This study evaluated the loss of Klotho in human lungs with COPD and the underlying mechanisms.
Keywords: bronchial epithelial cells, chronic obstructive pulmonary disease (COPD), cytokines, klotho protein, oxidative stress, smoke
Abstract
COPD (chronic obstructive pulmonary disease) is associated with sustained inflammation, excessive injury, and accelerated lung aging. Human Klotho (KL) is an anti-aging protein that protects cells against inflammation and damage. In the present study, we quantified KL expression in the lungs of COPD patients and in an ozone-induced mouse model of COPD, and investigated the mechanisms that control KL expression and function in the airways. KL distribution and levels in human and mouse airways were measured by immunohistochemistry and Western blotting. The effect of CSE (cigarette smoke extract) on KL expression was detected in human bronchial epithelial cells. Moreover, the effect of KL on CSE-mediated inflammation and hydrogen peroxide-induced cellular injury/apoptosis was determined using siRNAs. KL expression was decreased in the lungs of smokers and further reduced in patients with COPD. Similarly, 6 weeks of exposure to ozone decreased KL levels in airway epithelial cells. CSE and TNFα (tumour necrosis factor α) decreased KL expression and release from airway epithelial cells, which was associated with enhanced pro-inflammatory cytokine expression. Moreover, KL depletion increased cell sensitivity to cigarette smoke-induced inflammation and oxidative stress-induced cell damage. These effects involved the NF-κB (nuclear factor κB), MAPK (mitogen-activated protein kinase) and Nrf2 (nuclear factor erythroid 2-related factor 2) pathways. Reduced KL expression in COPD airway epithelial cells was associated with increased oxidative stress, inflammation and apoptosis. These data provide new insights into the mechanisms associated with the accelerated lung aging in COPD development.
CLINICAL PERSPECTIVES
-
•
Accelerated aging is linked to several chronic inflammatory disorders. The role of various anti-aging molecules in the pathophysiology and disease progression of respiratory diseases such as COPD are under extensive investigation.
-
•
Klotho (KL) protein is mainly expressed in human airway epithelium and its level is decreased in patients with COPD and in an animal model of the disease. These changes are associated with increased oxidative stress, inflammation and apoptosis in airway epithelial cells.
-
•
These functions of KL in human airway epithelial cells provide a means to better understand the molecular mechanisms and pathways involved in airway inflammation and open up new perspectives in potential novel therapeutic targets in the treatment of CS-related COPD.
INTRODUCTION
COPD (chronic obstructive pulmonary disease) is characterized by irreversible and progressive airflow limitation; this disease is caused by chronic inflammation and oxidative damage of the airways and lung parenchyma predominantly induced by chronic CS (cigarette smoke) exposure [1,2]. COPD is a syndrome that encompasses various degrees of chronic obstructive bronchitis and emphysema whereby emphysema is considered to be an important severity determinant [3]. COPD is also considered to be an ‘accelerated aging phenotype’ because of the increased prevalence of anatomical and physiological features of the aging lung that are observed even in younger patients with COPD [4,5]. Aging is associated with increased oxidative stress and shares several common genetic and molecular mechanisms with inflammation in COPD [5]. Notably, accelerated aging may increase a pathogenetic susceptibility of human lungs to develop COPD, but not in directly causing the disease itself.
Klotho (KL) is a type I transmembrane protein with restricted tissue distribution, notably the kidney and brain, that possesses potent biological activity [6]. KL protein exists as both a membrane and a secreted form due to either alternative RNA splicing [7] or proteolytic cleavage of the transmembrane protein by members of the ADAM (a disintegrin and metalloproteinase) family [8]. In mice, loss of KL expression results in various phenotypes, including atherosclerosis, pulmonary emphysema, skin atrophy, ectopic calcification, osteoporosis and a shortened lifespan [9,10], which are all observed in aging humans. Mice with a KL gene mutation also exhibit alveolar wall destruction, enlargement of air spaces and a longer expiration time, which closely resembles pulmonary emphysema in humans. Advanced age-associated emphysema can be reversed using an inducible KL expression system [10,11].
Humans also display decreased serous, urinary and renal KL protein levels with age or with age-related diseases [12–14]. The inflammatory cytokines TNFα (tumour necrosis factor α) and TWEAK (TNF-like weak inducer of apoptosis) down-regulate KL expression in the kidney [15]. In turn, KL regulates oxidative stress [16] and enables endothelial cells to reduce hydrogen peroxide (H2O2)-induced apoptosis and cellular senescence [17]. Recently, circulating KL was shown to protect the lung against injury partly through enhancing the endogenous antioxidative capacity of pulmonary epithelial cells [18]. KL can also suppress NF-κB (nuclear factor κB) activation and subsequent inflammatory cytokine production in kidney cells [19]. However, these studies never described how KL deficiency is involved in the pathogenesis of COPD. The expression and regulation of KL in human lung and the function of KL in the modulation of oxidative stress, inflammation and apoptosis in COPD remain unknown.
The airway epithelial cells are not only the first line of defence in the lungs, but also important effector cells in the pathogenesis of COPD [20]. We have shown previously in abstract form, for the first time, that KL is expressed in human bronchial epithelial cells [21]. We therefore hypothesized that the loss of KL reduces the protection of human lungs against oxidative damage and chronic inflammation, thus accelerating the formation of COPD.
MATERIALS AND METHODS
Additional details are provided in the Supplementary Online Data.
Ethics statement
The protocol was approved by the ethics committee of The First Affiliated Hospital of Nanjing Medical University. All of the human lung tissues were obtained from the tissue bank of the same institution. Animal experiments were performed under a Project License from the British Home Office, UK, under the Animals (Scientific Procedures) Act 1986.
Study subjects
Lung tissues were obtained from 59 subjects/patients who were subjected to resection surgery to treat solitary peripheral carcinoma in the First Affiliated Hospital of Nanjing Medical University, and were classified as healthy non-smokers, smokers with normal lung function and smokers with COPD (according to the GOLD guidelines [22]). In case of lung cancer, tissues were isolated from more than 5 cm away from the tumour border. Controls comprised smokers with normal lung function and non-smokers with normal lung function. No patients had a history of asthma or renal dysfunction. The clinical characteristics of the patients are shown in Supplementary Table S1. Additional details are provided in the Supplementary Online Data.
Emphysema induction in mice
Experiments were performed under a Project Licence from the British Home Office, U.K., under the Animals (Scientific Procedures) Act 1986. Eight-week-old male C57BL/6 mice were exposed to ozone at a concentration of 3 p.p.m. for 3 h a day, twice a week for a period of 1, 3 or 6 weeks as described previously [23]. Control animals were exposed to normal air. Lung tissues were obtained for morphological and histological analyses 24 h after the last exposure. BALF (bronchial alveolar lavage fluid) was collected to detect KL secretion by ELISA.
