Abstract
It is almost 40 years since the drug efflux pump P-glycoprotein (permeability glycoprotein or P-gp) was shown to confer multi-drug resistance in cancer cells. This protein has been one of the most extensively investigated transport proteins due to its intriguing mechanism and its affect in oncology. P-gp is known to interact with over 300 compounds and the ability to achieve this has not yet been revealed. Following the binding of substrate and nucleotide, a complex series of conformational changes in the membrane and cytosolic domains translocates substrate across the membrane. Despite over 30 years of biochemical investigation, the availability of structural data and a plethora of chemical tools to modulate its function, the molecular mechanism remains a mystery. In addition, overcoming its activity in resistant cancer cells has not been achieved in the clinic, thereby garnering some degree of pessimism in the field. This review highlights the progress that has been achieved in understanding this complex protein and the value of undertaking molecular studies.
Keywords: ATP-binding cassette (ABC) protein, cancer chemotherapy, membrane transport, molecular mechanism, multi-drug resistance, permeability (P)-glycoprotein
What's the point of molecular detail?
Recently I overheard a conversation between a departmental colleague and a graduate student regarding transport proteins. The student stated that she would measure substrate accumulation in a cell line and determine the Michaelis–Menten parameters for the transport process. The academic retorted: ‘I cannot understand why people measure KMs, VMs and all that stuff. Why would you bother?’ I resisted the temptation to pull out a soap box and harangue this colleague, but the question remained implanted in my thoughts.
Why do I bother pursuing molecular details on membrane proteins using a fundamental biochemistry approach? Perhaps recalling how I entered the fray may help. Near the end of my PhD tenure I was conducting a ritual browse through biochemical journals to pick a research topic for post-doctoral studies. Suddenly, an article in Scientific American on ‘primitive defence mechanism’ that had been hijacked by cancer cells to ensure their survival caught my attention [1]. The article described this phenotype from the point of view of a patient and how the problem was being tackled at a molecular level. Moreover, it displayed a captivating cartoon of a single protein, permeability glycoprotein (P-glycoprotein/P-gp), which was able to ensure the survival of cancer cells in the face of sustained attack from cytotoxic drugs. How could this be I wondered, with vivid recall of ‘locks and keys’ from my undergraduate biochemistry course? This topic has consumed my research career ever since and it was the thrill of the unexplained that caught my attention all those years ago.
I can understand that this topic is not high on everyone's list, but why the dismissive attitude towards fundamental research displayed by this colleague? It seems that a broader disinterest, disillusionment or dislike of fundamental biochemical research has emerged in the last decade (Figure 1). Are fundamental research questions and endeavours valued anymore? Should all biomedical research have a near immediate and tangible benefit from an economic standpoint? Have scientists also become obsessed with ‘budget bottom lines’ and pre-occupied with a desire for ‘bankable projects’ and ‘translational research’? Perhaps we have lost the desire to explore and provide an understanding of complex biological phenomena without justification on economic grounds? Should we simply ignore the intricacies and complexity of the natural world and merely view it as a tool to generate profit? There is nothing wrong with translational research, but surely it cannot completely replace fundamental research. This review provides a brief (and far from exhaustive) account of how research into the molecular mechanism of P-gp has progressed since its discovery in 1976. It is important to note that the field has also benefitted greatly from equally heroic efforts with other ATP-binding cassette (ABC) proteins and transporters from distinct families. This review cannot provide a wide sweep of these efforts and is restricted to studies exclusively with P-gp. Progress has been slow at times but the timeline demonstrates the creativity, resourcefulness, stirring debate and co-operation of scientists in the field. Hopefully it will provide an answer to the question posed by my colleague!
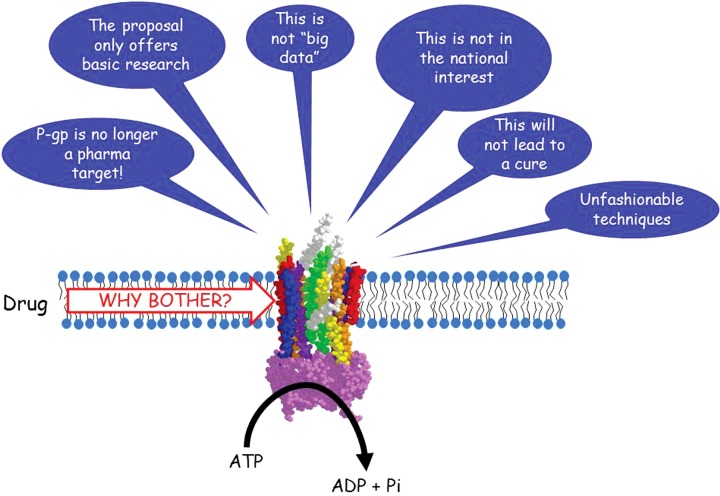
Figure 1. The frustrations of fundamental research.
A schematic representation of the low-resolution structure of membrane bound P-gp to illustrate its ability to bind and transport drugs in an ATP-dependent manner. The quotation bubbles represent a ‘rogue's gallery’ of statements from grant and/or manuscript reviewers received over the years by fundamental biochemists.
