Abstract
The protein kinase C (PKC) family of serine/threonine protein kinases share structural homology, while exhibiting substantial functional diversity. PKC isoforms are ubiquitously expressed in tissues which makes it difficult to define roles for individual isoforms, with complexity compounded by the finding that PKC isoforms can co-operate with or antagonize other PKC family members. A number of studies suggest the involvement of PKC family members in regulating leukaemic cell survival and proliferation. Chronic lymphocytic leukaemia (CLL), the most common leukaemia in the Western world, exhibits dysregulated expression of PKC isoforms, with recent reports indicating that PKCβ and δ play a critical role in B-cell development, due to their ability to link the B-cell receptor (BCR) with downstream signalling pathways. Given the prognostic significance of the BCR in CLL, inhibition of these BCR/PKC-mediated signalling pathways is of therapeutic relevance. The present review discusses the emerging role of PKC isoforms in the pathophysiology of CLL and assesses approaches that have been undertaken to modulate PKC activity.
Keywords: B-cell receptor (BCR) signalling, chronic lymphocytic leukaemia (CLL), leukaemia, protein kinase C (PKC)
Introduction
The protein kinase C (PKC) family of serine/threonine protein kinases regulate numerous cellular processes including survival, proliferation, differentiation, apoptosis and migration. Members of this family are composed of N-terminal regulatory and C-terminal catalytic domains that are structurally closely related, but can be further divided into three sub-classes based on their structural properties and co-factor requirements: conventional, novel and atypical PKCs (aPKCs). To date, ten distinct PKC isoforms have been identified, encoded by nine genes [1,2]. Conventional PKCs [cPKCs; α, β (alternatively spliced into βI and βII) and γ] contain diacylglycerol (DAG)-binding domains (C1A and C1B) and a calcium (Ca2+) binding domain (C2) in the regulatory region and require DAG and Ca2+ for activation. Novel PKCs (nPKCs; δ, ε, θ and η) require DAG and phosphatidylserine for activation, but contain a variant of the C2 domain, which is unable to bind Ca2+, rendering nPKCs Ca2+ independent. aPKCs (ζ and ι (λ in rodents) lack functional binding sites for DAG and Ca2+ [3].
Maturation and activation of PKCs require phosphorylation events at AGC kinase conserved sites [2]. In the case of cPKCs, the nascent protein associates with the plasma membrane where it is phosphorylated at the activation loop by phosphoinositide-dependent kinase-1 (PDK1). This event leads to further phosphorylation on the turn and hydrophobic motifs of the PKC, which serve to stabilize the protein, resulting in the release of the mature, primed but inactive, PKC into the cytoplasm [1]. Agonist-induced signals that result in hydrolysis of phosphatidylinositol-4,5-bisphosphate lead to the generation of the second messengers Ca2+ and DAG, which enable PKC translocation to the membrane. Engagement of PKC with the membrane leads to a conformational change and full activation of PKC, enabling the activation of PKC-mediated downstream signalling events. The affinity of PKC for the plasma membrane is dependent on the levels of available co-factors, and the maintenance of phosphorylation. Indeed, PKCs are sensitive to dephosphorylation by protein phosphatases such as PHLPP and PP2A, which has an impact on their stability [4]. In addition to translocating to the plasma membrane, PKCs can also translocate to discrete subcellular locations, binding to distinct receptors for activated C-kinase (RACK) proteins, such as the keratin cytoskeleton, tight junctions, Golgi apparatus and the endoplasmic reticulum [5]. Therefore selected translocation of the activated PKCs to specific cellular compartments can assist in the determination of cell fate decisions.
PKC isoforms are ubiquitously expressed in tissues, with studies demonstrating that PKCα, βI & II, δ, ε and ζ are expressed in all major tissues, whereas PKCγ expression is restricted to the central nervous system and spinal cord, PKCη in the skin, lung, spleen and brain, PKCθ in the skeletal muscle, lung, spleen, skin and brain [6–8]. These expression profiles make it difficult to define roles for individual isoforms, with the complexity compounded by the finding that PKC isoforms can co-operate with or antagonize other PKC family members [9,10]. Although genetic targeting of selective PKC isoforms has highlighted a degree of redundancy in the role of PKCs in normal cellular function, this approach has demonstrated the unique role played by individual PKC isoforms in specific cell functions. For example, PKCα has recently been shown to play a critical role in the interleukin (IL)-17A transcription in the Th17 helper T-cell subset, through the regulation of transforming growth factor β receptor 1 (TGFβR1)-mediated phosphorylation of SMAD2-3 [11], whereas the PKCβ-knockout mouse model revealed an immunodeficient phenotype with a pronounced block in B-cell development, reduced numbers of B1 cells and impaired antibody responses [12].
These studies highlight the complexity of PKC isoform regulation at the level of the expression profile within tissues/cells, differential regulation by co-factors and kinases and the distinct expression pattern of RACKs that has an impact on subcellular targeting. Each of these events is highly regulated to generate a signalling response that is unique to the cell/tissue of interest, leading to a finely tuned alteration in cell fate/functional response. In light of this tight regulation, it is perhaps unsurprising that dysregulation of PKC isoforms has been implicated in a plethora of cancers. Alterations in PKC isoenzyme expression have been implicated in transformation events in a number of cell types [13]. Interestingly, the PKCι-knockout mouse is the only model to generate an embryonic lethal phenotype [14]. In the present paper, we review the emerging important role of PKC isoforms in the pathophysiology of chronic lymphocytic leukaemia (CLL).
