Abstract
Chordin-mediated regulation of bone morphogenetic protein (BMP) family growth factors is essential in early embryogenesis and adult homoeostasis. Chordin binds to BMPs through cysteine-rich von Willebrand factor type C (vWC) homology domains and blocks them from interacting with their cell surface receptors. These domains also self-associate and enable chordin to target related proteins to fine-tune BMP regulation. The chordin–BMP inhibitory complex is strengthened by the secreted glycoprotein twisted gastrulation (Tsg); however, inhibition is relieved by cleavage of chordin at two specific sites by tolloid family metalloproteases. As Tsg enhances this cleavage process, it serves a dual role as both promoter and inhibitor of BMP signalling. Recent developments in chordin research suggest that rather than simply being by-products, the cleavage fragments of chordin continue to play a role in BMP regulation. In particular, chordin cleavage at the C-terminus potentiates its anti-BMP activity in a type-specific manner.
Keywords: bone morphogenetic protein (BMP) signalling, chordin, tolloid cleavage
Introduction
Bone morphogenetic proteins (BMPs) are homodimeric secreted growth factors of the transforming growth factor-β (TGF-β) superfamily with essential roles in dorsal-ventral axis patterning during early embryogenesis and most notably inducing growth of skeletal structures in post-natal tissues [1]. They are of clinical interest due to both their frequent dysregulation in cancer [2,3] and the increasing use of recombinant BMPs in orthopaedics [4]. BMPs signal by binding to cell surface BMP receptors (BMPRs) leading to gene regulation through phosphorylation of small mothers against decapentaplegic (Smad) transcription factors [5]. This in turn can induce a broad range of cellular responses including differentiation, migration, proliferation and apoptosis [6]. Following secretion, BMP signalling is closely regulated by a range of extracellular antagonists including noggin, the differential screening selected gene abberative in neuroblastoma (DAN) family, twisted gastrulation (Tsg) and chordin [7].
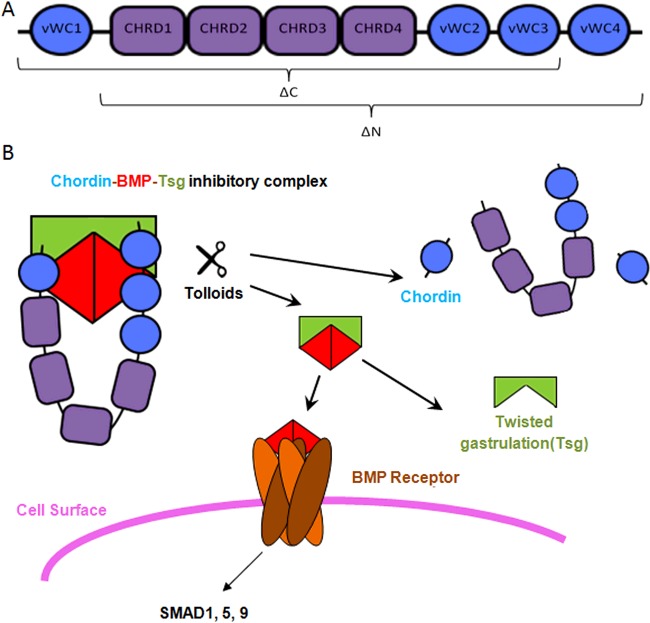
Chordin is a specific inhibitor of BMP-2, -4, -7 and anti-dorsalizing morphogenetic protein. During early embryogenesis, it is secreted from the dorsal embryonic pole, stabilizing a high-ventral to low-dorsal BMP morphogen gradient [8]. In addition, it is thought to play a key role in the adult brain [9]. It is a horseshoe-shaped protein [10] consisting of four cysteine rich von Willebrand factor type C (vWC) homology domains which are responsible for BMP binding and four chordin specific (CHRD) domains of unknown function which are unique to chordin and some bacterial proteins [11], shown in Figure 1(A). The terminal BMP-binding domains are spaced so that a BMP dimer can be positioned between them suggesting that chordin can bind BMPs co-operatively thereby covering two BMPR interaction sites simultaneously (Figure 1B). This may serve to stabilize the complex through multiple recognition sites and increase steric interference between BMPs and their receptors.
Figure 1. Summary of Chordin, Tsg and Tolloid Regulation of BMP Signalling.
