Abstract
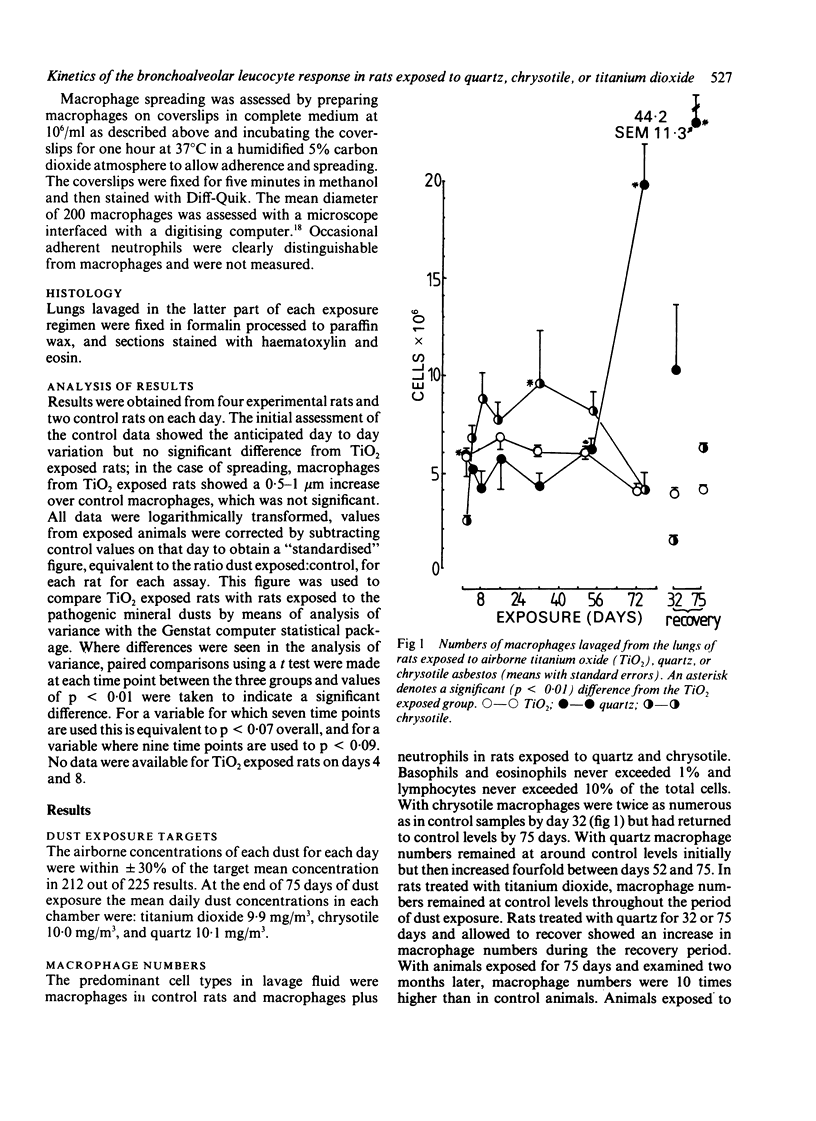
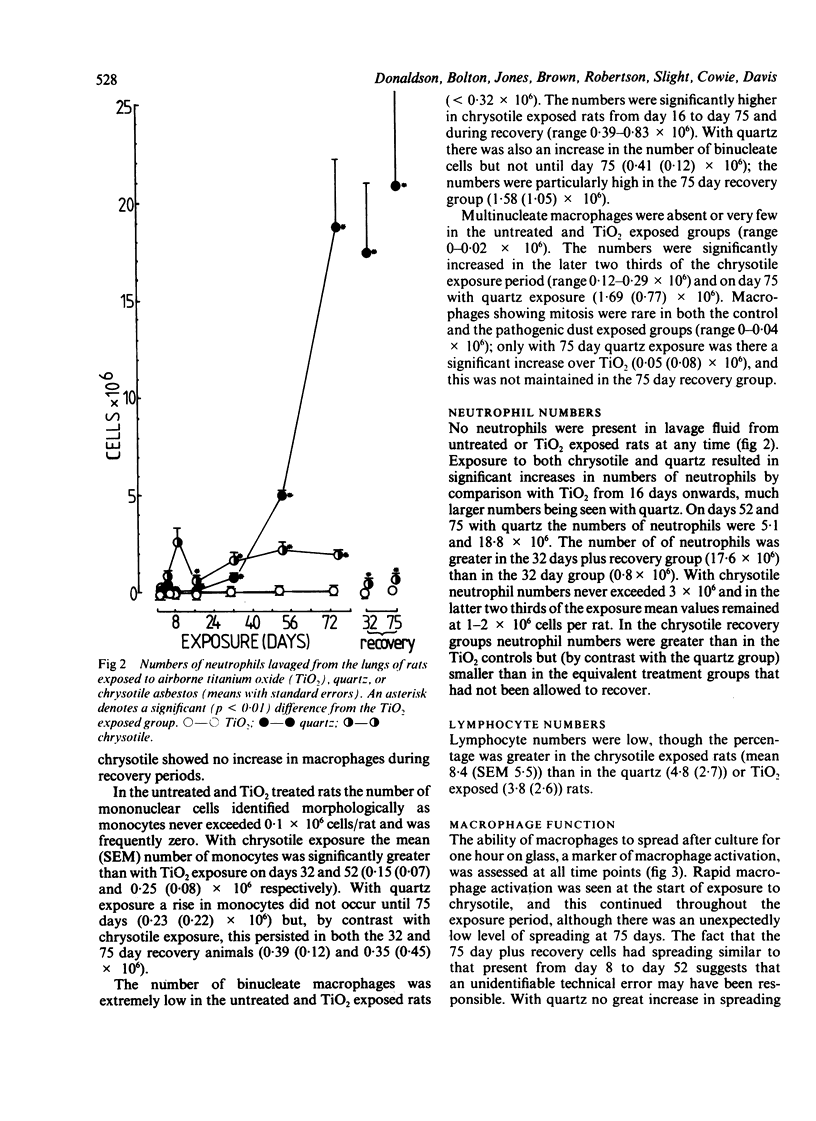
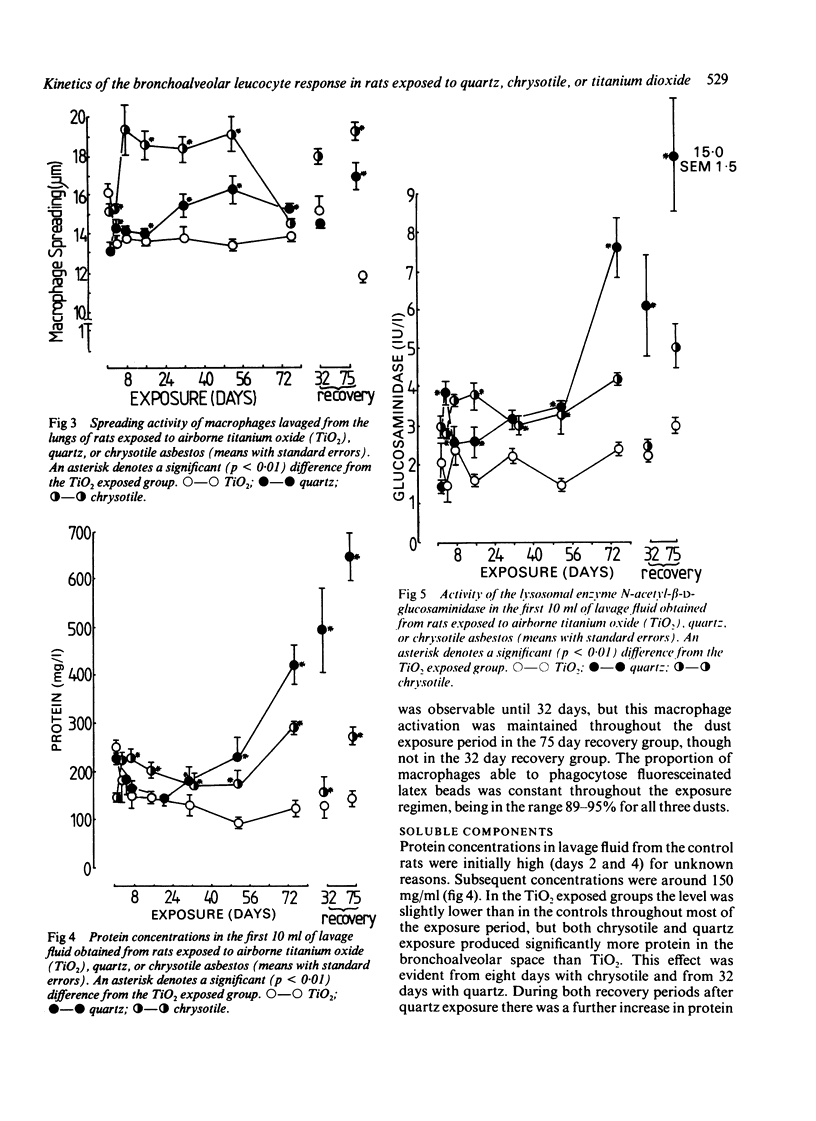
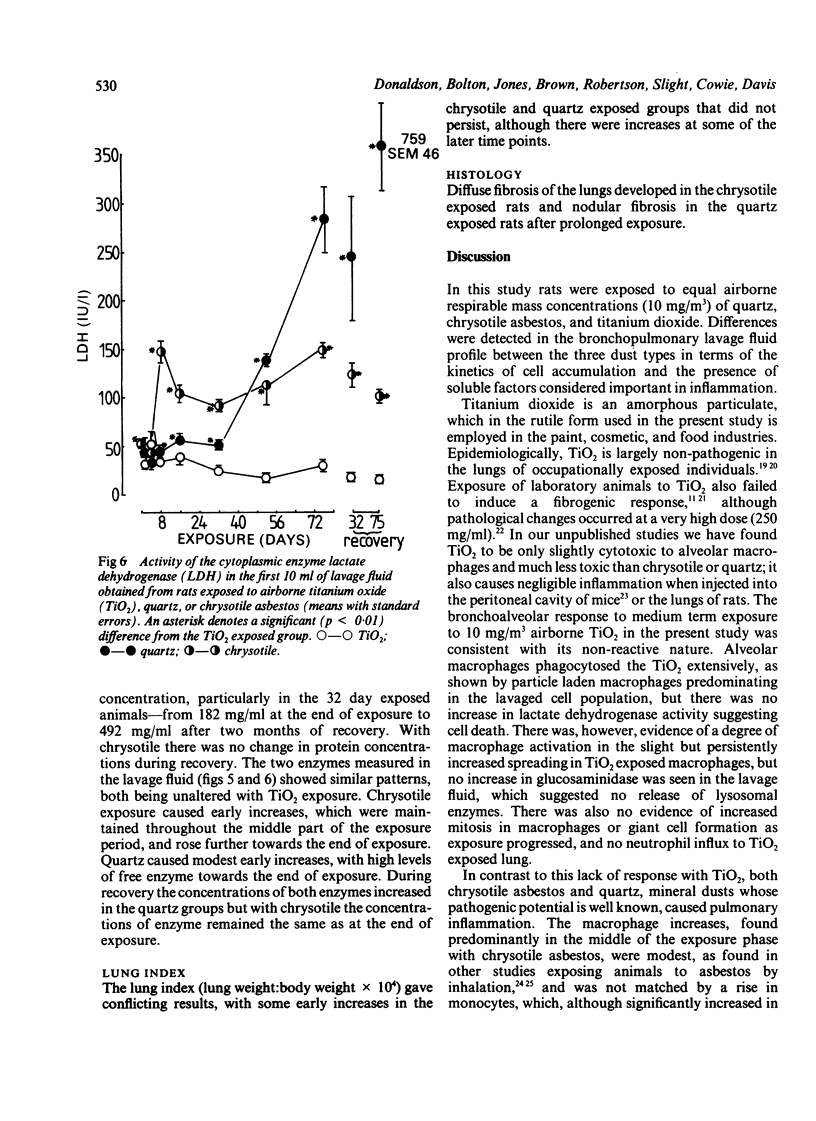
The kinetics of the bronchoalveolar response was assessed in rats exposed, at equal airborne mass concentration (10 mg/m3), to titanium dioxide--a non-pathogenic dust--and the two pathogenic mineral dusts quartz and chrysotile asbestos. Rats were killed at intervals over a 75 day exposure period and groups of rats exposed for 32 and 75 days after recovery for two months. Bronchoalveolar lavage was carried out and the lavage fluid characterised for cellular content, macrophage activation, and concentrations of free total protein, lactate dehydrogenase, and N-acetyl-beta-D-glucosaminidase. Inhalation exposure to the two pathogenic dusts resulted in an increased number of leucocytes, macrophage activation, and increased levels of free enzymes and total protein. The pattern and magnitude of the responses to quartz and chrysotile differed. Chrysotile caused less inflammation than quartz, and the main cellular response peaked around the middle of the period of dust exposure whereas the highest levels of enzymes occurred towards the end. The difference in timing suggests that macrophages were not available for lavage towards the end of the exposure, owing to their playing a part possibly in deposition of granulation tissue. Quartz caused a greater cellular and enzyme response than chrysotile, particularly towards the end of the dust exposure phase. There was a noticeable progression of inflammation in the quartz exposed groups left to recover for two months, but not in the chrysotile recovery groups.