Chronic lymphocytic leukaemia
CLL is the most common leukaemia in the Western world, accounting for 1% of new cancer cases in the U.K. and ~35% of all leukaemia types in adults [15]. CLL is characterized by the accumulation of long-lived mature monoclonal B-cells with the distinctive phenotype: CD19hi, CD5+, CD23+, IgMlo, FCM7− [15]. Although CLL was originally considered to be a malignancy of defective apoptosis following the discovery that Bcl-2 family of anti-apoptotic proteins were deregulated, it is now established to be a dynamic disorder, with rates of both proliferation and apoptosis of up to 2% of the clone size per day [16]. CLL cell division occurs within ‘proliferation centres’ in the lymph nodes through interaction with the stromal niche antigen and co-stimulation by activated CD4+ T lymphocytes expressing CD40 ligand (CD40L), and IL-4 attracted by Ki67+ CLL cells [16,17]. Two major prognostic subtypes of CLL are defined by mutational status of variable region within the immunoglobulin heavy chain gene (IgVH) of the B-cell receptor (BCR); mutated (M) IgVH genes are associated with favourable outcomes, whereas cases harbouring unmutated (UM) IgVH genes, which can also express ζ-chain (T-cell receptor)-associated protein kinase of 70 kDa (ZAP-70) and CD38, display more aggressive disease and more frequently require therapeutic intervention [18,19]. UM-CLL cells generally retain the ability to transmit signals through their BCR in comparison with M-CLL cells [20], with sustained BCR cross-linking resulting in a phosphoinositide 3-kinase (PI3K)/Akt-mediated up-regulation of Mcl1, which has been shown to increase CLL cell chemoresistance [21–23]. These studies highlight the central role played by BCR-mediated signalling in the pathogenesis of CLL [24]. Therefore a deeper understanding of the signalling pathways that regulate CLL survival and proliferation will highlight key therapeutic targets for inhibition.
PKC isoforms in CLL
The deregulation of PKC activity/expression in CLL cells was recognized in an early study demonstrating that total PKC activity in CLL was lower than that observed in acute myeloid leukaemia (AML) and acute lymphoblastic leukaemia (ALL) in the particulate and cytosolic fractions; however, a high level of PKCβ expression was associated with CLL in comparison with PKCα and PKCγ [25]. These findings were supported and extended by a study comparing PKC profiles in CLL cells [26]. In comparison with normal mature peripheral-blood-derived B-cells, PKCα and PKCβI expression was down-regulated in CLL cells, whereas PKCβII and PKCε expression was up-regulated. Indeed, PKCβII overexpression in CLL cells was indicated to play a role in CLL pathogenesis, as PKCβII protein levels increase with disease stage and tumour burden [26].
Initial studies assessing the role of PKC in CLL cells utilized reagents such as the macrocyclic lactone bryostatin and the phorbol ester PMA, which globally modulate PKC activities and signalling pathways. In this way, elevated PKC activity was shown to play a role in CLL cell differentiation [27,28], enhanced CLL immunomodulation [29] and increased cell survival and chemoresistance [30,31]. The role of PKC in these processes was inferred by the use of PKC selective inhibitors; however, these studies did not establish the function of individual PKC isoforms. As the complexity of the PKC family has become apparent, subsequent studies have utilized complementary approaches to confirm the role of specific PKC isoforms in CLL cell function.
PKCα
PKCα expression is down-regulated in CLL cells compared with normal mature B-cells [26]. Interestingly, we had previously shown that inhibiting PKCα activity in B-cell lineage progenitors resulted in the initiation of a CLL-like disease. This was achieved by stably expressing a plasmid-encoding kinase dead PKCα (PKCα-KR) in a haemopoietic stem and progenitor cell-enriched population from wild type mice and culturing these cells in B-cell generation systems in vitro and in vivo. The resultant cells exhibited hallmark characteristics of human CLL cells at the level of: (i) phenotype, e.g. CD19hi CD5+ CD23+ IgMlo; (ii) cell cycle phase (halted in G0/G1 ex vivo); and (iii) resistance to apoptosis [32]. CLL cells generated in vivo accumulated in the lymphoreticular system similar to that noted in CLL patients, demonstrating the applicability of this murine CLL model to the human disease. Our recent work indicates that the PKCα-KR-CLL model exhibits key characteristics of poor prognostic CLL, with cells expressing ZAP-70 and possessing enhanced proliferative capacity both in vivo and in vitro. Moreover, PKCβII expression is up-regulated during disease progression, suggesting that a functional down-regulation of PKCα signals may generate permissive conditions for the initiation of CLL [33].