Towards an experimental tool
Dano [2] demonstrated that a drug-resistant cancer cell line was associated with an ATP-dependent reduction in the accumulation of daunomycin. Juliano and Ling [3] took this key observation further and demonstrated that the phenotype was associated with expression of a 170000 Da surface glycoprotein in cell lines selected for anti-cancer drug resistance [3]. The identity of this P-gp was revealed by labelling cells with radioactive saccharides, fractionating with a cumbersome polymer separation process and detection with PAGE. Over the next few years, Ling and co-workers [4] (plus a rapidly expanding series of competitors) painstakingly revealed features of P-gp, such as its dependence on ATP, its stable cellular half-life, plasma membrane localization, a rich glycosylation pattern and extensive phosphorylation [5–7]. Today, many of these elements represent a throwaway line at the start of most manuscripts on P-gp, yet their genesis was born of numerous long outdated technologies.
The cell biology and pharmacology of P-gp continued to be investigated using an expanding number of cultured cell lines and a great deal of information regarding the substrate promiscuity of the protein was revealed. The transport process was ATP-dependent and presumably this was mediated by P-gp. In order to directly attribute the ATP hydrolysis to P-gp a method of isolating the protein was required. The first reported purification of P-gp used sequential lectin columns to make use of the rich glycosylation status of the protein [8]. However, this gallant procedure required the use of harsh, denaturing detergents and produced low yields. The protein was used to raise and validate the first antisera to P-gp. The first published account of active purified P-gp was obtained by Hamada and Tsuruo [9], in 1988, using affinity chromatography with a monoclonal antibody. The purified protein was not reconstituted into lipid vesicles, but was demonstrated to hydrolyse ATP at a rate of ∼2 nmol·min−1·mg−1 protein. This is roughly 500–1000-fold lower than the generally accepted rate of ATP hydrolysis by P-gp [10].
Progress was slow by current standards and two reports in 1992 reveal that partially purified P-gp displayed considerably greater, and drug stimulated, ATPase activity [11,12]. These procedures used mild detergents and conventional chromatography such as ion-exchange. By 1994–1995, the level of purification had been greatly increased and the activities retained an upward surge. Reconstitution procedures remained difficult and the exhaustive efforts of Rigaud et al. [13] with detergent adsorption by BioBeads as a general procedure for reconstitution caught the eyes of P-gp investigators. In addition, the importance of crude lipid mixtures during extraction and purification to prevent P-gp delipidation and inactivation were documented [14]. By the late 1990s, purification and reconstitution of P-gp from cancer cell lines using conventional chromatography media became de rigueur for the field. In 1995, a game-changing purification procedure was reported by Loo and Clarke [15]; namely the purification of P-gp containing a poly-histidine tag using metal affinity chromatography. The use of poly-histidine tags had only been developed by the early 1990s and rapidly supplanted conventional chromatography for routine protein purification, thereby rendering membrane protein purification a relatively simple, one-step procedure. Moreover, it is now possible to characterize multiple mutations of P-gp and bears stark contrast with the early heroic endeavours with a single isoform and cumbersome procedures.
P-gp: a hungry beast
The ATP-dependent cellular transport of drugs by P-gp suggested that the protein may be an ATPase, although the proof was not obtained for 12 years. It was not until the early 1990s that preparations of partially purified P-gp were used to show that anti-cancer drugs may stimulate ATP hydrolysis by P-gp [11,12]. This finally demonstrated that P-gp was a coupled, primary active transporter, which was only inferred from cellular studies.
The presence of two nucleotide-binding domains (NBDs) fostered considerable debate regarding the mechanism of hydrolysis and its conformational coupling to drug movement. The presence of endogenous cysteine residues in the NBDs, proximal to the WalkerA/B motifs, enabled the use of thiol-reactive compounds to covalently modify P-gp. Full inactivation of ATPase activity by P-gp was achieved with a stoichiometry of 1 mole covalent reagent per mole of P-gp [16]. This was the first evidence that both catalytic sites are essential to maintain P-gp function. Furthermore, photoaffinity-labelling with ATP analogues demonstrated equivalent distribution of label between the two NBDs; this indicates that both NBD domains are also catalytically active [17]. The use of vanadate to produce a highly stable Pgp-VO3-ADP (post-hydrolytic) intermediate, again at a 1:1 stoichiometry, proffered a model of catalysis that involved hydrolysis of nucleotide by the NBDs in an alternating sequence. These findings were confirmed (and extended) using isoforms of P-gp with one or more catalytic residues mutated. For example, the mutation of the two catalytic glutamate residues to alanine (E552A/E1197A) produced protein with an ‘occluded’ ATP molecule at one NBD [18]. This occluded model suggests that two ATP molecules are initially bound loosely by the NBDs. Dimerization ensues and one nucleotide is hydrolysed, with the other remaining tightly bound and ‘earmarked’ for hydrolysis in the subsequent translocation event. There are various modifications of this catalytic model (i.e. switch and constant contact) and the field continues to debate the exact mechanism [19].