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Role of polymorphonuclear leukocytes in silica-induced pulmonary fibrosis. Am J Pathol. 1984 Oct;117(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- Beckett S. T. The generation and evaluation of UICC asbestos clouds in animal exposure chambers. Ann Occup Hyg. 1975 Dec;18(3):187–198. doi: 10.1093/annhyg/18.3.187. [DOI] [PubMed] [Google Scholar]
- Bozelka B. E., Sestini P., Gaumer H. R., Hammad Y., Heather C. J., Salvaggio J. E. A murine model of asbestosis. Am J Pathol. 1983 Sep;112(3):326–337. [PMC free article] [PubMed] [Google Scholar]
- Bégin R., Rola-Pleszczynski M., Massé S., Nadeau D., Drapeau G. Assessment of progression of asbestosis in the sheep model by bronchoalveolar lavage and pulmonary function tests. Thorax. 1983 Jun;38(6):449–457. doi: 10.1136/thx.38.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis A. H., Sohnle P. G., Mandel G. S., Wiessner J., Mandel N. S. Kinetics of inflammatory and fibrotic pulmonary changes in a murine model of silicosis. J Lab Clin Med. 1985 May;105(5):547–553. [PubMed] [Google Scholar]
- Crystal R. G., Gadek J. E., Ferrans V. J., Fulmer J. D., Line B. R., Hunninghake G. W. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981 Mar;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Beckett S. T., Bolton R. E., Collings P., Middleton A. P. Mass and number of fibres in the pathogenesis of asbestos-related lung disease in rats. Br J Cancer. 1978 May;37(5):673–688. doi: 10.1038/bjc.1978.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K., Bolton R. E., Brown D., Douglas A. An improved macrophage spreading assay--a simple and effective measure of activation. Immunol Commun. 1984;13(3):229–244. doi: 10.3109/08820138409025464. [DOI] [PubMed] [Google Scholar]