PKCβ
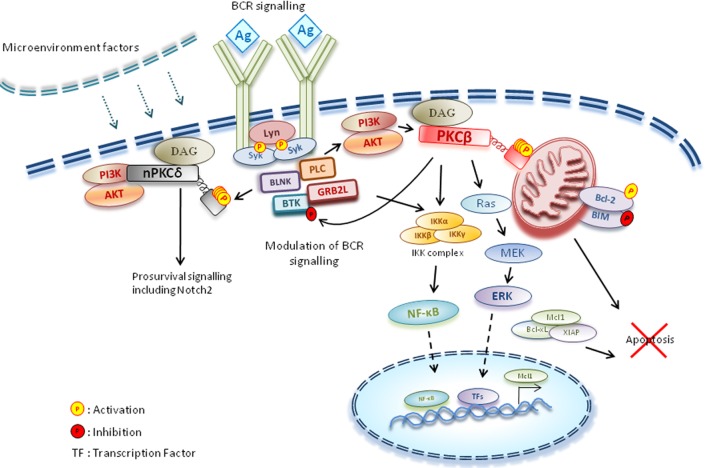
The BCR mediates key events during the life cycle of the B lymphocyte to regulate B-cell development and immune cell function. By coupling to the appropriate intracellular signalling molecules, BCR ligation triggers proliferative, survival, differentiation, anergic and/or apoptotic responses. PKCβ has previously been demonstrated to play a critical role in B-cell development, due to its ability to link the BCR with downstream signalling pathways [12]. Indeed, PKCβ has been demonstrated to regulate the nuclear factor κB (NF-κB) signalling pathway downstream of BCR activation [34], in addition to deactivating the non-receptor tyrosine kinase Bruton tyrosine kinase (Btk), by phosphorylating Ser180 and disrupting plasma membrane targeting [35] (Figure 1). Given the prognostic significance of the BCR in CLL [24], it is perhaps unsurprising that PKCβ overexpression, specifically PKCβII, is linked to poorer prognostic outcome [26]. PKCβ is also aberrantly up-regulated in other B-lymphocyte malignancies including diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma [36,37]. Of note, CLL development in the Tcl-1 transgenic CLL mouse model was abrogated in the absence of PKCβ, supporting a critical role for this PKC isoform in CLL pathogenesis [38].
Figure 1. Key events mediated by the BCR.
BCR signalling regulates B-cell development and function. Coupling of the BCR with PKCβ results in up-regulated MAPK and NF-κB signalling, leading to apoptotic resistance. In addition, PKCβ has been shown to deactivate the non-receptor tyrosine kinase Btk, by phosphorylating Ser180 and disrupting plasma membrane targeting. The PI3K-dependent PKCδ is activated by Syk, a tyrosine kinase proximal to the BCR. PKCδ activation is associated with elevated prosurvival signalling such as Notch2 and stabilization of anti-apoptotic factors such as Mcl-1 and XIAP. Ag, antigen; BLNK, B-cell linker; ERK, extracellular-signal-regulated kinase; GRB2L, Grb2 (growth-factor-receptor-bound protein 2)-like; IKK, inhibitor of NF-κB kinase; MEK, MAPK/ERK kinase; PLC, phospholipase C.
Assessment of expression levels demonstrated that PKCβII is the dominant PKC isoform expressed in CLL cells, and PKCβII is routinely present in the active form compared with normal B-cells, with membrane-association correlating with its activity. Supporting the role of PKCβ in the negative regulation of Btk, the levels of membrane-associated PKCβII are inversely related to membrane-associated Btk. Moreover, calcium responses after BCR ligation were lower in CLL cells that expressed higher levels of PKCβII [26]. Collectively, these findings demonstrate that PKCβII level/activity plays a central role in modulating signals proximal to the BCR in CLL cells.
PKC- and PI3K-mediated signalling pathways have been established to govern CLL cell survival [21,39]. PKCβ activity can promote phosphorylation/activation of Akt, thus promoting CLL cell survival [40]. In addition, PKCβ regulates Akt by modulating the expression of protein tyrosine phosphatase non-receptor type 22 (PTPN22), a phosphatase that is responsible for positively regulating Akt activity, which is overexpressed in CLL cells [41]. The PKCβ selective inhibitor ruboxistaurin down-regulated PTPN22 and enhanced apoptosis downstream of BCR ligation. Additional mechanisms supporting the pro-survival role of PKCβII are demonstrated in a study showing that PKCβII shuttles to the mitochondrial membrane where it phosphorylates Bcl-2, enabling it to sequester the pro-apoptotic protein BimEL, as well as phosphorylating BimEL and targeting it for proteosomal degradation. These actions skew the CLL cell fate towards apoptotic resistance [42].
Despite expressing high levels of Bcl-2, primary CLL cells require the presence of stromal cells during in vitro culture for long-term survival, suggesting that together with their autonomous apoptotic defect, they rely on paracrine signals from the tumour microenvironment [43,44]. PKCβII can also be activated downstream of growth factors such as vascular endothelial growth factor (VEGF) [45], indicating that the activation status of PKCβII and thus its pro-survival function is linked to the lymphoid organ microenvironment of CLL patients. Recently, it was demonstrated that incubation of CLL leukaemic cells with bone marrow stromal cells led to an up-regulation in PKCβII expression and subsequent activation of NF-κB signalling in the stromal cells, which was essential for CLL cell survival [44]. This study emphasizes the complex interplay that occurs between leukaemic cells and their stromal niche and highlights the potential for therapeutic intervention to disrupt both the malignancy and the tumour-associated microenvironment.
PKCδ
PKCδ is constitutively active in freshly isolated CLL cells. PKCδ activation, as indicated by Thr505 phosphorylation, is mediated through a PI3K-dependent pathway [46]. Inhibition of PKCδ resulted in the induction of caspase-dependent apoptosis, and reduction in expression of the anti-apoptotic factors Mcl-1 and X-linked inhibitor of apoptosis (XIAP) [47], suggesting that in addition to PKCβII, PKCδ may play a key role in regulating CLL cell survival. The phosphorylation status of PKCδ has also been linked to spleen tyrosine kinase (Syk), a tyrosine kinase that is proximal to the BCR. Syk activation results in PKCδ-mediated stabilization of Mcl-1, with inhibition of Syk leading to a decrease in PKCδ phosphorylation and an elevation in apoptosis [48] (Figure 1). These studies indicate that PKCδ is associated with CLL cell survival and regulation of Mcl-1 expression, a key antiapoptotic factor associated with chemoresistance [22]. PKCδ is responsible for activation of Notch2 signalling in CLL cells [49]. Increased Notch2 signalling is associated with elevated cell survival in CLL cells [50]. However, the activated Notch2 signal is significantly down-regulated in CLL cells after in vitro culture for 24 h, suggesting that the PKCδ/Notch2 signalling pathway is activated in response to microenvironmental stimuli [49].