While this gradual illumination of the catalytic process was underway, the field of structural biology endeavoured to describe the dimeric interaction between NBDs. The ‘murky waters’ of NBD dimerization were founded on the three distinct dimer interfaces obtained for the HisP, MalK and Rad50 proteins [20–22]. The three different dimer assemblies gave rise to distinct roles for motifs such as the signature sequence and the invariant histidine and D-loop. This was despite similar protein folds, structural resolution and overall topography of the monomer units. The accepted head-to-tail arrangement of the NBDs was only confirmed once the full-length structure of BtuCD was obtained [23]. The NBD dimer arrangement was similar to that of Rad50, wherein an ATP molecule is sandwiched between the Walker A/B motifs of one NBD and the signature sequence of the alternate NBD. The controversy with NBD dimer arrangement was frustrating at that point in time since changes in the structural model necessitated significant re-interpretation of much mutagenesis data. In the long run, the incremental development of our collective understanding stimulated new avenues of investigation and produced an elegant model for the structural disposition of ABC transporters.
The eternal question of how P-gp binds such an array of drugs
At current count, P-gp has been estimated to bind over 300 compounds, which certainly renders the protein an enigmatic one. Resolution of how P-gp interacts with so many compounds will provide a paradigm shift for the molecular basis of drug–protein interaction, applicable to transporters, receptors and enzymes.
The use of panels of cell lines and different chemotherapy agents ultimately led to the discovery of Mrp1 (ABCC1) [24] and BCRP (ABCG2) [25], which also exhibit the promiscuity of P-gp and together they form a triad of multi-drug efflux pumps conferring resistance in cancer. The promiscuity of P-gp led pharmacologists to posit that drugs unrelated to cancer chemotherapy may ‘compete’ with anti-cancer drugs for transport and thereby overcome the resistant phenotype, providing a potential clinical strategy.
A model was proposed whereby P-gp could be thought of as a ‘flippase’ that transports drugs between leaflets of the bilayer in a manner similar to the lipid translocase proteins [26]. This model could account for the broad substrate specificity of P-gp, particularly since it was thought that ‘…interactions with the substrate-binding site on the transport protein would be of secondary importance’ to the ability to intercalate into the bilayer. Another hypothesis was that P-gp was akin to a ‘hydrophobic vacuum cleaner’ that cleansed the bilayer of hydrophobic drugs. The mechanism relied on a hydrophobic surface and not the classical view of specific interactions between drugs and amino-acids (e.g., hydrogen bonds). These two proposals unleashed a veritable army of biochemical pharmacologists to elucidate the property of poly-specificity in P-gp.
One of the first attempts to address this issue used a photolabile probe [125I]-INA (iodonapthalene-1-azide) to specifically label P-gp in resistant cells [27]. The key property of this probe was that the photo-induced labelling could only be triggered by energy transfer from a proximal chromophore. The chromophores were doxorubicin and rhodamine 123, both substrates of P-gp. This demonstrated that substrates interact with P-gp or access the drug-binding site, from the lipid milieu of the bilayer. The second line of evidence was obtained with a series of fluorescent cell indicators (of pH and Ca2+) conjugated to an acetoxymethylester (AM) [28]. The AM derivatives of calcein and Fura-2 rapidly enter cells where they are avidly cleaved by esterases. The resultant free acid forms of probe cannot cross the plasma membrane, effectively being trapped within the cell. It was observed serendipitously that cellular accumulation of calcein–AM was considerably lower in P-gp expressing cells and could be increased following inhibition of the pump. Therefore, P-gp extrudes AM derivatives before they reach the cytoplasm; in other words, directly from the lipid milieu.
For receptor pharmacologists, there was a collective sigh of relief when Tamai and Safa [29] revealed classical non-competitive interactions on P-gp between azidpoine and inhibitors of P-gp. Photolabelling of [3H]-azidopine was described by a single binding site; however, the labelling was reduced by vinblastine and cyclosporine A in a non-competitive manner. This demonstrated the presence of multiple and discrete drug-binding sites on the transporter.
Over a 20-year period, photoaffinity analogues of P-gp substrates were widely used to locate the drug-binding site(s) [30–32]. Following photo-activation, P-gp was digested into large fragments to determine which were labelled by probe. Photolabelling was shown to occur in both halves of the protein; perhaps indicating multiple binding sites for a single ligand. An interesting study with flupentixol revealed that this drug specifically enhanced the photolabelling of P-gp by [125I]-IAAP at the C-terminus, with no alteration of labelling at the N-terminus [30]. This observation revealed that the two labelling sites were non-equivalent and the possible allostery between sites for flupentixol and IAAP binding. Another study used [125I]-IAAP photolabelling with a combination of enzymatic and chemical digestion of the protein to provide higher resolution of the region of P-gp labelled. The investigation concluded that [125I]-IAAP labelled P-gp at a single site with multiple spatial elements. Continuing the increasing sophistication of this process, the binding of another photoactive compound, [3H]-propafenone, was analysed by MALDI-TOF–MS [33]. This compound appeared to bind at the interfacial regions between the transmembrane domains and one mooted interpretation of the data is that the interface provides a ‘gate’ for substrate entrance to the central binding cavity.
