Abstract
The cellular functions of the trans-Golgi network protein TGN38 remain unknown. In this research, we studied the expression, localization and functions of TGN38 in the meiotic maturation of mouse oocytes. TGN38 was expressed at every stage of oocyte meiotic maturation and colocalized with γ-tubulin at metaphase I and metaphase II. The spindle microtubule disturbing agents nocodazole and taxol did not affect the colocalization of TGN38 and γ-tubulin. Depletion of TGN38 with specific siRNAs resulted in increased metaphase I arrest, accompanied with spindle assembly checkpoint activation and decreased first polar extrusion (PB1). In the oocytes that had extruded the PB1 after the depletion of TGN38, symmetric division occurred, leading to the production of 2 similarly sized cells. Moreover, the peripheral migration of metaphase I spindle and actin cap formation were impaired in TGN38-depleted oocytes. Our data suggest that TGN38 may regulate the metaphase I/anaphase I transition and asymmetric cell division in mouse oocytes.
Keywords: asymmetric cell division, Golgi apparatus, metaphase I/anaphase I transition, TGN38
Abbreviations
- GV
germinal vesicle
- GVBD
germinal vesicle breakdown
- MI
metaphase of the first meiosis
- MII
metaphase of the second meiosis
- TGN
trans-Golgi network
- PB1
first polar body extrusion
- MTOCs
microtubule organizing centers
Introduction
Meiosis is the important process to produce haploid gametes for sexual reproduction. During meiotic maturation, mammalian oocytes undergo 2 successive cell divisions without an intermediate replication of the genetic material. Meanwhile, the 2 divisions are highly asymmetric, generating a large oocyte and a small polar body. The asymmetric cell division maintains most maternal stores for the pre-implantation embryo development.1
After germinal vesicle breakdown (GVBD), microtubules accumulate near the center of the oocyte to form the spindle. Then the spindle migrates to the cell cortex in a microfilament-dependent manner.2,3 As the spindle migrating and anchoring to the cortex, cortical reorganization occurs, including the formation of actin-cap and redistribution of cortical granules.1,4 Anaphase I and first polar body extrusion are initiated soon after these events. Meiosis II spindle rapidly assembles below the first polar body and the oocyte will arrest at meiosis II metaphase until fertilization.5 Proteins involved in the process of spindle positioning and cortical reorganization are shown to affect asymmetric cell division of mammalian oocytes.6-8
Golgi apparatus is an important membrane-bound eukaryotic organelle.9 Modification and packaging of proteins and lipids are the principle functions of the Golgi. After proper processing, the proteins or lipids are sent to appropriate locations.10 Besides, Golgi apparatus is essential for the establishment of cell polarity and cell cycle regulation.11-13 In mouse oocytes, the Golgi is more accumulated near the nucleus than at the cortex at germinal vesicle (GV) stage. After GVBD, the Golgi is evenly diffused in the cytoplasm until metaphase II (MII).14 Treatment of mouse oocytes with brefeldin A, an inhibitor of membrane trafficking from the endoplasmic reticulum (ER) to the Golgi, resulted in impaired asymmetric spindle positioning at metaphase I (MI) and MII stages. Furthermore, the oocytes divided into 2 half-size cells instead of asymmetric division.15 Brefeldin A could inactivate ADP-ribosylation Factor 1 (ARF1) and expression of a dominant negative form of ARF1 in mouse oocytes resulted in symmetric cell division.16 Depletion of GM130, the cis-Golgi marker, by specific morpholino caused spindle abnormality and large polar body extrusion.17
TGN38 is a membrane-integrated protein of the trans-Golgi network (TGN)18,19 and a cargo protein of the retrograde transport with unknown cellular functions.20,21 In this study, we examined the roles of TGN38 during oocyte meiotic maturation. We show that RNAi of TGN38 resulted in metaphase I arrest or defects in asymmetric cell division in mouse oocytes.
