/Apobec1 KO/human apoB transgenic (LAhB) humanized mouse model that has a lipid profile more closely resembling that of humans. In all mouse models tested, Trib1 overexpression resulted in significant reductions in plasma cholesterol and TG. In the LAhB mice, Trib1 overexpression caused a significant reduction in plasma apoB protein, the main apolipoprotein component of VLDL and LDL. The investigators also measured many of the same lipid parameters in a previously reported Trib1 whole-body KO mouse model and saw the expected reciprocal results as compared with the AAV overexpression model.
When the investigators assessed VLDL–TG secretion into the plasma of these mice after treatment with Pluronic-407 (a detergent blocking lipolysis and thus clearance of plasma TG), they found that mice treated with hepatic overexpression of Trib1 had decreased TG secretion. Primary hepatocytes from these mice exhibited not only decreased TG secretion into the media, but also decreased cellular TG content. Indeed when the investigators examined the hepatic transcription of lipogenic genes, they found reduced expression of key genes in the fatty acid synthetic pathway such as acetyl-CoA carboxylase1 (Acc1), fatty acid synthase (Fasn) and stearoyl-coenzyme A desaturase1 (Scd1), among others. Primary hepatocytes expressing tribbles-1 were thus proven to have reduced de novo lipogenesis. Thus a model was formed that increased Trib1 expression can reduce lipogenesis and inhibit VLDL secretion, perhaps through insufficient lipidation of nascent apoB protein.
Mechanisms of TRIB1 regulation of lipid metabolism
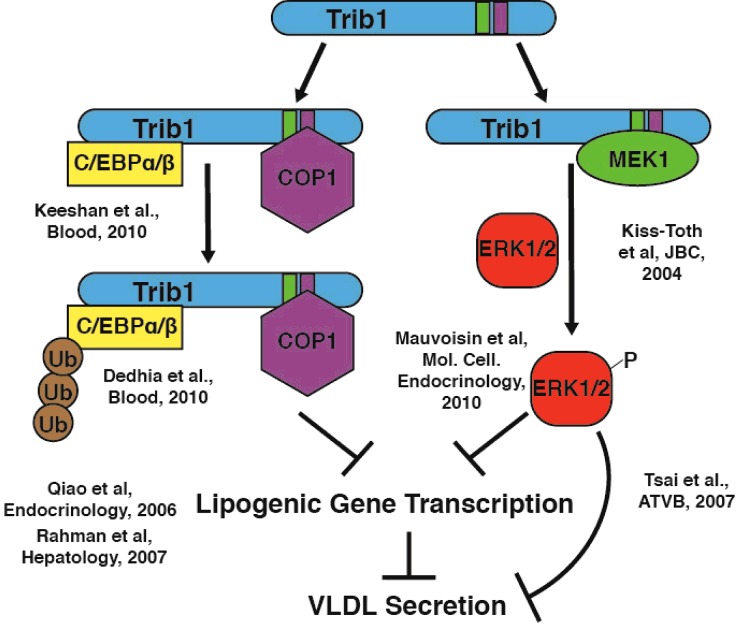
Although the work of Burkhardt et al. [23] helped shed light on the physiological roles of Trib1 in lipid metabolism, the exact molecular mechanism governing this regulation remains to be elucidated. Tribbles-1 has two well-defined functions in the literature: (1) facilitating the ubiquitination of the transcription factor CCAAT/enhancer-binding protein α (C/EBPα) and thus promoting its degradation [26] and (2) regulating mitogen-activated protein (MAP) kinase signalling by facilitating the phosphorylation of extracellular signal-regulated kinase (ERK)1/2 by the tyrosine/threonine kinase MAP kinase kinase 1 (MEK1) [27]. Both of these pathways have been shown to participate to some degree in the regulation of lipid metabolism (Figure 2).
Figure 2. Proposed mechanisms for tribbles-1 regulation of plasma lipids in humans.