(A) Schematic diagram of the domain layout of full-length chordin with the larger fragments produced by tolloid cleavage indicated. (B) Model showing the mechanism of BMP regulation by chordin. BMP binds to chordin in an inhibitory complex, which is strengthened in the presence of Tsg. Following cleavage of chordin by tolloids at both sites, Tsg competes with the residual fragments for binding to BMP and is thought to increase the rate of fragment turnover.
BMP inhibition is relieved by cleavage of chordin by tolloids at two specific sites, leaving the BMP-binding vWC domains intact [12]. Tsg acts as both an agonist and antagonist of BMP signalling in a context-dependent manner. It binds to chordin and BMPs strengthening the inhibitory complex, however in the presence of Tsg, chordin cleavage by tolloid is enhanced and the residual anti-BMP activity of the cleaved vWC domains is reduced [13]. A mechanism of facilitated diffusion is proposed for this pathway, whereby chordin and Tsg maintain BMP in an inactive state whereas they diffuse through the extracellular space [14]. Chordin then binds to cell surface anchored components such as BMP-binding endothelial cell precursor-derived regulator (BMPER) [15], integrins [16] and collagen IV [17], localizing captive BMP to a specific tissue for subsequent release by tolloids or promoting chordin endocytosis.
Individual vWC domains are less biologically active [18] and are thought be removed from the extracellular space more rapidly than full-length chordin [13,15]. In addition, Tsg competes for BMP-binding, causing the individual vWC domains to dissociate from BMP [13]. However, the larger fragments of chordin (∆N and ∆C in Figure 1A) appear to retain their BMP-inhibitory capacity [10]. Similarly truncated splice variants are expressed in a tissue specific manner and are likely to play a distinct role in BMP inhibition [19]. Although there is evidence that they may be subject to faster endocytic turnover than full-length chordin in some biological contexts, they are also predicted to exist at steady state levels in the developing embryo [20]. In this review, we focus on our emerging understanding of the contribution of the behaviour of these fragments compared with full-length chordin and discuss their role in BMP-inhibition.
Loss of the C-terminal vWC domain of chordin potentiates BMP antagonism
The first indication that truncation of chordin could potentially confer gain in function was observed in Drosophila [21]. The constructs, termed ‘Supersog’, are a range of C-terminally truncated fragments with increased in vivo activity, with the most effective Supersog terminating 80 residues prior to vWC2. However, removal of vWC1 eliminates Supersog activity entirely. This suggests that complete cleavage by tolloid is sufficient to ablate Sog anti-BMP activity, but Supersog was resistant to further tolloid processing [22]. Supersog does not appear to simply be a higher affinity version of Sog but rather have distinct broader-spectrum BMP inhibitory activity.
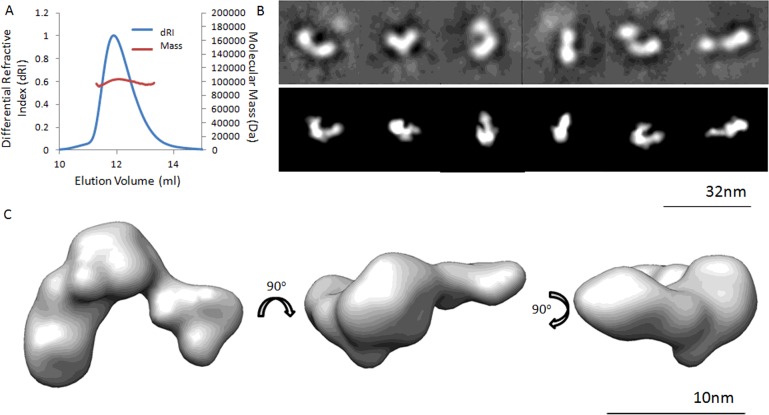
A subsequent study in zebrafish observed the effects of mutating the N-terminal, C-terminal or both cleavage sites of chordin to make them resistant to tolloids. N-terminally truncated chordin was a slightly less efficient BMP inhibitor [20]. However C-terminally truncated chordin was a more efficient in vivo BMP-inhibitor in vertebrates, similar to Supersog. It was also found to be present at steady-state levels in the developing embryo at comparable levels to full-length chordin, therefore likely to make a significant physiological contribution to BMP-inhibition. Tolloids are ubiquitous enzymes required for the processing of many essential matrix substrates including collagens. By utilizing either the N- or C-terminal chordin cleavage site tolloids may operate an intrinsic structural fine tune mechanism to yield either BMP-4 or -7 signalling. For instance, cleaving off the N-terminal vWC1 reduces BMP-7 but not BMP-4 inhibition, whereas C-terminal cleavage of vWC4 leads to stronger inhibition of BMP-4 while BMP-7 activity remains unaffected [10]. Our interaction studies with BMP growth factors and chordin cleavage variants combined with bioactivity assays employing human cell lines confirmed the reported in vivo property of chordin ∆C [10]. However, structurally there is little change following cleavage by tolloids either in chordin ∆N [10] or in chordin ∆C as shown in Figure 2. Multi angle light scattering (MALS) shows that, like full-length chordin, the protein is monomeric at 0.5 mg ml−1 (Figure 2A). Negative stain EM single particle analysis, shown in Figures 2(B) and 2(C), reveals a horseshoe-shaped nanoscale conformation similar to that previously described for full-length chordin.