- Elo R., Mättä K., Uksila E., Arstila A. U. Pulmonary deposits of titanium dioxide in man. Arch Pathol. 1972 Nov;94(5):417–424. [PubMed] [Google Scholar]
- Ferin J., Oberdörster G. Biological effects and toxicity assessment of titanium dioxides: anatase and rutile. Am Ind Hyg Assoc J. 1985 Feb;46(2):69–72. doi: 10.1080/15298668591394419. [DOI] [PubMed] [Google Scholar]
- Fogelmark B., Sjöstrand M., Bergström R., Rylander R. Pulmonary macrophage phagocytosis and enzyme production after in vivo exposure to silica dust. Toxicol Appl Pharmacol. 1983 Mar 30;68(1):152–159. doi: 10.1016/0041-008x(83)90364-2. [DOI] [PubMed] [Google Scholar]
- Gellert A. R., Langford J. A., Winter R. J., Uthayakumar S., Sinha G., Rudd R. M. Asbestosis: assessment by bronchoalveolar lavage and measurement of pulmonary epithelial permeability. Thorax. 1985 Jul;40(7):508–514. doi: 10.1136/thx.40.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassroth J. L., Bernardo J., Lucey E. C., Center D. M., Jung-Legg Y. J., Snider G. L. Interstitial pulmonary fibrosis induced in hamsters by intratracheally administered chrysotile asbestos. Histology, lung mechanics, and inflammatory events. Am Rev Respir Dis. 1984 Aug;130(2):242–248. doi: 10.1164/arrd.1984.130.2.242. [DOI] [PubMed] [Google Scholar]
- Harington J. S., Allison A. C., Badami D. V. Mineral fibers: chemical, physicochemical, and biological properties. Adv Pharmacol Chemother. 1975;12(0):291–402. doi: 10.1016/s1054-3589(08)60223-9. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Kagan E., Oghiso Y., Hartmann D. P. The effects of chrysotile and crocidolite asbestos on the lower respiratory tract: analysis of bronchoalveolar lavage constituents. Environ Res. 1983 Dec;32(2):382–397. doi: 10.1016/0013-9351(83)90120-2. [DOI] [PubMed] [Google Scholar]