Classical radioligand saturation and displacement assays also revealed reversible competitive or non-competitive interactions on P-gp [34–36]. An extensive investigation using multiple radioligands and modulators identified the presence of at least four pharmacologically distinct binding sites on P-gp [35]. Moreover, kinetic-binding assays demonstrated that the sites were linked by negative heterotropic allostery. Concurrently, investigations on drug transport and the drug stimulation of ATP hydrolysis also pointed to the existence of multiple drug-binding sites on the protein [37]. The transport studies examined the interaction of transported substrates and found positively co-operating interactions. The authors named the sites H (Hoechst33342) and R (rhodamine 123) based on their interacting ligands. Finally, using ATPase assays, another series of investigations concluded the presence of distinct sites for drug interaction on P-gp; some of which interacted and generated synergistic stimulation of hydrolysis [38,39].
Based on the number and distinct types of investigations over a 20-year period, it is clear that P-gp does have distinct sites for drug binding and does not simply rely on a non-specific hydrophobic adsorptive surface. Moreover, P-gp contains multiple sites for drug recognition or binding within the TMDs. In addition, the binding sites are connected by an allosteric communication network and drugs are extracted directly from the lipid milieu.
What has the structure of P-gp ever done for us?
‘Once we have the structure of the protein it will reveal the mechanism of action’.…….’Protein structures are static and cannot inform without solid biochemical evidence’……’Transporters live in membranes and X-ray crystal structures are from a non-membranous environment’.
And so the seemingly eternal ‘battle’ between biochemists and structural biologists continues. The truth is obviously at the mid-point of these diametrically opposing views. Obtaining the X-ray crystal structure of P-gp took almost 25 years from the time the amino-acid sequence was published. Was it worth the wait, why did it take so long and what has this process done for the field?
Back-to-back publication of the cDNA sequence for the mdr1-gene from human and mouse indicated that P-gp had considerable homology to a growing family of membrane transporters [40,41]. The P-gp sequences revealed the characteristic motifs of ABC proteins and consensus sites for glycosylation and phosphorylation. A topological model was developed for P-gp, subsequently known as the standard model of two transmembrane (each with six segments/helices) and two NBDs. Based on the topological model and information from bacterial transporters, Kartner and Ling [1] formulated what in retrospect was a prophetic depiction of the structure of P-gp in 1989.
Over the period 1991–1996 a number of teams set about to provide experimental verification of the standard model for the topology of P-gp. The predominant approach was to use a truncated protein containing various reporter groups (e.g. enzymes, antibody epitopes and biotinylation sequences) at the site of truncation. Functional assays for the truncated proteins were undertaken to ascertain whether the reporter was extra- or intracellular. Unfortunately, these investigations yielded an array of possible topologies for P-gp with little consensus and no agreement with the standard model [42]. Moreover, it was proposed that these topologies were in equilibrium, presumably at enormous energetic cost. Data began to emerge on the possible adverse effects of truncations and the stitching of large reporter groups to a protein, adding to the dilemma.
Loo and Clarke decided to adopt a relatively new procedure involving site directed cysteine mutagenesis in order to utilize the cell friendly chemistry of thiol-modification [15,42]. They generated the first version of a cysteine-less P-gp isoform that, crucially, retained full function. Cysteine residues were added to predicted intra- and extracellular loops and the relative accessibilities to covalent modification by membrane impermeant biotin-maleimide determined. The accessibility data were used to construct a topology that was in agreement with the standard model. A side-benefit of the tortuous path to the topology of P-gp was the development of site-directed mutagenesis studies; in particular, the development of the cysteine-directed mutagenesis investigative strategy to facilitate mechanistic studies.
In the mid to late 1990s, a number of X-ray crystal structures of the nucleotide-binding domains of ABC proteins were published. However, the first structure of a full-length ABC protein was obtained for P-gp using an EM approach [43]. P-gp was purified from hamster ovary cells using a mixed detergent system and a combination of ion-exchange and hydroxyapatite chromatography. The structure of the purified protein was determined using EM and single particle analysis. Although the structure was low resolution [25 Å (1 Å=0.1 nm)], it did provide overall dimensions of the protein. A prominent feature of the structure was the presence of a large central cavity, which was suggested to contain an aqueous environment. At the time, the presence of a central cavity in the structure caused considerable debate. To improve resolution, the teams embarked on the use of 2D crystals for P-gp, which has the advantage of retaining a membrane environment [44]. Continued improvements in the quality of protein and increasingly sophisticated analyses enabled improvement of resolution from 20 to 10 Å. New biochemical strategies enabled collection of structural data from multiple conformations; namely the nucleotide-bound and post-hydrolytic states of P-gp. This data revealed considerable conformational changes in the transmembrane domain of P-gp as it switched between the empty, ATP-bound and ADP-vanadate trapped conformations.