Cell culture
Cultured human bronchial epithelial (16HBE) cells were cultured as described previously [24]. In some cases, cells were pre-treated with SP600125 [JNK (c-Jun N-terminal kinase) inhibitor], SB203580 [p38 MAPK (mitogen-activated protein kinase) inhibitor], PD98059 {MEK [MAPK/ERK (extracellular-signal-regulated kinase) kinase] inhibitor} (all Sigma–Aldrich) or JSH-23 (NF-κB inhibitor) (Calbiochem) [25–27] before exposure to CSE (cigarette smoke extract). CSE was prepared essentially as described previously [28]. Cell viability was measured by CCK-8 assay (see Supplementary Figure S1). Subsequent experiments were conducted using CSE concentrations that did not affect cell viability. Additional details are provided in the Supplementary Online Data.
Immunohistochemistry and Western blotting
KL expression was detected using a polyclonal rabbit anti-human KL antibody (1:500 dilution) as described previously [29]. The preparation of whole-cell lysates, cytosolic extracts and nuclear extracts, and Western blot analysis in tissues and cells were as described previously [30]. Details of antibodies used are given in the Supplementary Online Data. Additional details are provided in the Supplementary Online Data.
RT (reverse transcription)–qPCR (quantitative PCR)
Total RNA was extracted from cells using TRIzol® (Invitrogen) according to the manufacturer's instructions and qPCR was performed using SYBR Green. Additional details are provided in the Supplementary Online Data.
Flow cytometry
Apoptotic rate and intracellular ROS (reactive oxygen species) production by 16HBE cells was determined by flow cytometry as previously described using annexin V–FITC and propidium iodide and DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate) respectively [31]. Additional details are provided in the Supplementary Online Data.
ELISA
Culture medium was centrifuged at 1255 g for 5 min and the supernatant was removed and stored at 80°C until analysis. KL, IL (interleukin)-1β and TNFα were detected using ELISA kits (IBL and R&D Systems) according to the manufacturers’ instructions.
Knockdown of KL expression using siRNA
Three siRNAs specific for human KL (siRNA1, siRNA2 and siRNA3) or control siRNA (siRNAc) were synthesized by Invitrogen and transfected into 16HBE cells using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer's instructions. Additional details are provided in the Supplementary Online Data.
Statistical analysis
Results were compared by Mann–Whitney U test, one-way ANOVA or Student's t test as appropriate. P<0.05 was considered statistically significant. Statistical analysis was performed using SPSS 13.0 and GraphPad Prism 5. Data are expressed as means±S.E.M. for three independent experiments.
RESULTS
KL expression is decreased in the lung of patients with COPD
A total of 59 subjects were divided into three groups according to lung function and smoking status: COPD group, healthy smoking group, and healthy control group. The clinical characteristics of the study subjects are shown in Supplementary Table S1. No difference was observed in pack years between COPD and healthy smoking groups. The three groups were similar in terms of age and body mass index.
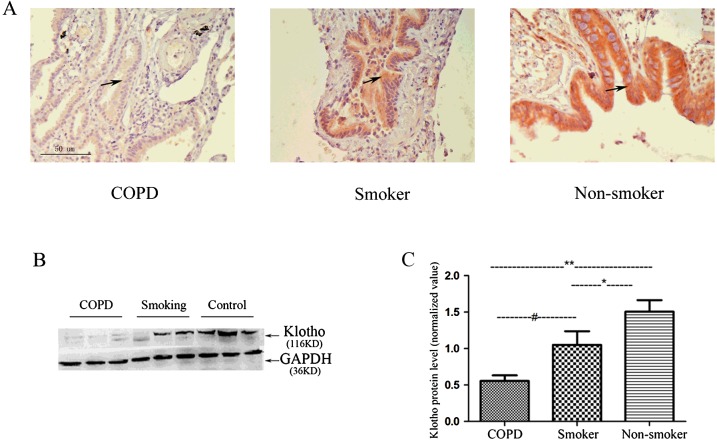
Immunohistochemistry results demonstrated that KL expression was mainly distributed along the airway epithelium. KL expression was reduced in healthy smokers and reduced further in COPD patients (Figure 1A). Western blot analysis confirmed a significant reduction in KL expression in healthy smokers compared with non-smokers (1.05±0.17 compared with 1.51±0.13, P<0.05). KL expression was reduced further in patients with COPD compared with healthy smoking subjects (0.53±0.07 compared with 1.05±0.17, P< 0.05). Overall, a significant 65% reduction occurred in KL expression in the lungs of patients with COPD compared with non-smokers (Figures 1B and 1C).
Figure 1. Klotho expression is significantly reduced in the lung of patients with COPD.
(A) KL immunostaining is markedly reduced in COPD patients compared with that in smoking control subjects and healthy non-smokers (original magnification ×100). (B) Representative Western blots were probed using an anti-KL antibody and normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as loading control. (C) The immunoblots of KL and the control GAPDH bands were quantified using a LiCOR image analyser and normalized to GAPDH. Results are means±S.D. of the normalized arbitrary scan values of 15 COPD patients, 22 healthy smokers and 22 healthy non-smoking controls. *P<0.05, **P<0.001, #P<0.05.
KL levels are reduced in the airway epithelium and briefly increased in the BALF of ozone-induced emphysematous mice
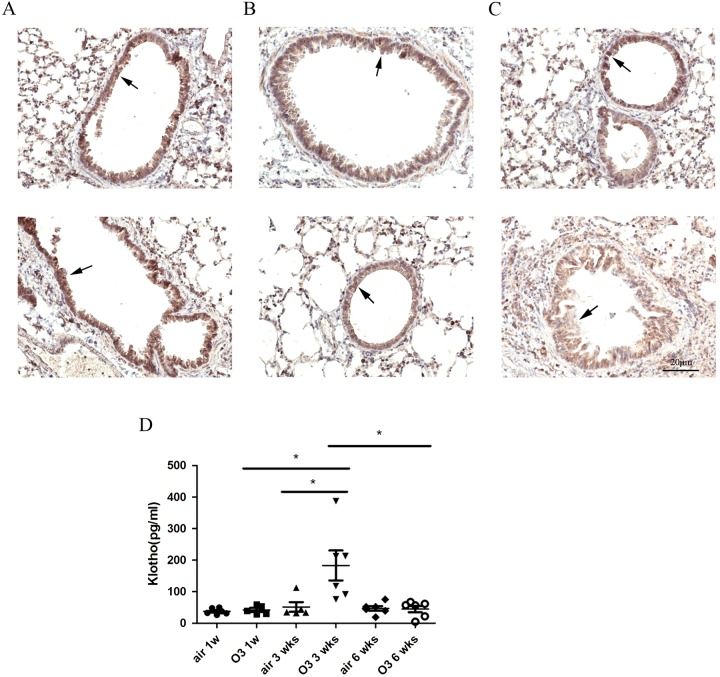
Lung tissue sections stained with haematoxylin/eosin showed irreversible destruction of the alveolar walls and enlargement of the alveolar spaces in C57B1/6 mice exposed to ozone compared with air controls (Supplementary Figure S2). A similar KL expression was found along the airway epithelium in 1- and 3-week ozone-exposed groups compared with air-exposed mice (Figures 2A and 2B), but a significant reduction occurred after 6 weeks of ozone exposure (Figure 2C). Secreted KL levels in BALF were significantly elevated in 3-week ozone-exposed groups (3.6-fold increase, P<0.05), but not in 1-week (1.1-fold increase) or 6-week (1.04-fold decrease) ozone-exposed animals (Figure 2D).