Previous work has identified roles for tribbles-1 in regulating both the ubiquitination and the turnover of the transcription factor C/EBPα, as well as the phosphorylation and activation of ERK1/2 by MEK kinase [26,27]. Both of these pathways have the potential to modulate plasma lipids in humans (relevant citations are shown); however, neither has been directly tested in a hepatic setting with modulation of TRIB1 protein or transcript.
The tribbles protein was originally identified in a Drosophila mutagenesis screen; the trb mutation results in defects in cell migration and mitosis during oogenesis [28–30]. These early studies determined that the role of Trb in Drosophila oogenesis is to promote the proteasomal degradation of String, Twine and Slbo, the latter of which is the Drosophila homologue of the human C/EBPα gene [31]. Subsequent work in the myeloblast 32D cell line has shown that tribbles-1 and tribbles-2 induce the proteasomal degradation of C/EBPα and C/EBPβ by promoting their ubiquitination by the E3 ligase constitutively photomorphogenic 1 (COP1) through direct binding to both the targets and the ligase [26,32]. C/EBPα has long been recognized as important for energy homoeostasis since Wang et al. [33] reported in 1995 that neonates with whole body deletion of C/EBPα died perinatally due to lack of glycogen and hypoglycaemia. These neonates also exhibited a distinct lack of lipids in their hepatocytes and adipocytes. Prior studies have implicated C/EBPα in the regulation of hepatic lipogenesis in mouse models of obesity. Matsusue et al. [34] showed that hepatic deficiency of C/EBPα in the leptin-deficient ob/ob mouse model of obesity abbrogated fatty liver caused by a high-carbohydrate diet. Additionally, the authors found that mice with hepatic C/EBPα deficiency had decreased lipogenic gene expression and decreased transcription of SREBP1, a master regulator of fatty acid synthesis. Qiao et al. [35] repeated most of these findings in the diabetic db/db mouse model by treating those mice with adenovirus containing siRNA directed to C/EBPα. Interestingly C/EBPα deficiency in ob/ob mice decreased plasma TGs but not TC, whereas in the db/db mice there was decreased plasma cholesterol but not plasma TGs. These reports support a role for C/EBPα in the regulation of lipid metabolism by TRIB1, and indeed this model was recently confirmed by a report from our group elucidating the roles of Trib1 and C/EBPα in hepatic lipid metabolism [36].
As previously mentioned, tribbles-1 has been shown to modulate MAP kinase signalling in HeLa and NIH3T3 cells by promoting the phosphorylation of ERK1/2 by MEK1, a finding not yet replicated in vivo [27]. To date, there has been no evidence for the direct phosphorylation of a target by tribbles proteins [37]. Phosphorylation of ERK1/2 has previously been shown in HepG2 cells to down-regulate the expression of Scd1, one of the lipogenic genes consistently differentially expressed in experimental models of Trib1 overexpression [38]. Additionally, Tsai et al. [39] showed that inhibition of MEK/ERK signalling using the ERK1/2 inhibitor U0126 corrected a defect in VLDL assembly in HepG2 cells, greatly increasing the secretion of apoB. The directionality of these observations is in agreement with the observed lipid phenotypes of mice with hepatic overexpression of Trib1, thus implicating altered MAP kinase signalling as a potential mechanism of metabolic regulation by tribbles-1.
More recently, Ishizuka et al. [17] published a Trib1 overexpression/knockdown study using adenovirus and these mice had physiological phenotypes consistent with the report from Burkhardt et al. [23]. They also showed that tribbles-1 could regulate both the mRNA and the protein levels of the transcription factor MLX-interacting protein-like (MLXIPL) (also known as carbohydrate-responsive element-binding protein (ChREBP), another known regulator of lipogenic gene expression [40]. Epitope-tagged tribbles-1 and MLXIPL interacted in both mammalian two-hybrid assays and overexpression pulldown studies in COS7 cells. The authors showed that protein levels of MLXIPL could be restored to WT levels in Trib1-overexpressing COS7 cells via treatment with proteasome inhibitors. The authors concluded that tribbles-1 affects lipogenesis through its interaction with MLXIPL. The extent to which all of these proposed mechanisms affect the regulation of lipid metabolism still needs to be dissected, ideally through genetic epistasis experiments in relevant animal models.