Figure 2. Structure of the C-terminally Cleaved Chordin Fragment Generated by Tolloid.
(A) MALS trace showing that following C-terminal cleavage the chordin fragment ∆C remains predominantly monomeric at 0.5 mg ml−1. Mr=102.3 kDa ± 0.038%. (B) Selected class averages (top) and re-projections of the 3D reconstruction (bottom) showing different views of chordin ∆C. (C) 3D TEM model of chordin ∆C generated from ∼10000 negatively stained particles from 20 micrographs using angular reconstruction with EMAN2, shown in three orthogonal orientations. Data collected on a Tecnai G2 Polara electron microscope at 300 kV and 5 Å (1 Å=0.1 nm) pixel. Resolution by Fourier shell correlation=24 Å. All data collected according to the methods described in Troilo et al. [10].
Using SAXS analysis of the CHRD domains and EM of chordin ∆N, the position of the domains were mapped. This demonstrated that the vWC domains protrude as prongs from the main body of the structure [10]. Based on this and the binding studies which have shown strong affinity between BMPs and both N- and C-terminal vWC domains [18,23], the BMP dimer is predicted to sit in the cleft (Figure 1B). Although the high prevalence of intradomain disulfide bonding renders secondary structure alteration unlikely and nanoscale structural analysis shows a broadly similar conformation, more subtle shifts in tertiary structure are possible. However, binding affinity to BMPs remains similar following cleavage [10], which suggests that the change in BMP-inhibitory activity is not necessarily the result of conformational change.
Self-affinity of von Willebrand factor type C domains
vWC domains are found not only within the chordin family but within a range of related proteins. In addition to binding to growth factors, they frequently display self-affinity which usually serves as the basis for common docking mechanisms between proteins containing them. These domains consist of 6–10 member cysteine rings and are ∼50–60 amino acids in length. The BMP-binding vWC domain of the chordin-family member BMPER determined by X-ray crystallography shows a β-sheet structure [24] as does a chordin-like vWC module from collagen IIa [25] determined by NMR. The vWC domain is composed of an N-terminal subdomain (SD)1 in which the BMP-binding activity resides and a C-terminal SD2 [26].
vWC self-association has been demonstrated between chordin and its fragments with binding partners, including BMPER. BMPER is important in long-range relocalization of chordin during mouse vertebral development [27]. This is predicted to localize chordin–BMP complexes to establish a vertebral morphogenetic field. Once bound to these complexes it exerts tolloid-independent anti-chordin activity, weakening chordin-BMP affinity promoting release of BMPs [28]. Unlike other secreted inhibitors such as noggin and gremlin, chordin associated to BMP is not internalized after binding to BMPER [15]. Moreover, BMPER binds preferentially to the chordin fragments generated by tolloids which may contribute to clean-up of residual anti-BMP activity following cleavage [29].
The Drosophila chordin equivalent, Sog, which has the same vWC domain layout as chordin has been shown to interact with collagen IV [17]. It is predicted that collagen IV assists in a stepwise manner with the assembly of the Sog–BMP complex. When Tsg binds in the vWC1 position this causes dissociation of Sog from collagen IV releasing the complex from the matrix. In addition, collagen IV appears to act as a scaffold to promote tolloid interaction with Sog, leading to enhanced Sog cleavage [30]. Due to conserved binding sequences for growth factor interaction, it is predicted that the role of collagen IV in BMP signalling in flies is conserved in vertebrates. Sog vWC1 and 4 both bind to collagen IV and it is likely that cleavage at either site would disrupt binding [17].