Figure 2. Klotho levels are reduced in the airway epithelium and transiently increased in BALF of ozone-induced emphysematous mice.
(A–C) Immunohistochemical analysis of the expression of KL protein in airway epithelium of mice exposed to air or ozone for 1, 3 and 6 weeks (original magnification ×200). KL immunostaining is similar in 1- or 3-week ozone-exposed mice compared with air controls (A and B), but there was slightest staining for KL in airway epithelial cells of 6-week-exposed mice (C). (D) BALF was obtained from all mice 24 h after the last exposure to ozone and the secreted KL was determined by ELISA. There was a significant but transient increase in KL secretion in 3-week ozone-exposed groups (3.6-fold increase, P<0.05), but not in 1-week (1.1-fold increase) or 6-week (1.04-fold decrease) ozone-exposed animals. Results are means±S.E.M., *P<0.05.
CSE and TNFα down-regulate KL expression in bronchial epithelial cells partly via the NF-κB pathway
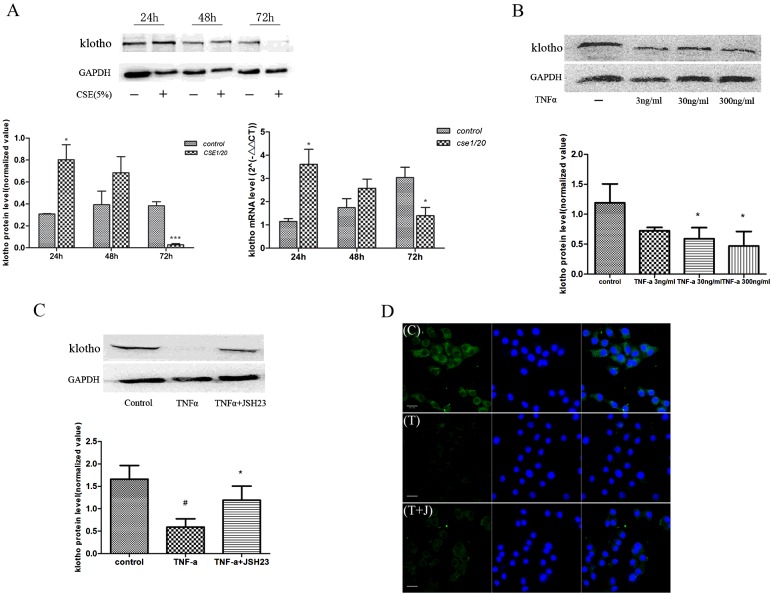
CS functions as a major risk factor for COPD, causes injury to the airway epithelial barrier and initiates various pathological processes. We initially demonstrated normal cellular viability after 48 or 72 h of CSE stimulation (Supplementary Figure S1). Treatment of 16HBE cells with 5% CSE resulted in a short-term increase in KL protein expression, followed by a significant reduction after 72 h exposure (Figure 3A). Similar results were observed at the mRNA level (Figure 3A). In addition, TNFα induced a concentration-dependent reduction in KL protein expression in 16HBE cells (Figure 3B), which was attenuated by the NF-κB inhibitor JSH23 (Figure 3C). This result was confirmed by immunocytochemical staining of KL within cells following TNFα stimulation in the presence and absence of JSH23 (Figure 3D).
Figure 3. Cigarette smoke extract (CSE) and TNFα reduce intracellular Klotho expression in an NF-κB-dependent manner.
Cells were treated with 5% CSE for 24, 48 and 72 h, and KL protein was measured by Western blotting. Immunoblots were quantified using a LiCOR image analyser and normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Similar results were seen at the KL mRNA level detected by RT–qPCR (A). Cultured 16HBE cells were exposed to TNFα (3, 30 and 300 ng/ml) for 24 h, and KL expression was determined by Western blotting (B). Quantification results are means±S.E.M. from three independent experiments (*P<0.05, ***P<0.001 compared with the control group). In some experiments, cells were pre-treated with the NF-κB inhibitor JSH23 (20 μM) for 30 min (C). (#P<0.01 compared with control and *P<0.05 compared with TNFα). Cells were exposed to medium alone (control, C) or TNFα (T, 30 ng/ml), or pre-treated with the NF-κB inhibitor JSH23 (20 μM) before TNFα stimulation (T+J) for 24 h and KL expression was determined by immunofluorescence (D). The green fluorescence in the left-hand panels indicates KL, the blue stain indicates the nucleus, and the merged panels are on the right. Immunocytochemistry results are representative of at least three independent experiments.
CSE and TNFα inhibit KL secretion in 16HBE cells with an increased release of pro-inflammatory cytokines
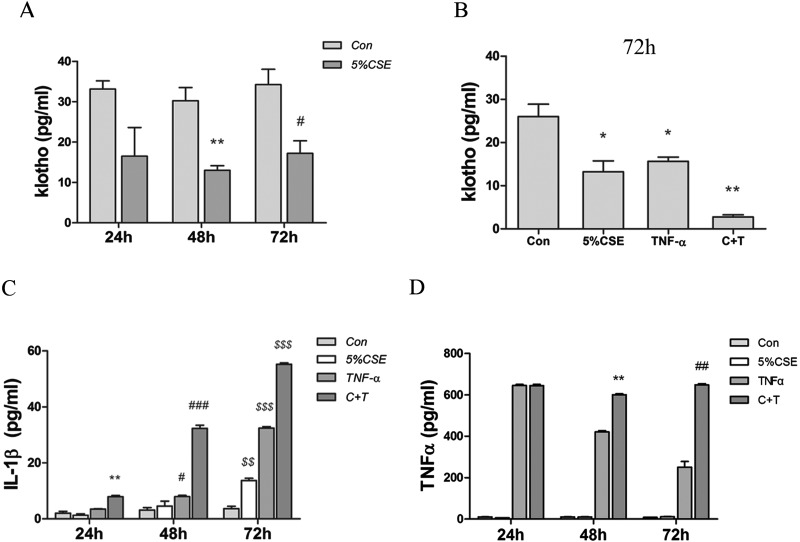
The effect of CSE and TNFα in combination on KL secretion was also measured. Secreted KL levels were reduced by 5% CSE (Figure 4A) and also exogenous TNFα (Figure 4B). The combination of CSE and TNFα resulted in almost complete suppression of KL release (Figure 4B). Meanwhile, the combination of CSE and TNFα exposure increased the expression of IL-1β to a much greater extent than observed with either stimulus alone (Figure 4C). Their combination also resulted in enhanced TNFα release after 48 and 72 h (Figure 4D). These results suggest an inhibited KL expression associated with increased inflammatory response upon CS exposure.
Figure 4. CSE and TNFα inhibit Klotho secretion and increase the release of primary pro-inflammatory cytokines in cultured human bronchial epithelial (16HBE) cells.