Tribbles-1 as a target for novel therapeutics
One of the main hopes for the unbiased genetic studies outlined earlier in this article has been to uncover novel biology pertaining to lipid metabolism that might be exploited for the production of novel therapeutics. Based on the above described research, therapeutics aimed at increasing levels of TRIB1 message or protein or small molecules that can increase the function of tribbles-1 could positively affect the plasma lipid profile and cardiovascular health of a patient. Indeed, a recent study from the Broad Institute identified a series of benzofuran-based compounds that could up-regulate transcription of TRIB1 in HepG2 cells [41]. Ultimately, these compounds had wide ranging effects on the lipid metabolism of these cells, many of which were independent of TRIB1 expression. The push for TRIB1-based therapeutics would benefit from a thorough elucidation of the upstream genetic regulators of this gene. Additionally, continued investigation into the important molecular functions of tribbles-1 and the portions of the protein required for such functions may help inform the development of small molecules that increase tribbles-1 function. Nevertheless, this study suggests that both the interest and potential exist for the development of TRIB1-based therapeutics.
Conclusions
The tribbles proteins are increasingly being shown to participate in a wide variety of fundamental cellular processes and tribbles-1 is no exception. Tribbles1 has the added appeal of being identified as significantly and reproducibly associated with multiple human pathologies by human genetics. Not only is the TRIB1 gene associated with lipid traits and CVD but also with Crohn's disease, plasma adiponectin levels (an adipokine linked to obesity) and plasma liver enzyme levels (a surrogate readout for fatty liver) in humans [14,42,43]. It remains unclear why the other tribbles proteins, known to affect metabolic signalling pathways, have not been identified in many of these GWAS. Nonetheless, tribbles-1 is increasingly recognized as a major regulator of human pathologies. Recent work has focused on identifying novel interactions between tribbles-1 and other proteins and it is likely that more of these interactions will be identified as the tool set for studying tribbles-1 expands [17,45]. However, it is important that researchers in the field attempt to dissect which physiological readouts are governed by which specific interactions. Clearly the tribbles-1 protein can significantly affect the normal biology of cells or tissues and thus one of the challenges moving forward will be discerning which of these molecular phenotypes are directly due to the actions of tribbles-1 itself, or are instead downstream of the primary mechanism of tribbles-1 action. These studies will help inform the development of novel therapeutics that take advantage of the fascinating biology governed by tribbles-1.
Abbreviations
- AAV
adeno-associated virus
- ALT
alanine transaminase
- apoB
apolipoprotein B
- C/EBP
CCAAT/enhancer-binding protein
- CAD
coronary artery disease
- CVD
cardiovascular disease
- ERK
extracellular signal-regulated kinase
- GLGC
Global Lipids Genetics Consortium
- GWAS
Genome-wide association studies
- KO
knockout
- LAhB
Ldlr KO/Apobec1 KO/human apoB transgenic
- LDL
low-density lipoprotein
- LDLR
LDL receptor
- MAP
mitogen-activated protein
- MEK1
mitogen-activated protein kinase kinase 1
- NAFLD
non-alcoholic fatty liver disease
- Scd1
stearoyl-coenzyme A desaturase1
- SNP
single nucleotide polymorphism
- TBG
thyroxin-binding globulin
- TC
total cholesterol
- TG
triglyceride
- VLDL
very low-density lipoprotein
- WT
wild-type
Footnotes
Tribbles Pseudokinases on the Crossroads of Metabolism, Cancer, Immunity and Development: Held at The Aquincum Hotel, Budapest, 22–24 April 2015
Funding
This work was supported by NIH R01-HL109489 (to D.J.R.), a Transatlantic Network of Excellence grant 10CVD03 from the Fondation Leducq (to D.J.R.), and American Heart Association postdoctoral fellowship 12POST12040456 (to R.C.B.).