Concurrent data using fluorescence and infrared spectroscopic analyses also demonstrated the major impetus for TMD conformational change was provided by ATP binding to the NBDs [45,46]. The structural data prompted the investigation of what affect the ATP-induced conformational changes have on the transport process. Equilibrium-binding assays with the transported substrate [3H]-vinblastine demonstrated that the binding affinity (KD) was markedly reduced as P-gp transitioned to the ATP-bound conformation [47]. The affinity was increased post-hydrolysis, but the initial high-affinity binding was only restored following release of inorganic phosphate. These data were used to generate a model of substrate translocation for P-gp whereby the binding of nucleotide causes re-orientation of the drug-binding site. The energy from ATP hydrolysis is used to reset P-gp for subsequent transport cycles. Moreover, this translocation model has also been demonstrated for the multi-drug efflux pumps Mrp1 (ABCC1) [48] and BCRP (ABCG2) [49].
The final reported structure obtained using EM focussed on P-gp trapped in the ATP bound configuration [50]. This state is highly stable and ensured homogeneous P-gp preparations for 2D crystallization. The structure was resolved to 8 Å, which is near the maximum possible with ‘conventional’ EM and direct evidence was provided for a 2×6 TM helical bundle and a narrow central cavity. In addition, this nucleotide bound conformation was characterized by close apposition of the NBDs. This spawned considerable debate on the mechanism by which NBDs generate and transmit energy from ATP hydrolysis to the translocation pathway. In particular, the reader is directed to a review outlining the ‘NBD switch’, the ‘alternating sites’ and ‘occluded ATP’ models for P-gp [19].
In 2009, the first 3D X-ray crystallographic structure of P-gp was published for the mouse isoform; a feat that was highly anticipated and the subject of intense international competition [51]. The structure of a Clostridium elegans isoform of P-gp was subsequently published in 2012, also in an nucleotide-free (basal) conformation [52]. The resolution of these structures was in the range 3.4–4.3 Å, thereby providing a greater insight into the peptide backbone organization of P-gp. Cross-over of helices between the two transmembrane domains was observed, which is in agreement with the structure of a bacterial multi-drug efflux pump Sav1866 and reflective of the coupling of translocation between domains. The NBDs are arranged in a head-to-tail orientation and the protein has a large central cavity. The dimensions of this cavity have caused considerable debate and it is likely that the crystallographic conditions and the location of the contact points between monomer units have exaggerated the distance between the NBDs. The central cavity is closed at the external face, a finding that was also observed in the EM structures and from cross-linking data for cysteine containing mutants of P-gp.
A consistent feature of research is that discoveries tend to open more doors than they close and the structures for P-gp are no exception. Recently, the initial mouse P-gp structure was refined, based on observations from the C. elegans structure [53]. Continued refinement of structural models will continue as new and higher resolution data become available. In addition, the powerful forces of the modelling fraternity have applied MD simulations to assess the stability of crystal structures and continue the refinements necessary to address key biochemical structures [54].
The structure obtained with mouse P-gp was also obtained in the presence of drug; in this case, a hitherto uncharacterized peptide. The resolution obtained does not yet allow unequivocal assignment of interacting residues, nor the forces that stabilize binding. However, the data have provided residues proximal to the bound inhibitor within the large binding domain. Many of these proximal residues have already been identified from mutagenesis and biochemical studies. These are exciting times as we progress towards defining the precise locations and molecular interactions of drug-binding sites in this enigmatic protein.
To date, the high resolution structure of nucleotide bound P-gp continues to be elusive. Provision of this data will generate a major leap in our understanding of the mechanism of translocation and facilitate the reconciliation of divergent biochemical observations.
Why bother?
This brief description of efforts to generate a molecular mechanism of P-gp have demonstrated the progress made since those initial experiments with cultured cancer cell lines that demonstrated chemoresistance. P-gp was discovered using methods long since consigned to history. Revealing the gene sequence provided the first topology, this led to topographical studies with EM and ultimately, to a high resolution crystal structure. These efforts were underpinned by the consistent improvements in the production of purified P-gp. This has progressed from using a ‘natural’ source of protein to the sophisticated expression systems that have enabled the examination of over 200 mutant versions of the protein. Early pharmacological studies identified the promiscuity of P-gp with drugs and identified a number of potential inhibitors. Many of these compounds ‘doubled-up’ as pharmacological tools and resulted in a steady progression to recognize the complex allosteric nature of substrate recognition by P-gp. This small list of achievements has enabled us to inch ever closer to defining the location and structural properties of drug interaction with P-gp.
The progression over 40 years reveals how disparate groups of scientists and disciplines merged and borrowed from each other to produce synergy in research. Technical advances were developed, often in unpredictable ways and avidly adopted to inexorably shift our understanding forward. Being part of an international ‘team’ of curious scientists embarking on a journey of discovery into the molecular world of the cell are one of many reasons for bothering with fundamental research.
Should we keep bothering?