The supernatant from the cells treated in (A) were collected and KL secretion detected by ELISA. In separate experiments, 16HBE cells were treated with CSE or TNFα, or both (C+T), for 72 h and KL release was measured by ELISA (B). (*P<0.05, **P<0.01 compared with the control group at 72 h). The 16HBE cells were treated as in (C) and the release of IL-1β was determined. (**P<0.01 compared with the control group at 24 h, #P<0.05, ###P<0.001 compared with the control group at 48 h, $$P<0.01, $$$P<0.001 compared with the control group at 72 h). Cells were cultured in medium containing 5% CSE, exogenous TNFα (30 ng/ml) or both for 24, 48 and 72 h (D). (**P<0.01 compared with the TNFα group at 48 h, ##P<0.01 compared with the TNFα group at 72 h). Results are means±S.E.M. for three independent experiments.
Endogenous KL plays roles in the inflammatory process and cell viability in human bronchial epithelial cells
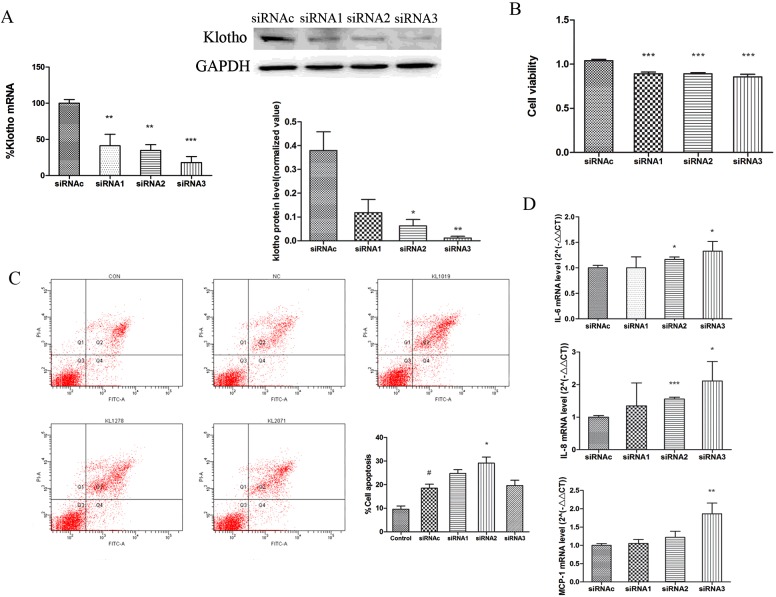
In reverse experiments, we examined the effect of KL knockdown on epithelial cell function by using selective siRNAs. KL knockdown resulted in an 80–95% suppression of KL protein and mRNA expression, with siRNA3 being the most effective (Figure 5A). KL knockdown showed a small, but significant, decrease in cell viability compared with the control siRNA (Figure 5B), and was associated with an increase in apoptosis (Figure 5C). The apoptosis level induced by control siRNA transfection alone (9.6±1.3% compared with 18.5±1.6%, P<0.05) was enhanced further with individual KL-directed siRNAs (siRNA2: 29.1±2.5%, P<0.05 compared with control siRNA; Figure 5C). In addition, the mRNA expression of inflammatory cytokines [IL-6, MCP-1 (monocyte chemoattractant protein 1) and IL-8] was significantly upregulated by KL knockdown (Figure 5D). However, no effect on protein secretion was observed (results not shown).
Figure 5. Klotho knockdown modulates epithelial cell survival and inflammatory gene expression.
(A) Transfection of 16HBE cells with KL siRNAs (siRNA1–siRNA3) significantly down-regulated KL protein and mRNA expression at 24 h compared with control siRNA (siRNAc) treatment. (B) CCK-8 staining shows that KL knockdown slightly, but significantly, reduced cell viability compared with control siRNA. (C) KL-directed siRNAs (siRNA1–siRNA3) induced apoptosis 24 h after transfection as determined by flow cytometry. Representative scatter plots of untreated control cells (CON), siRNAc-treated cells (NC) and siRNA1–siRNA3-treated cells (KL1019, KL1278 and KL2071) are shown. Results in the histogram are means±S.E.M. for three independent experiments. #P<0.05 compared with the control group, *P<0.05 compared with control siRNAc. (D) IL6, MCP1 and IL8 mRNA expression significantly increased after KL knockdown. Results are means±S.E.M. from three independent experiments. (*P<0.05, **P<0.01, ***P<0.001).
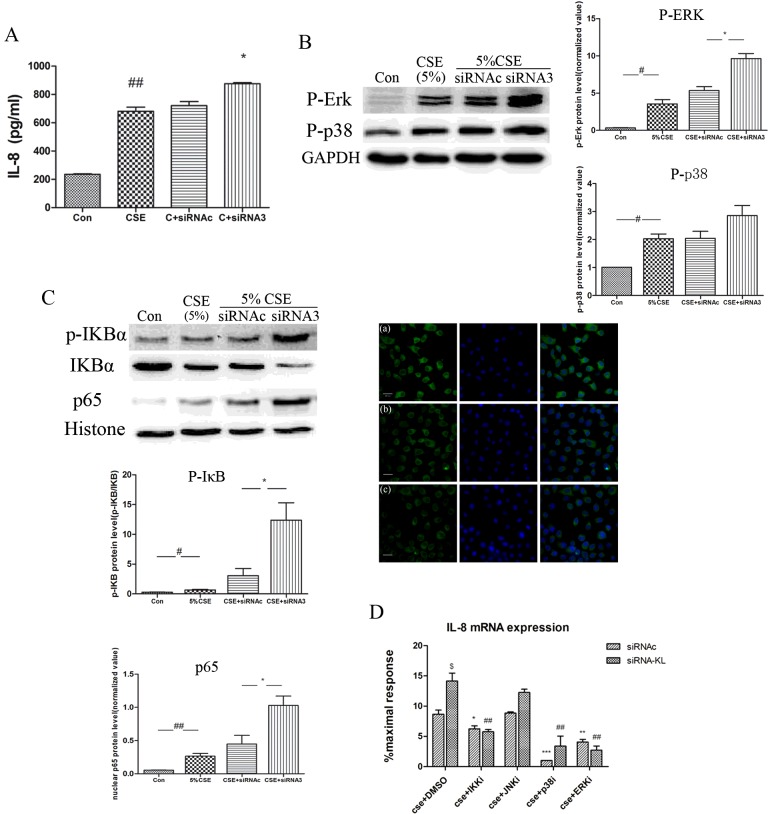
KL depletion amplifies the sensitivity of airway epithelial cells to CS-mediated inflammation
KL knockdown increased both IL-8 mRNA expression and protein release in response to 5% CSE (Figures 6A and 6D). This up-regulation was associated with increased MAPK phosphorylation (Figure 6B) and enhanced p65/NF-κB nuclear translocation (Figure 6C). We used the pharmacological inhibitors JSH23, SP600125, SB203580 and PD98059 (all at 20 μM) to investigate the pathways involved in KL's anti-inflammatory functions (Figure 6D). JSH23 and PD98059, but not SB203580 or SP600125, significantly inhibited CSE-induced IL8 mRNA expression. This result indicates that NF-κB and ERK1/2 signalling pathways were involved in the immunoinflammatory-regulatory functions of KL.