References
- 1.Kiss-Toth E. Tribbles: “puzzling” regulators of cell signalling. Biochem. Soc. Trans. 2011;39:684–687. doi: 10.1042/BST0390684. [DOI] [PubMed] [Google Scholar]
- 2.Lohan F., Keeshan K. The functionally diverse roles of tribbles. Biochem. Soc. Trans. 2013;41:1096–1100. doi: 10.1042/BST20130105. [DOI] [PubMed] [Google Scholar]
- 3.Hegedus Z., Czibula A., Kiss-Toth E. Tribbles: a family of kinase-like proteins with potent signalling regulatory function. Cell Signal. 2007;19:238–250. doi: 10.1016/j.cellsig.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama T., Nakamura T. Tribbles in disease: signaling pathways important for cellular function and neoplastic transformation. Cancer Sci. 2011;102:1115–1122. doi: 10.1111/j.1349-7006.2011.01914.x. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama T., Toki T., Aoki Y., Kanezaki R., Park M. Identification of TRIB1 R107L gain-of-function mutation in human acute megakaryocytic leukemia. Blood. 2012;19:2608–2611. doi: 10.1182/blood-2010-12-324806. [DOI] [PubMed] [Google Scholar]
- 6.Liang K.L., Rishi L., Keeshan K. Tribbles in acute leukemia. Blood. 2013;121:4265–4270. doi: 10.1182/blood-2012-12-471300. [DOI] [PubMed] [Google Scholar]
- 7.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Franco S., et al. Executive summary: heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 8.Glass C.K., Witztum J.L. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/S0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 9.Willer C.J., Sanna S., Jackson A.U., Scuteri A., Bonnycastle L.L., Clarke R., Heath S.C., Timpson N.J., Najjar S.S., Stringham H.M., et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varbo A., Benn M., Tybjaerg-Hansen A., Grande P., Nordestgaard B.G. TRIB1 and GCKR polymorphisms, lipid levels, and risk of ischemic heart disease in the general population. Arterioscler. Thromb. Vasc. Biol. 2011;31:451–457. doi: 10.1161/ATVBAHA.110.216333. [DOI] [PubMed] [Google Scholar]
- 11.Kraja A.T., Vaidya D., Pankow J.S., Goodarzi M.O., Assimes T.L., Kullo I.J., Sovio U., Mathias R.A., Sun Y.V., Franceschini N., et al. A bivariate genome-wide approach to metabolic syndrome: STAMPEED consortium. Diabetes. 2011;60:1329–1339. doi: 10.2337/db10-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teslovich T.M., Musunuru K., Smith A.V., Edmondson A.C., Stylianou I.M., Koseki M., Pirruccello J.P., Ripatti S., Chasman D.I., Willer C.J., et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global Lipids Genetics Consortium, Willer C.J., Schmidt E.M., Sengupta S., Peloso G.M., Gustafsson S., Kanoni S., Ganna A., Chen J., Buchkovich M.L., et al. Global Lipids Genetics Consortium. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers J.C., Zhang W., Sehmi J., Li X., Wass M.N., Van der Harst P., Holm H., Sanna S., Kavousi M., Baumeister S.E., et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat. Genet. 2011;43:1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratt D.S., Kaplan M.M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. New Engl. J. Med. 2000;342:1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 16.Nugent C., Younossi Z.M. Evaluation and management of obesity-related nonalcoholic fatty liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007;4:432–441. doi: 10.1038/ncpgasthep0879. [DOI] [PubMed] [Google Scholar]
- 17.Ishizuka Y., Nakayama K., Ogawa A., Makishima S., Boonvisut S., Hirao A., Iwasaki Y., Yada T., Yanagisawa Y., Miyashita H., et al. TRIB1 downregulates hepatic lipogenesis and glycogenesis via multiple molecular interactions. J. Mol. Endocrinol. 2014;52:145–158. doi: 10.1530/JME-13-0243. [DOI] [PubMed] [Google Scholar]