We are far from done with this enigmatic transporter. The precise residues involved in the binding site/cavity remain elusive. This information, coupled with higher resolution structural data, will describe the molecular interactions between drugs and the peptide chain; i.e., the much sought pharmacophoric elements for interaction with P-gp. There remains no plausible mechanism to describe the property of multi-drug recognition, which is used by proteins throughout nature. The number of ATP hydrolysed per compound translocated remains frustratingly elusive and a higher level kinetic description of the multi-drug process cannot be provided without this information. The physical mechanism of coupling drug-binding events and the ATP hydrolytic machinery is tantalisingly close. Once again, this information will be crucial for a detailed molecular mechanism. P-gp expression differs between normal healthy tissue and cancerous cells. The ability to regulate expression in either or both settings will also have considerable affect in our understanding of generic pharmacokinetics and potentially in modulating disease.
Can P-gp be considered a ‘pharma target’? The answer to this nebulous question is clearly dependent on perspective. Currently, it does not appear to be of interest due to the failures of past efforts. However, this may be a temporal issue. The possibility that the drug-binding site structure be elucidated and the pharmacophore for drug substrates revealed will engender renewed interest. Similarly, the ability to pharmacologically modulate its expression will undoubtedly kick-start efforts on this track. This is where fundamental science comes to the fore, i.e. by providing a language for translation.
It is worth remembering how fundamental research in cell biology can be slow and painstaking, but the journey towards understanding mechanisms in biology at a molecular level is rich in reward.
Abbreviations
- ABC
ATP-binding cassette
- AM
acetoxymethylester
- BCRP
breast cancer resistance protein
- BtuCD
vitamin B12 transporter
- IAAP
Iodo-aryl-azido prazosin
- MalK
maltose import protein K subunit
- Mrp1
multidrug resistance protein 1
- NBDs
nucleotide-bonding domains
- P-gp
permeability glycoprotein
- Rad50cd
DNA repair protein
Footnotes
ATP binding cassette transporters: from mechanism to organism: Held at University of Chester, U.K., 16–18 April 2015.
Funding
This work was supported by the Worldwide Cancer Research [grant number 12-0008]; and the Wellcome Trust [grant number WT094392MA].
References
- 1.Kartner N., Ling V. Multidrug resistance in cancer. Sci. Am. 1989;260:44–51. doi: 10.1038/scientificamerican0389-44. [DOI] [PubMed] [Google Scholar]
- 2.Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim. Biophys. Acta. 1973;323:466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- 3.Juliano R.L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 4.Kartner N., Riordan J.R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- 5.Chambers T.C., McAvoy E.M., Jacobs J.W., Eilon G. Protein kinase C phosphorylates P-glycoprotein in multidrug resistant human KB carcinoma cells. J. Biol. Chem. 1990;265:7679–7686. [PubMed] [Google Scholar]
- 6.Ichikawa M., Yoshimura A., Furukawa T., Sumizawa T., Nakazima Y., Akiyama S. Glycosylation of P-glycoprotein in a multidrug-resistant KB cell line, and in the human tissues. Biochim. Biophys. Acta. 1991;1073:309–315. doi: 10.1016/0304-4165(91)90136-5. [DOI] [PubMed] [Google Scholar]
- 7.Richert N.D., Aldwin L., Nitecki D., Gottesman M.M., Pastan I. Stability and covalent modification of P-glycoprotein in multidrug- resistant KB cells. Biochemistry. 1988;27:7607–7613. doi: 10.1021/bi00420a006. [DOI] [PubMed] [Google Scholar]
- 8.Riordan J.R., Ling V. Purification of P-glycoprotein from plasma membrane vesicles of chinese hamster ovary cell mutants with reduced colchicine permeability. J. Biol. Chem. 1979;254:12701–12705. [PubMed] [Google Scholar]
- 9.Hamada H., Tsuruo T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170- to 180-kilodalton membrane glycoprotein is an ATPase. J. Biol. Chem. 1988;263:1454–1458. [PubMed] [Google Scholar]
- 10.Taylor A.M., Storm J., Soceneantu L., Linton K.J., Gabriel M., Martin C., Woodhouse J., Blott E., Higgins C.F., Callaghan R. Detailed characterization of cysteine-less P-glycoprotein reveals subtle pharmacological differences in function from wild-type protein. Br. J. Pharmacol. 2001;134:1609–1618. doi: 10.1038/sj.bjp.0704400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambudkar S.V., Lelong I.H., Zhang J., Cardarelli C.O., Gottesman M.M., Pastan I. Partial purification and reconstitution of the human multidrug- resistance pump: characterization of the drug-stimulatable ATP hydrolysis. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8472–8476. doi: 10.1073/pnas.89.18.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doige C.A., Yu X., Sharom F.J. ATPase activity of partially purified P-glycoprotein from multidrug-resistant Chinese hamster ovary cells. Biochim. Biophys. Acta. 1992;1109:149–160. doi: 10.1016/0005-2736(92)90078-Z. [DOI] [PubMed] [Google Scholar]