Figure 6. Klotho knockdown modulates cigarette smoke-induced inflammation: role of intracellular signalling pathways.
(A) 16HBE cells were treated with KL-directed siRNA3 for 8 h before cells were exposed to 5% CSE for a further 6 h and IL-8 release was detected and compared with siRNAc-pre-treated cells. (B) The effects of siRNA3 knockdown of KL on 5% CSE-induced MEK/ERK (p-ERK) and p38 MAPK (p-p38) activation was determined after 30 min. (C) In similar experiments, NF-κB [p-IκB (inhibitor of NF-κB) and IκBα (IκB kinase α)] activation and p65 nuclear translocation was determined by Western blot analysis after 5 min in nuclear extracts. The p65 nuclear translocation was also studied by immunofluorescence. Cells were untreated (a) or treated with control siRNA (b) or KL-directed siRNA3 (c) for 8 h before cells were exposed to 5% CSE for 5 min. The green fluorescence in the left-hand panels indicates NF-κB/p65 staining, the blue stain indicates the nucleus, and the merged panels are on the right. Results in the histogram as means±S.E.M. for three independent experiments. ##P<0.01, #P<0.05 compared with the control group; *P<0.05 compared with siRNAc in response to 5% CSE. (D) The effect of 30 min pre-treatment of NF-κB (JSH23, 20 μM), JNK (SP600125, 20 μM), p38 MAPK (SB203580, 20 μM) and MEK/ERK (PD98059, 20 μM) inhibitors on the enhanced mRNA expression of IL-8 observed with 5% CSE and siRNA3-induced KL knockdown. Results are means±S.E.M. for three independent experiments. *P<0.05, **P<0.01, ***P<0.001 compared with CSE+siRNAc+DMSO-treated cells, ##P<0.01 compared with CSE+siRNA+DMSO-treated cells, $P<0.05.
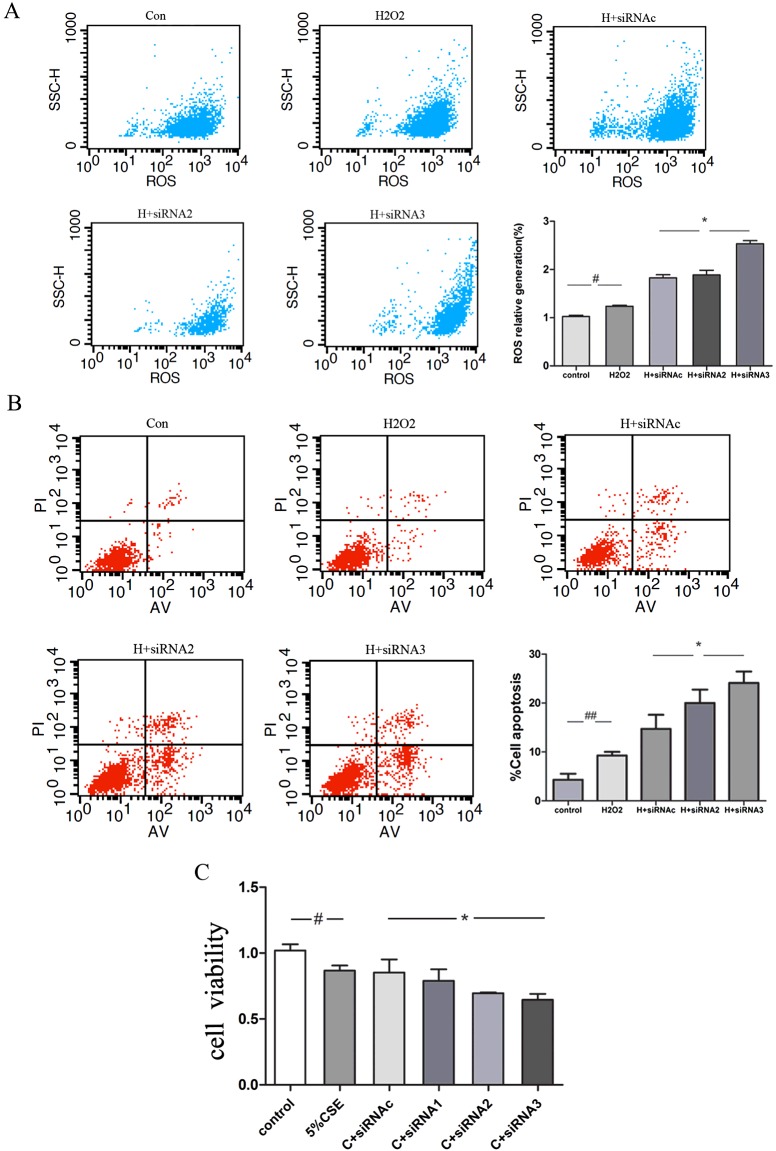
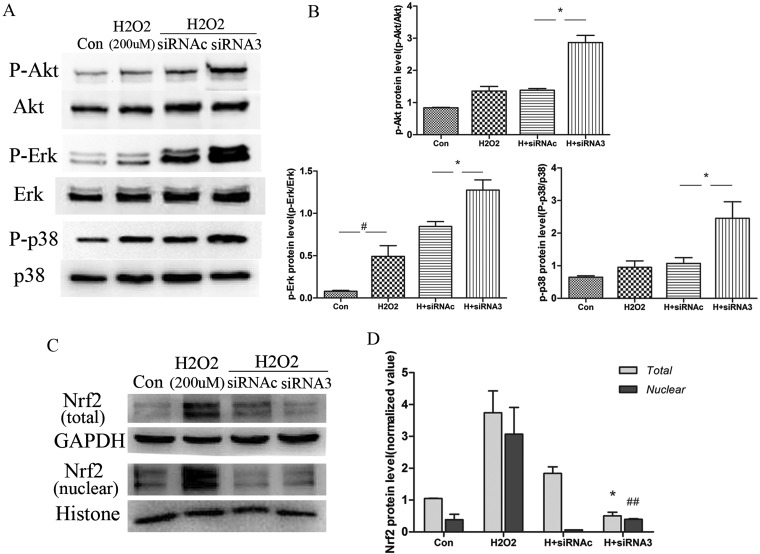
KL deficiency aggravates oxidative-stress-induced cellular injury partly via Nrf2 consumption in 16HBE cells
The effect of KL knockdown on oxidative damage was also assessed. Cells depleted of KL by using siRNA3 produced more ROS in response to 200 μM H2O2 than those treated with control siRNA (Figure 7A). Apoptosis was also significantly increased by KL knockdown in the presence of 200 μM H2O2 (Figure 7B), which may account for the reduced cell viability observed in response to 5% CSE (Figure 7C). An enhanced activation of Akt/PKB (protein kinase B), p38 MAPK and ERK1/2 pathways was observed in the presence of KL knockdown under the conditions of oxidative stress (Figure 8A and 8B). Furthermore, KL knockdown significantly accelerated Nrf2 (nuclear factor erythroid 2-related factor 2) consumption and reduced the ability of the cells to induce an endogenous antioxidant response, as measured by total Nrf2 expression and nuclear localization (Figure 8C). Our results suggest that the lack of intercellular KL increased sensitivity to oxidative stress and exacerbated cellular injury in bronchial epithelial cells.