- Katsnelson B. A., Privalova L. I. Recruitment of phagocytizing cells into the respiratory tract as a response to the cytotoxic action of deposited particles. Environ Health Perspect. 1984 Apr;55:313–325. doi: 10.1289/ehp.8455313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouffant L., Daniel H., Martin J. C., Bruyère S. Effect of impurities and associated minerals on quartz toxicity. Ann Occup Hyg. 1982;26(1-4):625–634. [PubMed] [Google Scholar]
- Lee K. P., Trochimowicz H. J., Reinhardt C. F. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol Appl Pharmacol. 1985 Jun 30;79(2):179–192. doi: 10.1016/0041-008x(85)90339-4. [DOI] [PubMed] [Google Scholar]
- Lemaire I. Characterization of the bronchoalveolar cellular response in experimental asbestosis. Different reactions depending on the fibrogenic potential. Am Rev Respir Dis. 1985 Jan;131(1):144–149. doi: 10.1164/arrd.1985.131.1.144. [DOI] [PubMed] [Google Scholar]
- Lugano E. M., Dauber J. H., Daniele R. P. Acute experimental silicosis. Lung morphology, histology, and macrophage chemotaxin secretion. Am J Pathol. 1982 Oct;109(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- Lugano E. M., Dauber J. H., Daniele R. P. Silica stimulation of chemotactic factor release by guinea pig alveolar macrophages. J Reticuloendothel Soc. 1981 Nov;30(5):381–390. [PubMed] [Google Scholar]
- Miller K. The effects of asbestos on macrophages. CRC Crit Rev Toxicol. 1978 Sep;5(4):319–354. doi: 10.3109/10408447809081010. [DOI] [PubMed] [Google Scholar]