- 13.Rigaud J.L., Paternostre M.T., Bluzat A. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 2. Incorporation of the light-driven proton pump bacteriorhodopsin. Biochemistry. 1988;27:2677–2688. doi: 10.1021/bi00408a007. [DOI] [PubMed] [Google Scholar]
- 14.Callaghan R., Berridge G., Ferry D.R., Higgins C.F. The functional purification of P-glycoprotein is dependent on maintenance of a lipid-protein interface. Biochim. Biophys. Acta. 1997;1328:109–124. doi: 10.1016/S0005-2736(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 15.Loo T.W., Clarke D.M. Rapid purification of human P-glycoprotein mutants expressed transiently in HEK 293 cells by nickel-chelate chromatography and characterization of their drug stimulated ATPase activities. J. Biol. Chem. 1995;270:21449–21452. doi: 10.1074/jbc.270.37.21449. [DOI] [PubMed] [Google Scholar]
- 16.al-Shawi M.K., Senior A.E. Characterization of the adenosine triphosphatase activity of Chinese hamster P-glycoprotein. J. Biol. Chem. 1993;268:4197–4206. [PubMed] [Google Scholar]
- 17.al-Shawi M.K., Urbatsch I.L., Senior A.E. Covalent inhibitors of P-glycoprotein ATPase activity. J. Biol. Chem. 1994;269:8986–8992. [PubMed] [Google Scholar]
- 18.Tombline G., Bartholomew L.A., Urbatsch I.L., Senior A.E. Combined mutation of catalytic glutamate residues in the two nucleotide binding domains of P-glycoprotein generates a conformation that binds ATP and ADP tightly. J. Biol. Chem. 2004;279:31212–31220. doi: 10.1074/jbc.M404689200. [DOI] [PubMed] [Google Scholar]
- 19.Jones P.M., O'Mara M.L., George A.M. ABC transporters: a riddle wrapped in a mystery inside an enigma. Trends Biochem. Sci. 2009;34:520–531. doi: 10.1016/j.tibs.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Diedrichs K., Diez J., Greller G., Muller C., Breed J., Schnell C., Vonrhein C., Boos W., Welte W. Crystal structure of MalK, the ATPase subunit of the trehalose/maltose ABC transporter of the archeon Thermococcus litoralis. EMBO J. 2000;19:5951–5961. doi: 10.1093/emboj/19.22.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopfner K.P., Karcher A., Shin D.S., Craig L., Arthur L.M., Carney J.P., Tainer J.A. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/S0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- 22.Hung L.W., Wang I.X., Nikaido K., Liu P.Q., Ames G.F., Kim S.H. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature. 1998;396:703–707. doi: 10.1038/25393. [DOI] [PubMed] [Google Scholar]
- 23.Dawson R.J., Locher K.P. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 24.Cole S.P.C., Bhardwaj G., Gerlach J.H., Mackie J.E., Grant C.E., Almquist K.C., Stewart A.J., Kurz E.U., Duncan A.M.V., Deeley R.G. Overexpression of a transporter gene in a multidrug resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 25.Doyle L.A., Yang W., Abruzzo L.V., Krogmann T., Gao Y., Rishi A.K., Ross D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins C.F., Gottesman M.M. Is the multidrug transporter a flippase? Trends Biochem. Sci. 1992;17:18–21. doi: 10.1016/0968-0004(92)90419-A. [DOI] [PubMed] [Google Scholar]
- 27.Raviv Y., Pollard H.B., Bruggemann E.P., Pastan I., Gottesman M.M. Photosensitized labeling of a functional multidrug transporter in living drug-resistant tumor cells. J. Biol. Chem. 1990;265:3975–3980. [PubMed] [Google Scholar]
- 28.Homolya L., Hollo Z., Germann U.A., Pastan I., Gottesman M.M., Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J. Biol. Chem. 1993;268:21493–21496. [PubMed] [Google Scholar]
- 29.Tamai I., Safa A.R. Azidopine noncompetitively interacts with vinblastine and cyclosporin A binding to P-glycoprotein in multidrug resistant cells. J. Biol. Chem. 1991;266:16796–16800. [PubMed] [Google Scholar]
- 30.Dey S., Ramachandra M., Pastan I., Gottesman M.M., Ambudkar S.V. Evidence for two nonidentical drug-interaction sites in the human P- glycoprotein. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10594–10599. doi: 10.1073/pnas.94.20.10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberger L.M. Major photoaffinity drug labeling sites for iodoaryl azidoprazosin in P-glycoprotein are within, or immediately C-terminal to, transmembrane domains 6 and 12. J. Biol. Chem. 1993;268:11417–11425. [PubMed] [Google Scholar]
- 32.Safa A.R. Photoaffinity labelling of P-glycoprotein in multidrug resistant cells. Cancer Invest. 1992;10:295–305. doi: 10.3109/07357909209032754. [DOI] [PubMed] [Google Scholar]
- 33.Pleban K., Kopp S., Csaszar E., Peer M., Hrebicek T., Rizzi A., Ecker G.F., Chiba P. P-glycoprotein substrate binding domains are located at the transmembrane domain/transmembrane domain interfaces: a combined photoaffinity labeling-protein homology modeling approach. Mol. Pharmacol. 2005;67:365–374. doi: 10.1124/mol.104.006973. [DOI] [PubMed] [Google Scholar]