Figure 7. KL deficiency aggravates oxidative-stress-induced cellular injury.
(A and B) Cells were transfected with control siRNA (siRNAc) and three KL-directed siRNAs (siRNA1–siRNA3) for 6–8 h. After KL knockdown, cells were treated with H2O2 (200 μM) for 24 h, and flow cytometry was performed to determine the intracellular ROS content using the fluorescent probe DCFH-DA (A) and the apoptotic ratio using annexin V–FITC double staining (B). Results in the histogram are means±S.E.M. for three independent experiments. #P<0.05 compared with the control, *P<0.05 compared with H2O2+siRNAc-treated cells. (C) In some experiments, the cells were subsequently exposed to 5% CSE, and cell viability was determined using the CCK-8 method after 24 h. Results are means±S.E.M. for three independent experiments. #P<0.05 compared with the control group, *P<0.05 compared with CSE+siRNAc-treated cells.
Figure 8. KL deficiency aggravates oxidative-stress-induced cellular injury partly via Nrf2 consumption in 16HBE cells.
(A) The ability of H2O2 (200 μM) to activate stress kinases was strengthened and the antioxidant transcription factor Nrf2 was attenuated in siRNA3-pre-treated cells compared with siRNAc-pre-treated cells. 16HBE cells were transfected with control siRNA (siRNAc) and KL-directed siRNA3 for 6–8 h before these cells were treated with H2O2 (200 μM). After 30 min, cell extracts were collected to detect the phosphorylation of several stress kinases [ERK, p38 and PI3K (phosphoinositide 3-kinase)/PI3K-activated serine/threonine kinase Akt/PKB] by Western blotting. (B) Results are means±S.E.M. from three independent experiments (#P<0.05 compared with the control group, *P<0.05 compared with the H2O2 + siRNAc-treated cells). (C) The effect of treatments on Nrf2 expression and translocation, with (D) results shown as means±S.E.M. from three independent experiments (*P<0.05 compared with the H2O2+siRNAc-treated total cell extracts, ##P<0.01 compared with the H2O2+siRNAc-treated nuclear extracts). Con, control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
The present study is the first to demonstrate that KL is mainly expressed along the human airway epithelium and decreased in the lungs of healthy smokers compared with non-smokers, but reduced further in the lungs of COPD patients. KL expression was also lower in the airway epithelium of ozone-induced emphysematous mice than in control mice. Intercellular and secreted KL was down-regulated in 16HBE cells in response to CS exposure and inflammation. Conversely, KL down-regulation promoted the increased expression of pro-inflammatory cytokines, apoptosis and decreased cell viability of human bronchial epithelial cells. This feedback effect of KL protein aggravates chronic inflammation and oxidative injury in the lungs in response to tobacco smoking or oxidative stress, which are pathological hallmarks of COPD [32]. Thus these data provide new insights into the mechanisms associated with the accelerated lung aging in the COPD development.
We also for the first time recapitulate the reduction in KL expression in the ozone-induced mouse model of COPD which possess many features of COPD [23]. Ozone exposure in mice induces a persistent airway inflammatory response and emphysematous changes associated with an increased oxidative damage in airway epithelial cells. Decreased KL expression was observed in the lung along with an alveolar enlargement and damage after 6 weeks of ozone exposure, suggesting that loss of airway KL expression may be linked to the elevated levels of inflammation and oxidative injury. We observed a transient increase in secreted KL in BALF after 3 weeks of ozone exposure only, which correlated with the previous induction of antioxidant responses such as Nrf2 induction observed after 3 weeks in this model [23].
The lung is the only organ that reveals age-exacerbated degenerative changes, including air space enlargement, which suggests that it has a greater sensitivity to KL's actions [10]. KL expression was decreased in the airway epithelium of healthy smokers and decreased further in COPD patients, suggesting that factors additional to CS may be involved in the pathological processes associated with KL suppression. We speculated that chronic inflammation and sustained oxidative injury following CS exposure probably contributed to further KL deficiency in the lung. Consequently, we examined the processes involved in KL suppression in airway epithelial cells. The results showed that intracellular KL levels were reduced by chronic exposure to CSE. In contrast, acute CSE exposure (24 h) resulted in a transient up-regulation of KL protein and mRNA. In addition, KL expression was reduced in a concentration-dependent manner by TNFα both acutely and after more chronic exposure. This indicates that stimuli known to be important in COPD pathogenesis, such as inflammation and oxidative stress, could drive the loss of KL expression [33].
Using a pharmacological approach [16], we demonstrated a key role for the NF-κB pathway in mediating this decrease in KL expression seen in airway epithelial cells. Although we did not explore the mechanism of this further, we speculate that NF-κB may either bind directly to the KL promoter to prevent KL transcription or that NF-κB acts indirectly by inhibiting PPARγ (peroxisome-proliferator-activated receptor γ) or Egr-1 (early growth response-1) which are known to activate the KL promoter [16,34–36]. KL secretion was also reduced by CSE and TNFα, and this was accompanied by an increase in pro-inflammatory mediators. Considering the two pathways involved in KL secretion [7,8], we speculated that CS and inflammatory cytokine reduce soluble KL either by inhibiting KL shedding by affecting ADAM levels or proteolytic activity in addition to suppressing KL gene transcription.
Given the fact that KL exerts various beneficial functions, including its cytoprotection of pulmonary epithelia, it is plausible that the KL deficiency seen in COPD may either predispose to, or exacerbate the effects of, exogenous insults causing lung injury [19]. To determine the potential biological roles of reduced KL in COPD pathogenesis, we established an in vitro human airway epithelial cell model of KL depletion and stimulated it with noxious stimuli such as CSE. Intracellular KL deficiency contributed to up-regulation of pro-inflammatory cytokine expression (IL-8, IL-6 and MCP-1) and the increased sensitivity of cells to CSE induced inflammatory processes such as enhanced IL-8 expression, augmented MAPK phosphorylation and p65/NF-κB nuclear translocation. Unexpectedly, we found that KL knockdown had no effect on the secretion of cytokines and chemokines. This may reflect the limited numbers of genes examined and be linked to various mechanisms controlling their expression. It is possible that the effect of KL knockdown is to leave the cells primed to respond to a second signal such as CSE which enhances the release of these inflammatory mediators from epithelial cells (Figure 6). These results indicate an intrinsic anti-inflammatory property of KL and suggest the possibility of a feedforward mechanism to amplify the loss of KL over time.
One previous study has shown an anti-inflammatory effect of KL associated with the reduction in NF-κB activity in kidney cells [19]. In the present study, pharmacological inhibition of the pathways decreased IL-8 expression, with a greater effect observed with the NF-κB and ERK inhibitors in KL-depleted cells compared with control siRNA-exposed cells, providing a supplement to previous studies and novel intracellular signalling pathways involved in KL function. Overall, the loss of KL expression impairs the resistance of the cells to persistent airway insults and exacerbates irreversible inflammation, which may accelerate the progression of COPD [37].