Results
Expression and localization of TGN38 during mouse oocyte meiotic maturation
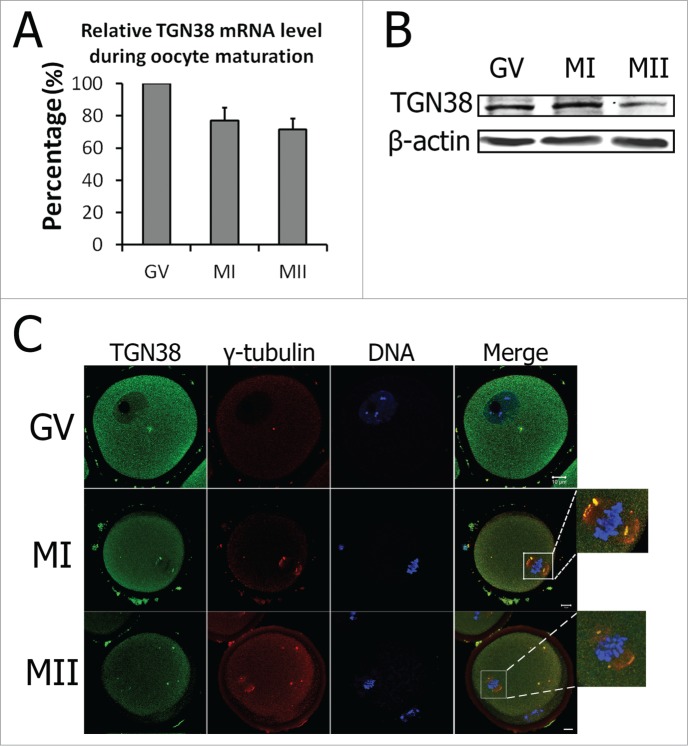
The TGN38 mRNA level was investigated by real-time PCR. As shown in Figure 1A, TGN38 mRNA expression was detected from GV to MII stages. In detail, the TGN38 mRNA level at MI and MII stages were 77.17 ± 7.82% and 71.51 ± 6.86% of that at GV stage. Subcellular localization of TGN38 was studied by TGN38 antibody immunofluorescent staining. Western blot showed that TGN38 protein level was similar at GV and MI stages, with a decrease at MII stage in mouse oocytes (Fig. 1B). As the spatial and functional relationships between the Golgi and centrosomes were reported in previous studies (reviewed by Sutterlin et al.),12 we co-stained γ-tubulin, the protein essential for microtubule nucleation. At GV stage, TGN38 was dispersed in the cytoplasm (Fig. 1C). Occasionally, we found the accumulation of TGN38 in the cytoplasm, colocalizing with γ-tubulin (Fig. 1C). At MI and MII stages, TGN38 localized to the spindle poles and colocalized with γ-tubulin (Fig. 1C).
Figure 1.
Expression and localization of TGN38 in mouse oocytes during meiotic maturation. (A) Relative level of TGN38 mRNA in mouse oocytes during meiotic maturation. TGN38 mRNA levels were normalized to the maximum levels at GV stage. Samples were collected for quantitative RT-PCR when the oocytes were cultured for 0 h, 8 h or 12 h, corresponding to GV, MI or MII stages, respectively. Each sample contains 50 oocytes. (B) Protein level of TGN38 in mouse oocytes at GV, MI and MII stages. Each sample contains 200 oocytes. (C) Oocytes at GV, MI or MII stages were co-stained anti-TGN38 (green) and anti-γ-tubulin (red) antibodies. Besides the localization in the cytoplasm, TGN38 co-localized with γ-tubulin during oocyte maturation. Bars, 10 μm.
Subcellular localization of TGN38 in mouse oocytes after treatment with spindle disturbing drugs
Colocalization of TGN38 and γ-tubulin indicates that TGN38 may be a component of the microtubule organizing centers (MTOCs). We then analyzed the interaction of TGN38 localization and microtubule dynamics by employing spindle disturbing drugs. In the control group, TGN38 colocalized with γ-tubulin at the spindle poles (Fig. 2). Nocodazole is a reversible inhibitor of microtubule polymerization. After treated with nocodazole, the spindle thoroughly disassembled in mouse oocytes, but the colocalization of TGN38 and γ-tubulin was not altered (Fig. 2). Taxol promotes microtubule assembly and stabilizes polymerized microtubules. Treatment of mouse oocytes with taxol lead to enlarged spindles and multiple asters in the cytoplasm of mouse oocytes (Fig. 2). TGN38 colocalized with γ-tubulin at the multiple MTOCs in taxol treated mouse oocytes (Fig. 2).
Figure 2.
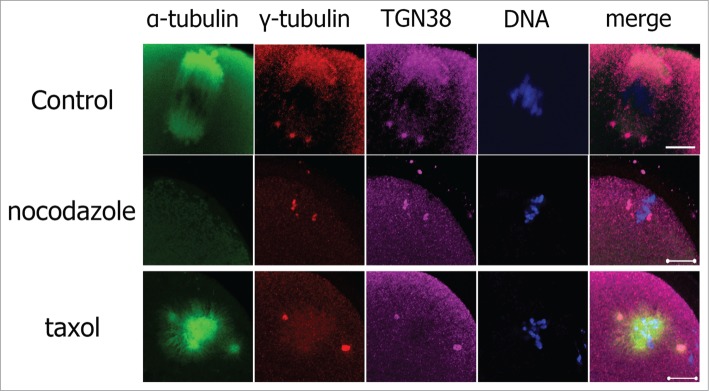
Localization of TGN38 in mouse oocytes after treatment with nocodazole or taxol. Oocytes were incubated in M2 medium containing nocodazole (20 μg/ml; 10 min) or taxol (10 μM; 45 min). Then the oocytes were washed thoroughly with M2 medium and co-stained with antibodies against TGN38 (purple) and γ-tubulin (red). Hoechst 33342 was used to stain DNA (Blue). Bars, 10 μm.