- 34.Ferry D.R., Russell M.A., Cullen M.H. P-glycoprotein possesses a 1,4-dihydropyridine selective drug acceptor site which is allosterically coupled to a vinca alkaloid selective binding site. Biochem. Biophys. Res. Commun. 1992;188:440–445. doi: 10.1016/0006-291X(92)92404-L. [DOI] [PubMed] [Google Scholar]
- 35.Martin C., Berridge G., Higgins C.F., Mistry P., Charlton P., Callaghan R. Communication between multiple drug binding sites on P-glycoprotein. Mol. Pharmacol. 2000;58:624–632. doi: 10.1124/mol.58.3.624. [DOI] [PubMed] [Google Scholar]
- 36.Martin C., Berridge G., Mistry P., Higgins C., Charlton P., Callaghan R. The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br. J. Pharmacol. 1999;128:403–411. doi: 10.1038/sj.bjp.0702807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shapiro A.B., Ling V. Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur. J. Biochem. 1997;250:130–137. doi: 10.1111/j.1432-1033.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 38.Garrigos M., Mir L.M., Orlowski S. Competitive and non-competitive inhibition of the multidrug-resistance-associated P-glycoprotein ATPase. Further experimental evidence for a multisite model. Eur. J. Biochem. 1997;244:664–673. doi: 10.1111/j.1432-1033.1997.00664.x. [DOI] [PubMed] [Google Scholar]
- 39.Pascaud C., Garrigos M., Orlowski S. Multidrug resistance transporter P-glycoprotein has distinct but interacting binding sites for cytotoxic drugs and reversing agents. Biochem. J. 1998;333:351–358. doi: 10.1042/bj3330351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C.J., Chin J.E., Ueda K., Clark D.P., Pastan I., Gottesman M.M., Roninson I.B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47:381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- 41.Gros P., Croop J., Houseman D.E. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986;47:371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- 42.Loo T.W., Clarke D.M. Membrane topology of a cysteine-less mutant of human P-glycoprotein. J. Biol. Chem. 1995;270:843–848. doi: 10.1074/jbc.270.2.843. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg M.F., Callaghan R., Ford R.C., Higgins C.F. Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J. Biol. Chem. 1997;272:10685–10694. doi: 10.1074/jbc.272.16.10685. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg M.F., Velarde G., Ford R.C., Martin C., Berridge G., Kerr I.D., Callaghan R., Schmidlin A., Wooding C., Linton K.J., Higgins C.F. Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. EMBO J. 2001;20:5615–5625. doi: 10.1093/emboj/20.20.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonveaux N., Vigano C., Shapiro A.B., Ling V., Ruysschaert J.M. Ligand-mediated tertiary structure changes of reconstituted P- glycoprotein. A tryptophan fluorescence quenching analysis. J. Biol. Chem. 1999;274:17649–17654. doi: 10.1074/jbc.274.25.17649. [DOI] [PubMed] [Google Scholar]
- 46.Vigano C., Julien M., Carrier I., Gros P., Ruysschaert J.M. Structural and functional asymmetry of the nucleotide-binding domains of P-glycoprotein investigated by attenuated total reflection Fourier transform infrared spectroscopy. J. Biol. Chem. 2002;277:5008–5016. doi: 10.1074/jbc.M107928200. [DOI] [PubMed] [Google Scholar]
- 47.Martin C., Higgins C.F., Callaghan R. The vinblastine binding site adopts high- and low-affinity conformations during a transport cycle of P-glycoprotein. Biochemistry. 2001;40:15733–15742. doi: 10.1021/bi011211z. [DOI] [PubMed] [Google Scholar]
- 48.Rothnie A., Callaghan R., Deeley R.G., Cole S.P. Role of GSH in estrone sulfate binding and translocation by the multidrug resistance protein 1 (MRP1/ABCC1) J. Biol. Chem. 2006;281:13906–13914. doi: 10.1074/jbc.M600869200. [DOI] [PubMed] [Google Scholar]
- 49.Clark R., Kerr I.D., Callaghan R. Multiple drug-binding sites on the R482G isoform of the ABCG2 transporter. Br. J. Pharmacol. 2006;149:506–515. doi: 10.1038/sj.bjp.0706904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberg M.F., Callaghan R., Modok S., Higgins C.F., Ford R.C. Three-dimensional Structure of P-glycoprotein: the transmembrane regions adopt an asymmetric configuration in the nucleotide-bound state. J. Biol. Chem. 2005;280:2857–2862. doi: 10.1074/jbc.M410296200. [DOI] [PubMed] [Google Scholar]
- 51.Aller S.G., Yu J., Ward A., Weng Y., Chittaboina S., Zhuo R., Harrell P.M., Trinh Y.T., Zhang Q., Urbatsch I.L., Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin M.S., Oldham M.L., Zhang Q., Chen J. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature. 2012;490:566–569. doi: 10.1038/nature11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Jaimes K.F., Aller S.G. Refined structures of mouse P-glycoprotein. Protein Sci. 2014;23:34–46. doi: 10.1002/pro.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Mara M.L., Mark A.E. Structural characterization of two metastable ATP-bound states of P-glycoprotein. PLoS One. 2014;9:e91916. doi: 10.1371/journal.pone.0091916. [DOI] [PMC free article] [PubMed] [Google Scholar]