Excessive ROS generation initiates inflammatory responses, regulates cell proliferation, induces apoptosis or causes direct lung injury, which is involved in COPD pathology [32]. Previous studies have reported that KL increases resistance to oxidative injury in endothelial cells via different mechanisms [17,38,39]. In the present study, KL depletion increased the sensitivity of the airway epithelial cells to oxidative-stress-induced damage measured by further ROS production, reduced cell viability and enhanced apoptosis. Phosphorylation of Akt/PKB, ERK1/2 and p38 MAPK pathways, which possess critical functions in regulating inflammation and oxidative stress in COPD [40], were also found in KL-depleted cells upon H2O2 exposure.
Nrf2 plays a pivotal role in antioxidant protection in CS-exposed lungs [41], whereas its gene disruption results in elevated susceptibility to emphysema after CS exposure [42]. In the present study, oxidative stress activated Nrf2 in a KL-dependent manner. KL depletion resulted in decreased total Nrf2 expression and increased nuclear translocation of Nrf2 upon H2O2 exposure. Lau et al. [43] demonstrated that translocating Nrf2 to the nucleus corresponds to a defensive response in BEAS-2B cells under oxidative stress, which is also involved in IL-8 expression. Our results provide evidence suggesting that KL may function as an antioxidant to delay the consumption of Nrf2 and attenuate pro-inflammatory cytokine expression. Although the effect observed on Nrf2 translocation was relatively small, it may, at least in part, provide a novel antioxidant function for KL that could contribute to COPD pathogenesis.
The present study has several limitations. The work was performed only in the 16HBE cell line, whereas, ideally, primary cells from COPD patients and healthy controls should be used. Several biological roles for KL have been determined, but the underlying molecular mechanisms are not fully understood. Whether the up-regulation of KL or the transgenic modification of KL slows the progression of COPD in models of disease should also be determined in future studies.
In conclusion, we are the first to demonstrate that KL deficiency induced by CS or other related risk factors may be a significant factor to increase the susceptibility of the lung to develop COPD. The study not only provides data for KL expression in the epithelial cells of human COPD lung, but also explores the possible role of KL in COPD formation in animal and in vitro studies, which might provide new insights into the mechanisms associated with the accelerated lung aging in the COPD development. Moreover, considering the role of bronchial epithelial cells in orchestrating various downstream responses to CS [20], drugs targeting KL deficiency in the cells may improve excessive inflammation and oxidative injury without the need for separate therapies.
Acknowledgments
We thank Rongbin Yu, Ji Zhou and Hui Bi for their suggestions and comments during the study.
Abbreviations
- ADAM
a disintegrin and metalloproteinase
- BALF
bronchial alveolar lavage fluid
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- CSE
cigarette smoke extract
- DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- ERK
extracellular-signal-regulated kinase
- IL
interleukin
- JNK
c-Jun N-terminal kinase
- KL
Klotho
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein 1
- MEK
MAPK/ERK kinase
- NF-κB
nuclear factor κB
- Nrf2
nuclear factor erythroid 2-related factor 2
- PKB
protein kinase B
- qPCR
quantitative PCR
- ROS
reactive oxygen species
- RT
reverse transcription
- TNF
tumour necrosis factor
AUTHOR CONTRIBUTION
All authors read and met ICMJE criteria for authorship. Wei Gao, Ian Adcock and Xin Yao designed this study. Wei Gao, Cheng Yuan, Jingying Zhang, Coen Wiegman and Like Yu acquired the data of the study. Wei Gao, Lingling Li and Mao Huang performed the analysis and interpretation. Wei Gao wrote the first draft of the paper, and Xin Yao, Ian Adcock and Peter Barnes critically revised the paper. All authors read and approved the final paper.
FUNDING
This work was supported by the National Nature Science Foundation [grant numbers 81470237 and 81070025] and the Priority Academic Program Development of Jiangsu Higher Education Institutions [PAPD, grant number JX10231802]. I.M.A. and P.J.B. are supported by the Medical Research Council [grant number G0801266] and Wellcome Trust [grant number 093080/Z/10/Z]. C.H.W. is a Research Councils UK (RCUK) Fellow.
References
- 1.Barnes P.J. Chronic obstructive pulmonary disease. N. Engl. J. Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 2.Brusselle G.G., Joos G.F., Bracke K.R. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378:1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- 3.Shaker S.B., Stavngaard T., Hestad M., Bach K.S., Tonnesen P., Dirksen A. The extent of emphysema in patients with COPD. Clin. Respir. J. 2009;3:15–21. doi: 10.1111/j.1752-699X.2008.00102.x. [DOI] [PubMed] [Google Scholar]
- 4.Ito K., Barnes P.J. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 5.Provinciali M., Cardelli M., Marchegiani F. Inflammation, chronic obstructive pulmonary disease and aging. Curr. Opin. Pulm. Med. 2011;17:S3–S10. doi: 10.1097/01.mcp.0000410742.90463.1f. [DOI] [PubMed] [Google Scholar]
- 6.Kurosu H., Kuro-o M. The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol. Cell. Endocrinol. 2009;299:72–78. doi: 10.1016/j.mce.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 7.Matsumura Y., Aizawa H., Shiraki-Iida T., Nagai R., Kuro-o M., Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted Klotho protein. Biochem. Biophys. Res. Commun. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 8.Chen C.D., Podvin S., Gillespie E., Leeman S.E., Abraham C.R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 10.Suga T., Kurabayashi M., Sando Y., Ohyama Y., Maeno T., Maeno Y., Aizawa H., Matsumura Y., Kuwaki T., Kuro-o M., et al. Disruption of the klotho gene causes pulmonary emphysema in mice: defect in maintenance of pulmonary integrity during postnatal life. Am. J. Respir. Cell Mol. Biol. 2000;22:26–33. doi: 10.1165/ajrcmb.22.1.3554. [DOI] [PubMed] [Google Scholar]