- 18.Speliotes E.K., Yerges-Armstrong L.M., Wu J., Hernaez R. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CardioGRAMplusC4D Consortium, Deloukas P., Kanoni S., Willenborg C., Farrall M., Assimes T.L., Thompson J.R., Ingelsson E., Saleheen D., Erdmann J., et al. CARDIoGRAMplusC4D Consortium. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musunuru K., Romaine S., Lettre G., Wilson J.G. Multi-ethnic analysis of lipid-associated loci: the NHLBI CARe project. PLoS One. 2012;7:e36473. doi: 10.1371/journal.pone.0036473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walia G.K., Gupta V., Aggarwal A., Asghar M., Dudbridge F., Timpson N., Sign N.S., Kumar M.R., Kinra S., Prabhakaran D., et al. Association of common genetic variants with lipid traits in the Indian population. PLoS ONE. 2014;9:e101688–e101688. doi: 10.1371/journal.pone.0101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douvris A., Soubeyrand S., Naing T., Martinuk A., Nikpay M., Williams A., Buick J., Yauk C., McPherson R. Functional analysis of the TRIB1 associated locus linked to plasma triglycerides and coronary artery disease. J. Am. Heart Assoc. 2014;3:e000884. doi: 10.1161/JAHA.114.000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkhardt R., Toh S-A, Lagor W.R., Birkeland A., Levin M., Li X., Robblee M., Fedorov V.D., Yamamoto M., Satoh T., et al. Trib1 is a lipid- and myocardial infarction-associated gene that regulates hepatic lipogenesis and VLDL production in mice. J. Clin. Invest. 2010;120:4410–4414. doi: 10.1172/JCI44213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagor W.R., Johnston J.C., Lock M., Vandenberghe L.H., Rader D.J. Adeno-associated viruses as liver-directed gene delivery vehicles: focus on lipoprotein metabolism. Methods Mol. Biol. 2013;1027:273–307. doi: 10.1007/978-1-60327-369-5. [DOI] [PubMed] [Google Scholar]
- 25.Bauer R.C., Stylianou I.M., Rader D.J. Functional validation of new pathways in lipoprotein metabolism identified by human genetics. Curr. Opin. Lipidol. 2011;22:123–128. doi: 10.1097/MOL.0b013e32834469b3. [DOI] [PubMed] [Google Scholar]
- 26.Dedhia P.H., Keeshan K., Uljon S., Xu L., Vega M.E., Shestova O., Zaks-Zilberman M., Romany C., Blacklow S.C., Pear W.S. Differential ability of Tribbles family members to promote degradation of C/EBPalpha and induce acute myelogenous leukemia. Blood. 2010;116:1321–1328. doi: 10.1182/blood-2009-07-229450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiss-Toth E., Bagstaff S.M., Sung H.Y., Jozsa V., Dempsey C., Caunt J.C., Oxley K.M., Wyllie D.H., Polgar T., Harte M., et al. Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. J. Biol. Chem. 2004;279:42703–42708. doi: 10.1074/jbc.M407732200. [DOI] [PubMed] [Google Scholar]
- 28.Seher T.C., Leptin M. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr. Biol. 2000;10:623–629. doi: 10.1016/S0960-9822(00)00502-9. [DOI] [PubMed] [Google Scholar]
- 29.Grosshans J., Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell. 2000;101:523–531. doi: 10.1016/S0092-8674(00)80862-4. [DOI] [PubMed] [Google Scholar]
- 30.Mata J., Curado S., Ephrussi A., Rørth P. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell. 2000;101:511–522. doi: 10.1016/S0092-8674(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 31.Rørth P., Szabo K., Texido G. The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation. Mol. Cell. 2000;6:23–30. doi: 10.1016/S1097-2765(05)00008-0. [DOI] [PubMed] [Google Scholar]