Targeting PKCs: current and future directions
As the PKC family has long been considered to play a major role in the pathogenesis of a number of diseases, there has been a focus to develop compounds that selectively modulate the activity of individual isoforms. Therapeutic targeting of individual PKC isoforms represents a significant challenge because PKC isoforms share 70% homology at the ATP binding site, making it extremely difficult to develop isoform-specific drugs [51]. In spite of the initial promise of PKC modulators as a treatment for human diseases, the outcome at clinical trial has been varied and for the most part negative [51]. Most small-molecule inhibitors exhibit undesired off/on target effects making it difficult to translate adequate drug concentrations from animal studies to human clinical trials. Moreover, a lack of pharmacological biomarkers to predict drug efficacy affects the cost and success of clinical trials [51]. Indeed rotterlin, marketed as a PKCδ and Ca2+/calmodulin-dependent protein kinase II (CaMKIII) inhibitor, was later shown to also inhibit protein kinases such as checkpoint kinase 2 (CHK2), Polo-like kinase 1 (PLK1) and mitogen-activated protein kinase (MAPK)-activated protein kinase 2 (MAPKAPK2). The cellular effects observed with rotterlin treatment did not correlate with results generated using kinase dead PKCδ, siRNA knockdown of PKCδ or other more selective PKC inhibitors [52].
Early studies showing that bryostatin increased susceptibility of CLL cells to induce apoptosis and/or differentiation [27,28] prompted the clinical assessment of this PKC modulator either alone or in combination with fludarabine or vincristine [53,54]. Patients tolerated bryostatin well, and the in vitro findings were supported, with CLL cells undergoing differentiation and a reduction in PKC activity during treatment. Bryostatin was also studied in combination with the anti-CD20 monoclonal antibody rituximab in a Phase II clinical trial (NCT00087425), but the study did not produce data and is now closed. Increased clinical activity of rituximab with conventional chemotherapeutic agents likely made bryostatin-based therapies difficult to justify in CLL.
The first PKC inhibitors used in clinical trial were the staurosporine-derivatives midostaurin (PKC412) and UCN-01 (7-hydroxysporine). Midostaurin was found to be selectively toxic towards the CLL cells compared with peripheral blood lymphocytes in vitro [55]. In a Phase II clinical trial, PKC412 was well tolerated by patients, and a reduction in circulating tumour load was noted in more than half of the patients enrolled in the trial and the majority showed a decrease in PKC activity [56]. In addition, PKC412 enhanced cell killing induced by chlorambucil in vitro [55]. UCN-01 induced apoptosis in CLL cells both in vivo and in vitro alone or in combination with fludarabine [57,58]. These studies suggest that targeting PKC may provide a valid therapeutic strategy in CLL and other haematological malignancies.
In CLL, the BCR holds significant prognostic importance, therefore inhibiting the signalling pathways that emanate downstream of BCR ligation are of therapeutic relevance. Indeed, a variety of emerging novel agents are being developed that target BCR signalling components or downstream effectors [59]. Given the evidence supporting a central role for PKCβII in regulating CLL cell survival downstream of the BCR and within tumour microenvironment, this PKC isoform represents a very attractive therapeutic target. Enzastaurin (LY317615), a small-molecule inhibitor targeted towards PI3K/PKCβ pathways, has been demonstrated to induce apoptosis in CLL cells in vitro [38,42]; however, there is limited published data available from clinical trials in CLL patients. Enzastaurin showed promise in Phase II clinical trials as monotherapy in DLBCL patients [60]; however, at Phase III in the PRELUDE trial, no clinical significance was observed. It will be interesting to determine whether enzastaurin may be better used as part of a combination therapy, as it has previously been shown to enhance the apoptosis in CLL cells in vitro upon combination with fludarabine, vincristine, doxorubicin and bendamustine [42,61]. The use of the PKCβ-selective inhibitor ruboxistaurin (LY333531) in a clinical trial for treatment of leukaemia/lymphoma has not been reported. However, although this inhibitor induced significant apoptosis of CLL cells in response to BCR ligation, survival was unaffected by drug treatment in unstimulated CLL cells [40,41]. These findings indicate that although isoform-selective inhibitors may inhibit certain aspects of disease progression, they will not be curative, suggesting that less-selective PKC inhibitors may hold the key to a promising therapy.
Marked apoptosis has been noted in CLL cells treated with RO32-0432, a pan PKC inhibitor [26]. Sotrastaurin (AEB071), an inhibitor of conventional and novel PKC isotypes, is currently being developed as an immunosuppressive agent for organ transplantation. However, sotrastaurin has been shown to have anti-tumour effects in DLBCL resulting in cell cycle arrest and cell death both in vitro and in vivo [62] and is currently being used in a Phase I clinical trial for DLBCL and melanoma. Encouragingly, sotrastaurin has recently been shown to display selective cytotoxicity towards CLL cells and can inhibit their proliferation by altering the BCR-dependant survival pathways (MAPK, PI3K and NF-κB). Sotrastaurin not only reduced the levels of survival stimuli within the CLL cells, but also disrupted signals from the microenvironment that contribute to CLL cell survival [63]. Although this latter effect is likely to occur through targeting PKCβII activity, the potential success of pan PKC inhibitors will be due to the inhibition of additional PKC isoforms including PKCδ and PKCε.