- 11.Masuda H., Chikuda H., Suga T., Kawaguchi H., Kuro-o M. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech. Ageing Dev. 2005;126:1274–1283. doi: 10.1016/j.mad.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki Y., Imura A., Urakawa I., Shimada T., Murakami J., Aono Y., Hasegawa H., Yamashita T., Nakatani K., Saito Y., et al. Establishment of sandwich ELISA for soluble α-Klotho measurement: age-dependent change of soluble α-Klotho levels in healthy subjects. Biochem. Biophys. Res. Commun. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu M.C., Shi M., Zhang J., Quiñones H., Griffith C., Kuro-o M., Moe O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh N., Fujimori T., Nishiguchi S., Tamori A., Shiomi S., Nakatani T., Sugimura K., Kishimoto T., Kinoshita S., Kuroki T., Nabeshima Y. Severely reduced production of Klotho in human chronic renal failure kidney. Biochem. Biophys. Res. Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 15.Moreno J.A., Izquierdo M.C., Sanchez-Niño M.D., Suárez-Alvarez B., Lopez-Larrea C., Jakubowski A., Blanco J., Ramirez R., Selgas R., Ruiz-Ortega M., et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NF-κB. J. Am. Soc. Nephrol. 2011;22:1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto M., Clark J.D., Pastor J.V., Gurnani P., Nandi A., Kurosu H., Miyoshi M., Ogawa Y., Castrillon D.H., Rosenblatt K.P., Kuro-o M. Regulation of oxidative stress by the anti-aging hormone Klotho. J. Biol. Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maekawa Y., Ohishi M., Ikushima M., Yamamoto K., Yasuda O., Oguro R., Yamamoto-Hanasaki H., Tatara Y., Takeya Y., Rakugi H. Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr. Gerontol. Int. 2011;11:510–516. doi: 10.1111/j.1447-0594.2011.00699.x. [DOI] [PubMed] [Google Scholar]
- 18.Ravikumar P., Ye J., Zhang J., Pinch S.N., Hu M.C., Kuro-o M., Hsia C.C., Moe O.W. α-Klotho protects against oxidative damage in pulmonary epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307:L566–L575. doi: 10.1152/ajplung.00306.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Banerjee S., Dey N., LeJeune W.S., Sarkar P.S., Brobey R., Rosenblatt K.P., Tilton R.G., Choudhary S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine)536 phosphorylation. Diabetes. 2011;60:1907–1916. doi: 10.2337/db10-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao W., Li L., Wang Y., Zhang S., Adcock I.M., Barnes P.J., Huang M., Yao X. Bronchial epithelial cells: the key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology. 2015;20:722–729. doi: 10.1111/resp.12542. [DOI] [PubMed] [Google Scholar]
- 21.Yao X., Yuan C., Zhang J., Zhou L., Huang M., Adcock I., Barnes P. Klotho: an important protein in the formation and development of emphysema. Eur. Respir. J. 2012;40(Suppl. S6):4536. [Google Scholar]
- 22.Pauwels R.A., Buist A.S., Calverley P.M., Jenkins C.R., Hurd S.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am. J. Respir. Crit. Care. Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 23.Wiegman C.H., Li F., Clarke C.J., Jazrawi E., Kirkham P., Barnes P.J., Adcock I.M., Chung K.F. A comprehensive analysis of oxidative stress in the ozone-induced lung inflammation mouse model. Clin. Sci. 2014;126:425–440. doi: 10.1042/CS20130039. [DOI] [PubMed] [Google Scholar]
- 24.Hodge S., Hodge G., Scicchitano R., Reynolds P.N., Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 2003;81:289–96. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L., Gallup M., Zlock L., Basbaum C., Finkbeiner W.E., McNamara N.A. Cigarette smoke disrupts the integrity of airway adherens junctions through the aberrant interaction of p120-catenin with the cytoplasmic tail of MUC1. J. Pathol. 2013;229:74–86. doi: 10.1002/path.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White S.R., Tse R., Marroquin B.A. Stress-activated protein kinases mediate cell migration in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2005;32:301–310. doi: 10.1165/rcmb.2004-0118OC. [DOI] [PubMed] [Google Scholar]
- 27.Ren S., Zhang S., Li M., Huang C., Liang R., Jiang A., Guo Y., Pu Y., Huang N., Yang J., Li Z. NF-κB p65 and c-Rel subunits promote phagocytosis and cytokine secretion by splenic macrophages in cirrhotic patients with hypersplenism. Int. J. Biochem. Cell Biol. 2012;45:335–343. doi: 10.1016/j.biocel.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Carp H., Janoff A. Possible mechanisms of emphysema in smokers: in vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am. Rev. Respir. Dis. 1978;118:617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- 29.He X.J., Ma Y.Y., Yu S., Jiang X.T., Lu Y.D., Tao L., Wang H.P., Hu Z.M., Tao H.Q. Up-regulated miR-199a-5p in gastric cancer functions as an oncogene and targets klotho. BMC Cancer. 2014;14:218. doi: 10.1186/1471-2407-14-218. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Zimarino V., Wu C. Induction of sequence-specific binding of Drosophila heat shock activator protein without protein synthesis. Nature. 1987;327:727–730. doi: 10.1038/327727a0. [DOI] [PubMed] [Google Scholar]
- 31.Liu E., Du X., Ge R., Liang T., Niu Q., Li Q. Comparative toxicity and apoptosis induced by diorganotins in rat pheochromocytoma (PC12) cells. Food Chem. Toxicol. 2013;60:302–308. doi: 10.1016/j.fct.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 32.Rahman I., Adcock I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 33.Mitobe M., Yoshida T., Sugiura H., Shirota S., Tsuchiya K., Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp. Nephrol. 2005;101:e67–e74. doi: 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- 34.Barnes P.J. Mediators of chronic obstructive pulmonary disease. Pharmacol. Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H., Li Y., Fan Y., Wu J., Zhao B., Guan Y., Chien S., Wang N. Klotho is a target gene of PPAR-γ. Kidney Int. 2008;74:732–739. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 36.Choi B.H., Kim C.G., Lim Y., Lee Y.H., Shin S.Y. Transcriptional activation of the human Klotho gene by epidermal growth factor in HEK293 cells: role of Egr-1. Gene. 2010;450:121–127. doi: 10.1016/j.gene.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Barnes P.J., Shapiro S.D., Pauwels R.A. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur. Respir. J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 38.Ohta J., Rakugi H., Ishikawa K., Yang J., Ikushima M., Chihara Y., Maekawa Y., Oguro R., Hanasaki H., Kida I., et al. Klotho gene delivery suppresses oxidative stress in vivo. Geriatr. Gerontol. Int. 2007;7:293–299. doi: 10.1111/j.1447-0594.2007.00406.x. [DOI] [Google Scholar]
- 39.Rakugi H., Matsukawa N., Ishikawa K., Yang J., Imai M., Ikushima M., Maekawa Y., Kida I., Miyazaki J., Ogihara T. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82–87. doi: 10.1007/s12020-007-0016-9. [DOI] [PubMed] [Google Scholar]
- 40.Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: cellular and molecular mechanisms. Cell Biochem. Biophys. 2005;43:167–188. doi: 10.1385/CBB:43:1:167. [DOI] [PubMed] [Google Scholar]
- 41.Yao H., Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol. Appl. Pharmacol. 2011;254:72–85. doi: 10.1016/j.taap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louhelainen N., Myllarniemi M., Rahman I., Kinnula V.L. Airway biomarkers of the oxidant burden in asthma and chronic obstructive pulmonary disease: current and future perspectives. Int. J. Chron. Obstruct. Pulmon. Dis. 2008;3:585–603. doi: 10.2147/copd.s3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau W.K., Chan S.C., Law A.C., Ip M.S., Mak J.C. The role of MAPK and Nrf2 pathways in ketanserin-elicited attenuation of cigarette smoke-induced IL-8 production in human bronchial epithelial cells. Toxicol. Sci. 2012;125:569–577. doi: 10.1093/toxsci/kfr305. [DOI] [PubMed] [Google Scholar]