- 32.Keeshan K., He Y., Wouters B.J., Shestova O., Xu L., Sai H., Rodriguez C.G., Maillard I., Tobias J.W., Valk P., et al. Tribbles homolog 2 inactivates C/EBPα and causes acute myelogenous leukemia. Cancer Cell. 2006;10:401–411. doi: 10.1016/j.ccr.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang N.D., Finegold M.J., Bradley A., Ou C.N., Abdelsayed S.V., Wilde M.D., Taylor L.R., Wilson D.R., Darlington G.J. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- 34.Matsusue K., Gavrilova O., Lambert G., Brewer H.B., Ward J.M., Inoue Y., LeRoith D., Gonzalez F.J. Hepatic CCAAT/enhancer binding protein mediates induction of lipogenesis and regulation of glucose homeostasis in leptin-deficient mice. Mol. Endocrinol. 2004;18:2751–2764. doi: 10.1210/me.2004-0213. [DOI] [PubMed] [Google Scholar]
- 35.Qiao L., MacLean P.S., You H., Schaack J., Shao J. Knocking down liver CCAAT/enhancer-binding protein by adenovirus-transduced silent interfering ribonucleic acid improves hepatic gluconeogenesis and lipid homeostasis in db/db mice. Endocrinology. 2006;147:3060–3069. doi: 10.1210/en.2005-1507. [DOI] [PubMed] [Google Scholar]
- 36.Bauer R.C., Sasaki M., Cohen D.M., Cui J., Smith M.A., Yenilmez B.O., Steger D.J., Rader D.J. Tribbles-1 regulates hepatic lipogenesis through post-transcriptional regulation of C/EBPα. J. Clin. Invest. (In Press) 2015 doi: 10.1172/JCI77095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowers A.J., Scully S., Boylan J.F. SKIP3, a novel Drosophila tribbles ortholog, is overexpressed in human tumors and is regulated by hypoxia. Oncogene. 2003;22:2823–2835. doi: 10.1038/sj.onc.1206367. [DOI] [PubMed] [Google Scholar]
- 38.Mauvoisin D., Prévost M., Ducheix S., Arnaud M-P, Mounier C. Key role of the ERK1/2 MAPK pathway in the transcriptional regulation of the stearoyl-CoA desaturase (SCD1) gene expression in response to leptin. Mol. Cell. Endocrinol. 2010;319:116–128. doi: 10.1016/j.mce.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 39.Tsai J., Qiu W., Kohen-Avramoglu R., Adeli K. MEK-ERK inhibition corrects the defect in VLDL assembly in HepG2 cells: potential role of ERK in VLDL-ApoB100 particle assembly. Arterioscler. Thromb. Vasc. Biol. 2007;27:211–218. doi: 10.1161/01.ATV.0000249861.80471.96. [DOI] [PubMed] [Google Scholar]
- 40.Dentin R., Benhamed F., Hainault I., Fauveau V., Foufelle F., Dyck J.R.B., Girard J., Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 41.Nagiec M.M., Skepner A.P., Negri J., Eichhorn M., Kuperwasser N., Comer E., Muncipinto G., Subramanian A., Clish C., Musunuru K., et al. Modulators of hepatic lipoprotein metabolism identified in a search for small-molecule inducers of tribbles pseudokinase 1 expression. PLoS One. 2015;10:e0120295–e0120226. doi: 10.1371/journal.pone.0120295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franke A., McGovern D.P.B., Barrett J.C., Wang K., Radford-Smith G.L., Ahmad T., Lees C.W., Balschun T., Lee J., Roberts R., et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dastani Z., Hivert M.F., Timpson N., Perry J., Yuan X. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pruim R.J., Welch R.P., Sanna A., Teslovich T.M., Chines P.S., Giledt T.P., Boehnke M., Abecasis G.R., Willer C.J. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makishima S., Boonvisut S., Ishizuka Y., Watanabe K., Nakayama K., Iwamoto S. Sin3A-associated protein, 18 kDa, a novel binding partner of TRIB1, regulates MTTP expression. J. Lipid Res. 2015;56:1145–1152. doi: 10.1194/jlr.M057802. [DOI] [PMC free article] [PubMed] [Google Scholar]