As our knowledge of PKC biology deepens, it is evident that specific isoenzyme inhibitors may be insufficient for a drug to have optimal efficacy and minimal off target effects [51]. Indeed, it may be possible to develop drugs that inhibit the interaction of PKC with specific binding partners. This approach requires a detailed understanding of the molecular events that regulate PKC activation. Although the development of separation of function inhibiting peptides that target substrates downstream of a single PKC isoenzyme is at an early stage, peptides have been developed that have biological effect in disease states, holding promise for the future [51]. Finally, early studies suggest that a future approach towards PKC mediated therapies may be to restore PKC activity rather than inhibiting it. One study established that correction of a loss of function point mutation within the regulatory domain of PKCβ in a colon cancer cell line resulted in reduced tumour growth in vitro and in vivo [64]. Therefore it is important to establish whether modulation of the upstream or downstream targets of the PKC, either inhibition or restoration of activity, would be sufficient to change the course of the malignancy. To achieve this, additional translational research is required: the importance of PKC in cell fate decisions and the limitations of current therapies justify the continued quest for PKC-directed treatments.
Abbreviations
- aPKC
atypical PKC
- BCR
B-cell receptor
- Btk
Bruton tyrosine kinase
- CLL
chronic lymphocytic leukaemia
- cPKC
conventional PKC
- DAG
diacylglycerol
- DLBCL
diffuse large B-cell lymphoma
- IL
interleukin
- M
mutated
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor κB
- nPKC
novel PKC
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- PTPN22
protein tyrosine phosphatase non-receptor type 22
- RACK
receptor for activated C-kinase
- Syk
spleen tyrosine kinase
- UM
unmutated
- XIAP
X-linked inhibitor of apoptosis
- ZAP-70
ζ-chain (T-cell receptor)-associated protein kinase of 70 kDa
Footnotes
Protein Kinase C Signalling in Health and Disease: A Biochemical Society Focused Meeting held at Science Gallery, Trinity College Dublin, Ireland, 24–25 June 2014. Organized and Edited by Aideen Long (Trinity College Dublin, Ireland) and Maria O’Connell (University of East Anglia, U.K.).
Funding
A.T. is funded by a Leukaemia & Lymphoma Research project grant [grant number 13012].
References
- 1.Newton A.C. Protein kinase C: structure, function, and regulation. J. Biol. Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 2.Parekh D.B., Ziegler W., Parker P.J. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson J.E., Giorgione J., Newton A.C. The C1 and C2 domains of protein kinase C are independent membrane targeting modules, with specificity for phosphatidylserine conferred by the C1 domain. Biochemistry. 2000;39:11360–11369. doi: 10.1021/bi000902c. [DOI] [PubMed] [Google Scholar]
- 4.Gao T., Brognard J., Newton A.C. The phosphatase PHLPP controls the cellular levels of protein kinase C. J. Biol. Chem. 2008;283:6300–6311. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- 5.Kheifets V., Mochly-Rosen D. Insight into intra- and inter-molecular interactions of PKC: design of specific modulators of kinase function. Pharmacol. Res. 2007;55:467–476. doi: 10.1016/j.phrs.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacher N., Zisman Y., Berent E., Livneh E. Isolation and characterization of PKC-L, a new member of the protein kinase C-related gene family specifically expressed in lung, skin, and heart. Mol. Cell. Biol. 1991;11:126–133. doi: 10.1128/mcb.11.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osada S., Mizuno K., Saido T.C., Suzuki K., Kuroki T., Ohno S. A new member of the protein kinase C family, nPKCθ, predominantly expressed in skeletal muscle. Mol. Cell. Biol. 1992;12:3930–3938. doi: 10.1128/mcb.12.9.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W.S., Heckman C.A. The sevenfold way of PKC regulation. Cell. Signal. 1998;10:529–542. doi: 10.1016/S0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 9.Lutz-Nicoladoni C., Thuille N., Wachowicz K., Gruber T., Leitges M., Baier G. PKCα and PKCβ cooperate functionally in CD3-induced de novo IL-2 mRNA transcription. Immunol. Lett. 2013;151:31–38. doi: 10.1016/j.imlet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thuille N., Wachowicz K., Hermann-Kleiter N., Kaminski S., Fresser F., Lutz-Nicoladoni C., Leitges M., Thome M., Massoumi R., Baier G. PKCθ/β and CYLD are antagonistic partners in the NFκB and NFAT transactivation pathways in primary mouse CD3+ T lymphocytes. PLoS ONE. 2013;8:e53709. doi: 10.1371/journal.pone.0053709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meisel M., Hermann-Kleiter N., Hinterleitner R., Gruber T., Wachowicz K., Pfeifhofer-Obermair C., Fresser F., Leitges M., Soldani C., Viola A., et al. The kinase PKCα selectively upregulates interleukin-17A during Th17 cell immune responses. Immunity. 2013;38:41–52. doi: 10.1016/j.immuni.