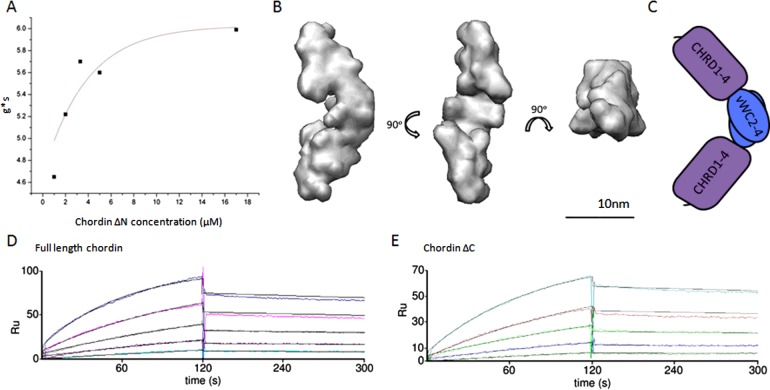
Chordin itself shows a propensity to reversibly self-associate in a concentration-dependent manner shown in Figure 3. In order to establish the strength of the interaction in solution, the changes in the differential sedimentation coefficient distribution (Is-g*s) with respect to concentration was experimentally determined using analytical ultracentrifugation and analysed within the Sedfit analysis package [31]. Fitting the apparent sedimentation coefficients to an experimental association curve give as a value of 3.3 μM for the Kd. In Xenopus, chordin reaches a concentration of 33 nM in the embryo though at the dorsal pole this is predicted to be substantially higher [32]. This indicates that the presence of self-associated chordin is probably sparse and transient in vivo. However, in Figures 3(D) and 3(E) surface plasmon resonance (SPR) analysis of full-length chordin and chordin ∆C respectively show self-affinity in the low nanomolar range. We predict this to be a product of immobilization of the ligand and it raises the possibility that chordin immobilized on the cell surface may be more prone to oligomerization.
Figure 3. Chordin Self-Association.
(A) Self association was quantified using sedimentation velocity where the Is-g*s determined sedimentation coefficients were plotted against chordin concentration. The resulting dissociation constant was 3.3 μM. (B) Ab initio model rendered at 25 Å, generated using DAMMIN software applying P2 symmetry shown in three orthogonal views. Model with lowest normalized standard deviation (NSD) (0.779) shown (mean NSD from 20 models=0.843±0.074). (C) Schematic diagram showing chordin ∆N dimer in end-to-end orientation. The CHRD1–4 domains (purple) are not able to self-associate therefore one orientation is possible. SPR analysis showing self-association between full-length chordin ligand and analyte (D) and chordin ∆C ligand and analyte (E). Analyte concentration=0—80 nM in 1 M urea. Kd=3.05 nM and 3.15 nM respectively. SPR experiments performed according to the methods described in Troilo et al. [10].
SAXS analysis of the fragments at concentrations exceeding 1 mg ml−1 reveals that the chordin oligomers are able to form in an end-to-end orientation, shown for ∆N in Figure 3(B). Flexibility analysis using normalized Kratky and Porod–Debye plots shows that the oligomers are inflexible (Supplementary Figures S1 and S2). ∆N forms a dimer at high concentrations (147 kDa) according to reference-independent mass assessment using Scatter software whereas ∆C preferentially forms a larger oligomer (302 kDa). We have shown that the CHRD1–4 region is monomeric [10]; therefore, self-interaction must occur via the vWC domains. In the case of ∆N, there is only one orientation for end-to-end association (Figure 3C). However ∆C and full-length chordin have vWC domains at both ends allowing higher order assemblies to form. These oligomers have maximum dimensions of 23 nm and 25 nm and a radius of gyration (Rg) of 7.3 nm and 6.15 nm for ∆C and ∆N respectively. This small decrease in overall length for ∆C compared with ∆N suggests that while C-terminal-mediated dimerization results in an end-to-end extended conformation, interaction involving vWC1 does not lead to further extension.
Concluding remarks
Taken together, the available evidence suggests that the increase in BMP-antagonist activity in C-terminally truncated chordin and Sog, observed by several groups, is unlikely to be the result of intrinsic changes in BMP interaction [20,21]. It is possible that the increase in BMP inhibitory activity of ∆C arises due to the loss of a vWC4-mediated interaction. Alternatively, it is possible that vWC4 may have an inhibitory effect on chordin binding to BMP types which have a higher affinity to vWC3 and that vWC4 removal may render vWC3 more easily accessible. BMP-4 has been shown to bind more effectively to vWC3 than vWC4, whereas the reverse is true for BMP-7 which supports this hypothesis [18].