- Morgan A., Moores S. R., Holmes A., Evans J. C., Evans N. H., Black A. The effect of quartz, administered by intratracheal instillation, on the rat lung. I. The cellular response. Environ Res. 1980 Jun;22(1):1–12. doi: 10.1016/0013-9351(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Pritchard J. N., Holmes A., Evans J. C., Evans N., Evans R. J., Morgan A. The distribution of dust in the rat lung following administration by inhalation and by single intratracheal instillation. Environ Res. 1985 Apr;36(2):268–297. doi: 10.1016/0013-9351(85)90025-8. [DOI] [PubMed] [Google Scholar]
- Richards R. J., White L. R., Eik-Nes K. B. Biological reactivity of different crystalline forms of titanium dioxide in vitro and in vivo. Scand J Work Environ Health. 1985 Aug;11(4):317–320. doi: 10.5271/sjweh.2217. [DOI] [PubMed] [Google Scholar]
- Schoenberger C. I., Hunninghake G. W., Kawanami O., Ferrans V. J., Crystal R. G. Role of alveolar macrophages in asbestosis: modulation of neutrophil migration to the lung after acute asbestos exposure. Thorax. 1982 Nov;37(11):803–809. doi: 10.1136/thx.37.11.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbrell V., Hyett A. W., Skidmore J. W. A simple dispenser for generating dust clouds from standard reference samples of asbestos. Ann Occup Hyg. 1968 Oct;11(4):273–281. doi: 10.1093/annhyg/11.4.273. [DOI] [PubMed] [Google Scholar]
- Voisin C., Gosselin B., Ramon P., Wallaert B., Aerts C., Lenoir L. Le lavage broncho-alvéolaire dans la pneumoconiose des mineurs de charbon. Aspects cytologiques. Rev Fr Mal Respir. 1983;11(4):455–466. [PubMed] [Google Scholar]
- WOOLLEN J. W., HEYWORTH R., WALKER P. G. Studies on glucosaminidase. 3. Testicular N-acetyl-beta-glucosaminidase and N-acetyl-beta-galactosaminidase. Biochem J. 1961 Jan;78:111–116. doi: 10.1042/bj0780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]