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitges M., Schmedt C., Guinamard R., Davoust J., Schaal S., Stabel S., Tarakhovsky A. Immunodeficiency in protein kinase Cβ-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- 13.Michie A.M., Nakagawa R. The link between PKCα regulation and cellular transformation. Immunol. Lett. 2005;96:155–162. doi: 10.1016/j.imlet.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Farese R.V., Sajan M.P., Yang H., Li P., Mastorides S., Gower W.R., Jr, Nimal S., Choi C.S., Kim S., Shulman G.I., et al. Muscle-specific knockout of PKC-λ impairs glucose transport and induces metabolic and diabetic syndromes. J. Clin. Invest. 2007;117:2289–2301. doi: 10.1172/JCI31408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dighiero G. CLL biology and prognosis. Hematology Am. Soc. Hematol. Educ. Program. 2005;2005:278–284. doi: 10.1182/asheducation-2005.1.278. [DOI] [PubMed] [Google Scholar]
- 16.Messmer B.T., Messmer D., Allen S.L., Kolitz J.E., Kudalkar P., Cesar D., Murphy E.J., Koduru P., Ferrarini M., Zupo S., et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J. Clin. Invest. 2005;115:755–764. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghia P., Chiorazzi N., Stamatopoulos K. Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J. Intern. Med. 2008;264:549–562. doi: 10.1111/j.1365-2796.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- 18.Hamblin T.J., Davis Z., Gardiner A., Oscier D.G., Stevenson F.K. Unmutated Ig VH genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 19.Morilla A., Gonzalez de Castro D., Del Giudice I., Osuji N., Else M., Morilla R., Brito Babapulle V., Rudenko H., Matutes E., Dearden C., et al. Combinations of ZAP-70, CD38 and IGHV mutational status as predictors of time to first treatment in CLL. Leuk. Lymphoma. 2008;49:2108–2115. doi: 10.1080/10428190802360810. [DOI] [PubMed] [Google Scholar]
- 20.Lanham S., Hamblin T., Oscier D., Ibbotson R., Stevenson F., Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. 2003;101:1087–1093. doi: 10.1182/blood-2002-06-1822. [DOI] [PubMed] [Google Scholar]
- 21.Petlickovski A., Laurenti L., Li X., Marietti S., Chiusolo P., Sica S., Leone G., Efremov D.G. Sustained signaling through the B-cell receptor induces mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105:4820–4827. doi: 10.1182/blood-2004-07-2669. [DOI] [PubMed] [Google Scholar]
- 22.Pepper C., Lin T.T., Pratt G., Hewamana S., Brennan P., Hiller L., Hills R., Ward R., Starczynski J., Austen B., et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008;112:3807–3817. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- 23.McCaig A.M., Cosimo E., Leach M.T., Michie A.M. Dasatinib inhibits B cell receptor signalling in chronic lymphocytic leukaemia but novel combination approaches are required to overcome additional pro-survival microenvironmental signals. Br. J. Haematol. 2011;153:199–211. doi: 10.1111/j.1365-2141.2010.08507.x. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson F.K., Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103:4389–4395. doi: 10.1182/blood-2003-12-4312. [DOI] [PubMed] [Google Scholar]
- 25.Komada F., Nishikawa M., Uemura Y., Morita K., Hidaka H., Shirakawa S. Expression of three major protein kinase C isozymes in various types of human leukemic cells. Cancer Res. 1991;51:4271–4278. [PubMed] [Google Scholar]
- 26.Abrams S.T., Lakum T., Lin K., Jones G.M., Treweeke A.T., Farahani M., Hughes M., Zuzel M., Slupsky J.R. B-cell receptor signaling in chronic lymphocytic leukemia cells is regulated by overexpressed active protein kinase CβII. Blood. 2007;109:1193–1201. doi: 10.1182/blood-2006-03-012021. [DOI] [PubMed] [Google Scholar]
- 27.al-Katib A., Mohammad R.M., Dan M., Hussein M.E., Akhtar A., Pettit G.R., Sensenbrenner L.L. Bryostatin 1-induced hairy cell features on chronic lymphocytic leukemia cells in vitro. Exp. Hematol. 1993;21:61–65. [PubMed] [Google Scholar]
- 28.Drexler H.G., Gignac S.M., Jones R.A., Scott C.S., Pettit G.R., Hoffbrand A.V. Bryostatin 1 induces differentiation of B-chronic lymphocytic leukemia cells. Blood. 1989;74:1747–1757. [PubMed] [Google Scholar]
- 29.Hammond C., Shi Y., Mena J., Tomic J., Cervi D., He L., Millar A.E., Debenedette M., Schuh A.C., Baryza J.L., et al. Effect of serum and antioxidants on the immunogenicity of protein kinase C-activated chronic lymphocytic leukemia cells. J. Immunother. 2005;28:28–39. doi: 10.1097/00002371-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Thomas A., Pepper C., Hoy T., Bentley P. Bryostatin induces protein kinase C modulation, mcl-1 up-regulation and phosphorylation of bcl-2 resulting in cellular differentiation and resistance to drug-induced apoptosis in B-cell chronic lymphocytic leukemia cells. Leuk. Lymphoma. 2004;45:997–1008. doi: 10.1080/10428190310001639470. [DOI] [PubMed] [Google Scholar]