Further study is needed to determine the exact biological function of the partially cleaved chordin fragments and the truncated chordin splice-variants. What is clear is that partial cleavage of chordin by tolloids is insufficient to activate BMPs and that the fragments, present at significant levels in vivo, are relevant contributors to BMP-inhibition [20]. Truncated chordin appears to be more stable and, being smaller, easier to produce than full-length chordin and greater understanding of their function may unlock future therapeutic applications, such as inhibiting BMP signalling in cancer.
Acknowledgments
We thank Dr Maxim Petouckhov (beamline X33) for assistance during SAXS data collection.
Abbreviations
- BMP
bone morphogenetic protein
- BMPER
BMP-binding endothelial cell precursor-derived regulator
- BMPR
BMP receptor
- MALS
multi angle light scattering
- SD
subdomain
- SPR
surface plasmon resonance
- Tsg
twisted gastrulation
- vWC
von Willebrand factor type C
Footnotes
Repetitive, Non-Globular Proteins: Nature to Nanotechnology: Held at the University of York, U.K., 30 March 2015–1 April 2015.
Funding
This work was supported by the Biotechnology and Biological Sciences Research Council [grant number BB/I019286/1 (to C.B.)]; the Wellcome Trust Studentship [grant number 106503/Z/14/Z (to A.B.)]; and the Deutsche Forschungsgemeinschaft [grant number SFB829/Project B12 (to G.S.)].
References
- 1.Medeiros D.M., Crump J.G. New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Dev. Biol. 2012;371:121–135. doi: 10.1016/j.ydbio.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng H., Makizumi R., Ravikumar T.S., Dong H., Yang W., Yang W.L. Bone morphogenetic protein-4 is overexpressed in colonic adenocarcinomas and promotes migration and invasion of HCT116 cells. Exp. Cell Res. 2007;313:1033–1044. doi: 10.1016/j.yexcr.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Yan H., Zhu S., Song C., Liu N., Kang J. Bone morphogenetic protein (BMP) signaling regulates mitotic checkpoint protein levels in human breast cancer cells. Cell Signal. 2012;24:961–968. doi: 10.1016/j.cellsig.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Guo J., Liu J., Wei L., Wu G. BMP-functionalised coatings to promote osteogenesis for orthopaedic implants. Int. J. Mol. Sci. 2014;15:10150–10168. doi: 10.3390/ijms150610150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawabata M., Imamura T., Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/S1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 6.Kallioniemi A. Bone morphogenetic protein 4-a fascinating regulator of cancer cell behavior. Cancer Genetics. 2012;205:267–277. doi: 10.1016/j.cancergen.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Brazil D.P., Church R.H., Surae S., Godson C., Martin F. BMP signalling: agony and antagony in the family. Trends Cell Biol. 2015;25:249–264. doi: 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Reversade B., De Robertis E.M. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikawa S., Sato K. Chordin expression in the adult rat brain. Neuroscience. 2014;258:16–33. doi: 10.1016/j.neuroscience.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Troilo H., Zuk A.V., Tunnicliffe R.B., Wohl A.P., Berry R., Collins R.F., Jowitt T.A., Sengle G., Baldock C. Nanoscale structure of the BMP antagonist chordin supports cooperative BMP binding. Proc. Natl. Acad. Sci. US.A. 2014;111:13063–13068. doi: 10.1073/pnas.1404166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyvonen M. CHRD, a novel domain in the BMP inhibitor chordin, is also found in microbial proteins. Trends Biochem. Sci. 2003;28:470–473. doi: 10.1016/S0968-0004(03)00171-3. [DOI] [PubMed] [Google Scholar]
- 12.Piccolo S., Agius E., Lu B., Goodman S., Dale L., De Robertis E.M. Cleavage of chordin by xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/S0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larrain J., Oelgeschlager M., Ketpura N.I., Reversade B., Zakin L., De Robertis E.M. Proteolytic cleavage of chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128:4439–4447. doi: 10.1242/dev.128.22.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Zvi D., Shilo B.Z., Fainsod A., Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453:1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- 15.Kelley R., Ren R., Pi X., Wu Y., Moreno I., Willis M., Moser M., Ross M., Podkowa M., Attisano L., Patterson C. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J. Cell Biol. 2009;184:597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larrain J., Brown C., De Robertis E.M. Integrin-alpha3 mediates binding of chordin to the cell surface and promotes its endocytosis. EMBO Rep. 2003;4:813–818. doi: 10.1038/sj.embor.embor902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawala A., Sutcliffe C., Ashe H.L. Multistep molecular mechanism for bone morphogenetic protein extracellular transport in the Drosophila embryo. Proc. Natl. Acad. Sci. U.S.A. 2012;109:11222–11227. doi: 10.1073/pnas.1202781109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larrain J., Bachiller D., Lu B., Agius E., Piccolo S., De Robertis E.M. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000;127:821–830. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millet C., Lemaire P., Orsetti B., Guglielmi P., Francois V. The human chordin gene encodes several differentially expressed spliced variants with distinct BMP opposing activities. Mech. Dev. 2001;106:85–96. doi: 10.1016/S0925-4773(01)00423-3. [DOI] [PubMed] [Google Scholar]
- 20.Xie J., Fisher S. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development. 2005;132:383–391. doi: 10.1242/dev.01577. [DOI] [PubMed] [Google Scholar]
- 21.Yu K., Srinivasan S., Shimmi O., Biehs B., Rashka K.E., Kimelman D., O'Connor M.B., Bier E. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development. 2000;127:2143–2154. doi: 10.1242/dev.127.10.2143. [DOI] [PubMed] [Google Scholar]
- 22.Yu K., Kang K.H., Heine P., Pyati U., Srinivasan S., Biehs B., Kimelman D., Bier E. Cysteine repeat domains and adjacent sequences determine distinct bone morphogenetic protein modulatory activities of the Drosophila Sog protein. Genetics. 2004;166:1323–1336. doi: 10.1534/genetics.166.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J.L., Huang Y., Qiu L.Y., Nickel J., Sebald W. von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J. Biol. Chem. 2007;282:20002–20014. doi: 10.1074/jbc.M700456200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J.L., Qiu L.Y., Kotzsch A., Weidauer S., Patterson L., Hammerschmidt M., Sebald W., Mueller T.D. Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev. Cell. 2008;14:739–750. doi: 10.1016/j.devcel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 25.O'Leary J.M., Hamilton J.M., Deane C.M., Valeyev N.V., Sandell L.J., Downing A.K. Solution structure and dynamics of a prototypical chordin-like cysteine-rich repeat (von Willebrand Factor type C module) from collagen IIA. J. Biol. Chem. 2004;279:53857–53866. doi: 10.1074/jbc.M409225200. [DOI] [PubMed] [Google Scholar]
- 26.Fujisawa T., Huang Y., Sebald W., Zhang J.L. The binding of von Willebrand factor type C domains of Chordin family proteins to BMP-2 and Tsg is mediated by their SD1 subdomain. Biochem. Biophys. Res. Commun. 2009;385:215–219. doi: 10.1016/j.bbrc.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Zakin L., Chang E.Y., Plouhinec J.L., De Robertis E.M. Crossveinless-2 is required for the relocalization of Chordin protein within the vertebral field in mouse embryos. Dev. Biol. 2010;347:204–215. doi: 10.1016/j.ydbio.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J.L., Patterson L.J., Qiu L.Y., Graziussi D., Sebald W., Hammerschmidt M. Binding between crossveinless-2 and chordin von Willebrand factor type C domains promotes BMP signaling by blocking chordin activity. PLoS One. 2010;5:e12846. doi: 10.1371/journal.pone.0012846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambrosio A.L., Taelman V.F., Lee H.X., Metzinger C.A., Coffinier C., De Robertis E.M. Crossveinless-2 is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev. Cell. 2008;15:248–260. doi: 10.1016/j.devcel.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winstanley J., Sawala A., Baldock C., Ashe H.L. Synthetic enzyme-substrate tethering obviates the Tolloid-ECM interaction during Drosophila BMP gradient formation. eLife. 2015;4 doi: 10.7554/eLife.05508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuck P., Rossmanith P. Determination of the sedimentation coefficient distribution by least-squares boundary modeling. Biopolymers. 2000;54:328–341. doi: 10.1002/1097-0282(20001015)54:5<328::AID-BIP40>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Lee H.X., Ambrosio A.L., Reversade B., De Robertis E.M. Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]