- 31.Barragan M., Bellosillo B., Campas C., Colomer D., Pons G., Gil J. Involvement of protein kinase C and phosphatidylinositol 3-kinase pathways in the survival of B-cell chronic lymphocytic leukemia cells. Blood. 2002;99:2969–2976. doi: 10.1182/blood.V99.8.2969. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa R., Soh J.W., Michie A.M. Subversion of protein kinase Cα signaling in hematopoietic progenitor cells results in the generation of a B-cell chronic lymphocytic leukemia-like population in vivo. Cancer Res. 2006;66:527–534. doi: 10.1158/0008-5472.CAN-05-0841. [DOI] [PubMed] [Google Scholar]
- 33.Tarafdar A., Vukovic M., Nakagawa R., Cosimo E., Dunn K., Michie A. Subversion of PKCα signalling in B cell progenitors results in an upregulation of PKCβII expression and leukaemogenesis. 2014. http://www.biochemistry.org/Portals/0/Conferences/Abstracts/SA165/SA165P008.pdf (Abstract)
- 34.Su T.T., Guo B., Kawakami Y., Sommer K., Chae K., Humphries L.A., Kato R.M., Kang S., Patrone L., Wall R., et al. PKC-β controls IκB kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 2002;3:780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- 35.Kang S.W., Wahl M.I., Chu J., Kitaura J., Kawakami Y., Kato R.M., Tabuchi R., Tarakhovsky A., Kawakami T., Turck C.W., et al. PKCβ modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 2001;20:5692–5702. doi: 10.1093/emboj/20.20.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hans C.P., Weisenburger D.D., Greiner T.C., Chan W.C., Aoun P., Cochran G.T., Pan Z., Smith L.M., Lynch J.C., Bociek R.G., et al. Expression of PKC-β or cyclin D2 predicts for inferior survival in diffuse large B-cell lymphoma. Mod. Pathol. 2005;18:1377–1384. doi: 10.1038/modpathol.3800434. [DOI] [PubMed] [Google Scholar]
- 37.Decouvelaere A.V., Morschhauser F., Buob D., Copin M.C., Dumontet C. Heterogeneity of protein kinase Cβ2 expression in lymphoid malignancies. Histopathology. 2007;50:561–566. doi: 10.1111/j.1365-2559.2007.02666.x. [DOI] [PubMed] [Google Scholar]
- 38.Holler C., Pinon J.D., Denk U., Heyder C., Hofbauer S., Greil R., Egle A. PKCβ is essential for the development of chronic lymphocytic leukemia in the TCL1 transgenic mouse model: validation of PKCβ as a therapeutic target in chronic lymphocytic leukemia. Blood. 2009;113:2791–2794. doi: 10.1182/blood-2008-06-160713. [DOI] [PubMed] [Google Scholar]
- 39.Longo P.G., Laurenti L., Gobessi S., Sica S., Leone G., Efremov D.G. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–855. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 40.Barragan M., de Frias M., Iglesias-Serret D., Campas C., Castano E., Santidrian A.F., Coll-Mulet L., Cosialls A.M., Domingo A., Pons G., Gil J. Regulation of Akt/PKB by phosphatidylinositol 3-kinase-dependent and -independent pathways in B-cell chronic lymphocytic leukemia cells: role of protein kinase Cβ. J. Leukoc. Biol. 2006;80:1473–1479. doi: 10.1189/jlb.0106041. [DOI] [PubMed] [Google Scholar]
- 41.Negro R., Gobessi S., Longo P.G., He Y., Zhang Z.Y., Laurenti L., Efremov D.G. Overexpression of the autoimmunity-associated phosphatase PTPN22 promotes survival of antigen-stimulated CLL cells by selectively activating AKT. Blood. 2012;119:6278–6287. doi: 10.1182/blood-2012-01-403162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.zum Buschenfelde C.M., Wagner M., Lutzny G., Oelsner M., Feuerstacke Y., Decker T., Bogner C., Peschel C., Ringshausen I. Recruitment of PKC-βII to lipid rafts mediates apoptosis-resistance in chronic lymphocytic leukemia expressing ZAP-70. Leukemia. 2010;24:141–152. doi: 10.1038/leu.2009.216. [DOI] [PubMed] [Google Scholar]
- 43.Burger J.A., Tsukada N., Burger M., Zvaifler N.J., Dell’Aquila M., Kipps T.J. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. [PubMed] [Google Scholar]
- 44.Lutzny G., Kocher T., Schmidt-Supprian M., Rudelius M., Klein-Hitpass L., Finch A.J., Durig J., Wagner M., Haferlach C., Kohlmann A., et al. Protein kinase C-β-dependent activation of NF-κB in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer. Cell. 2013;23:77–92. doi: 10.1016/j.ccr.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrams S.T., Brown B.R., Zuzel M., Slupsky J.R. Vascular endothelial growth factor stimulates protein kinase CβII expression in chronic lymphocytic leukemia cells. Blood. 2010;115:4447–4454. doi: 10.1182/blood-2009-06-229872. [DOI] [PubMed] [Google Scholar]
- 46.Ringshausen I., Schneller F., Bogner C., Hipp S., Duyster J., Peschel C., Decker T. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cδ. Blood. 2002;100:3741–3748. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 47.Ringshausen I., Oelsner M., Weick K., Bogner C., Peschel C., Decker T. Mechanisms of apoptosis-induction by rottlerin: therapeutic implications for B-CLL. Leukemia. 2006;20:514–520. doi: 10.1038/sj.leu.2404113. [DOI] [PubMed] [Google Scholar]
- 48.Baudot A.D., Jeandel P.Y., Mouska X., Maurer U., Tartare-Deckert S., Raynaud S.D., Cassuto J.P., Ticchioni M., Deckert M. The tyrosine kinase Syk regulates the survival of chronic lymphocytic leukemia B cells through PKCδ and proteasome-dependent regulation of Mcl-1 expression. Oncogene. 2009;28:3261–3273. doi: 10.1038/onc.2009.179. [DOI] [PubMed] [Google Scholar]
- 49.Hubmann R., Duchler M., Schnabl S., Hilgarth M., Demirtas D., Mitteregger D., Holbl A., Vanura K., Le T., Look T., et al. NOTCH2 links protein kinase Cδ to the expression of CD23 in chronic lymphocytic leukaemia (CLL) cells. Br. J. Haematol. 2010;148:868–878. doi: 10.1111/j.1365-2141.2009.08024.x. [DOI] [PubMed] [Google Scholar]
- 50.Rosati E., Sabatini R., Rampino G., Tabilio A., Di Ianni M., Fettucciari K., Bartoli A., Coaccioli S., Screpanti I., Marconi P. Constitutively activated notch signaling is involved in survival and apoptosis resistance of B-CLL cells. Blood. 2009;113:856–865. doi: 10.1182/blood-2008-02-139725. [DOI] [PubMed] [Google Scholar]
- 51.Mochly-Rosen D., Das K., Grimes K.V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug Discov. 2012;11:937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu-Zhang A.X., Newton A.C. Protein kinase C pharmacology: refining the toolbox. Biochem. J. 2013;452:195–209. doi: 10.1042/BJ20130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varterasian M.L., Mohammad R.M., Shurafa M.S., Hulburd K., Pemberton P.A., Rodriguez D.H., Spadoni V., Eilender D.S., Murgo A., Wall N., et al. Phase II trial of bryostatin 1 in patients with relapsed low-grade non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Clin. Cancer Res. 2000;6:825–828. [PubMed] [Google Scholar]
- 54.Roberts J.D., Smith M.R., Feldman E.J., Cragg L., Millenson M.M., Roboz G.J., Honeycutt C., Thune R., Padavic-Shaller K., Carter W.H., et al. Phase I study of bryostatin 1 and fludarabine in patients with chronic lymphocytic leukemia and indolent (non-Hodgkin's) lymphoma. Clin. Cancer Res. 2006;12:5809–5816. doi: 10.1158/1078-0432.CCR-05-2730. [DOI] [PubMed] [Google Scholar]
- 55.Ganeshaguru K., Wickremasinghe R.G., Jones D.T., Gordon M., Hart S.M., Virchis A.E., Prentice H.G., Hoffbrand A.V., Man A., Champain K., et al. Actions of the selective protein kinase C inhibitor PKC412 on B-chronic lymphocytic leukemia cells in vitro. Haematologica. 2002;87:167–176. [PubMed] [Google Scholar]
- 56.Virchis A., Ganeshaguru K., Hart S., Jones D., Fletcher L., Wright F., Wickremasinghe R., Man A., Csermak K., Meyer T., et al. A novel treatment approach for low grade lymphoproliferative disorders using PKC412 (CGP41251), an inhibitor of protein kinase C. Hematol. J. 2002;3:131–136. doi: 10.1038/sj.thj.6200165. [DOI] [PubMed] [Google Scholar]
- 57.Byrd J.C., Shinn C., Willis C.R., Flinn I.W., Lehman T., Sausville E., Lucas D., Grever M.R. UCN-01 induces cytotoxicity toward human CLL cells through a p53-independent mechanism. Exp. Hematol. 2001;29:703–708. doi: 10.1016/S0301-472X(01)00649-X. [DOI] [PubMed] [Google Scholar]
- 58.Marti G.E., Stetler-Stevenson M., Grant N.D., White T., Figg W.D., Tohnya T., Jaffe E.S., Dunleavy K., Janik J.E., Steinberg S.M., Wilson W.H. Phase I trial of 7-hydroxystaurosporine and fludararbine phosphate: in vivo evidence of 7-hydroxystaurosporine induced apoptosis in chronic lymphocytic leukemia. Leuk. Lymphoma. 2011;52:2284–2292. doi: 10.3109/10428194.2011.589547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Byrd J.C., Jones J.J., Woyach J.A., Johnson A.J., Flynn J.M. Entering the era of targeted therapy for chronic lymphocytic leukemia: impact on the practicing clinician. J. Clin. Oncol. 2014;32:3039–3047. doi: 10.1200/JCO.2014.55.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robertson M.J., Kahl B.S., Vose J.M., de Vos S., Laughlin M., Flynn P.J., Rowland K., Cruz J.C., Goldberg S.L., Musib L., et al. Phase II study of enzastaurin, a protein kinase Cβ inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol. 2007;25:1741–1746. doi: 10.1200/JCO.2006.09.3146. [DOI] [PubMed] [Google Scholar]
- 61.Liffraud C., Quillet-Mary A., Fournie J.J., Laurent G., Ysebaert L. Protein phosphatase-2A activation is a critical step for enzastaurin activity in chronic lymphoid leukemia cells. Leuk. Lymphoma. 2012;53:966–972. doi: 10.3109/10428194.2011.634041. [DOI] [PubMed] [Google Scholar]
- 62.Naylor T.L., Tang H., Ratsch B.A., Enns A., Loo A., Chen L., Lenz P., Waters N.J., Schuler W., Dorken B., et al. Protein kinase C inhibitor sotrastaurin selectively inhibits the growth of CD79 mutant diffuse large B-cell lymphomas. Cancer Res. 2011;71:2643–2653. doi: 10.1158/0008-5472.CAN-10-2525. [DOI] [PubMed] [Google Scholar]
- 63.El-Gamal D., Williams K., LaFollette T.D., Cannon M., Blachly J.S., Zhong Y., Woyach J.A., Williams E., Awan F.T., Jones J., et al. PKC-β as a therapeutic target in CLL: PKC inhibitor AEB071 demonstrates preclinical activity in CLL. Blood. 2014;124:1481–1491. doi: 10.1182/blood-2014-05-574830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antal C., Kang E., Stephenson N.L., Trotter E.W., Zanca C., Gallegos L.L., Furnari F.B., Hunter T., Brognard J., Newton A.C. Reversing the paradigm: PKC as a tumour supressor. 2014. http://www.biochemistry.org/Portals/0/Conferences/Abstracts/SA165/SA165P007.pdf (Abstract)