Depletion of TGN38 in mouse oocytes induced MI arrest and decreased percentage of first polar body emission
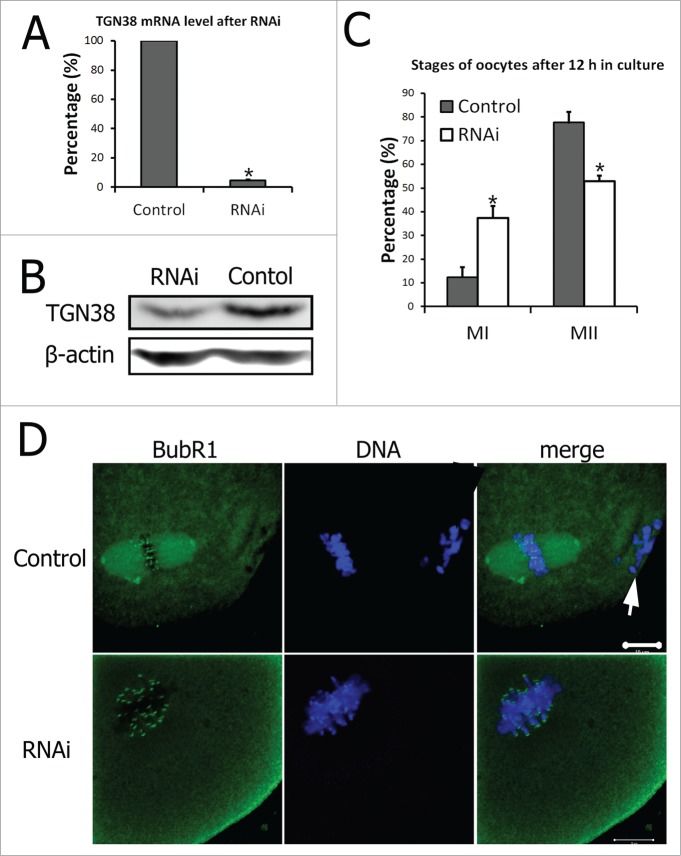
RNAi was employed to study the functions of TGN38 in mouse oocyte meiotic maturation. After injection of TGN38 specific siRNAs (RNAi group), oocytes were collected for real-time PCR analysis to determine the relative level of TGN38 mRNA. Expression of TGN38 mRNA in mouse oocytes after TGN38 siRNAs injection was significantly decreased compared with that in the control group (scrambled siRNAs injected group, 4.61 ± 0.67% vs. 100%, P < 0.001, Fig. 3A). And the TGN38 protein level was also considerably declined in the TGN38 RNAi group (Fig. 3B). After 12 h of culture, the oocytes in both groups were collected and immunostained for α-tubulin and DNA to check the stages of oocyte maturation. In the control group (n = 118), most oocytes emitted the first polar body and arrested at MII stage, while in the TGN38 RNAi group (n = 132), the percentage of oocytes at MII stage was critically lower (52.88 ± 2.32% vs. 77.67 ± 4.61%, P < 0.05, Fig. 3C). In the meantime, the percentage of oocytes arrested at MI stage in the RNAi group was evidently higher than that in the control group (37.29 ± 2.32% vs 12.36 ± 4.30%, P < 0.05, Fig. 3C). These oocytes in the TGN38 depleted group were immunostained against BubR1. And localization of BubR1 to the centromeres were detected, implicating the spindle assembly checkpoint (SAC) activation (Fig. 3D). While in the control group, oocytes were arrested at MII stage and BubR1 localized to the centromeres (Fig. 3D).
Figure 3.
Depletion of TGN38 in mouse oocytes caused MI arrest and decreased percentage of first polar body extrusion. (A) Relative level of TGN38 mRNA in mouse oocytes after injection of scrambled siRNAs or TGN38 siRNAs. Following injection, oocytes were cultured in M2 medium containing 2.5 μM milrinone for 24 h before collected for quantitative RT-PCR. Fifty oocytes were collected in each sample. *, P < 0.001. (B) TGN38 protein level in control and TGN38 RNAi groups. Each sample contains 200 oocytes. (C) Percentages of oocytes at different stages when cultured for 12 h after TGN38 RNAi. *, P < 0.05. (D) Representative images of BubR1 localization in TGN38 depleted oocytes. Arrow: PB1. Bar, 10 μm.
TGN38 depletion caused failure of asymmetric cell division in mouse oocytes
We then examined the oocytes that emitted the first polar body both in the control group and the TGN38 RNAi group. Compared to that in the control group (n = 116), a significantly higher proportion of oocytes in the TGN38 depleted group (n = 79) underwent symmetric cell division (43.73 ± 4.87% vs 14.44 ± 2.72%, P < 0.05, Fig. 4A and B). Moreover, the spindles in the symmetrically divided daughter cells were aberrant, with loose spindle poles (Fig. 4B).
Figure 4.
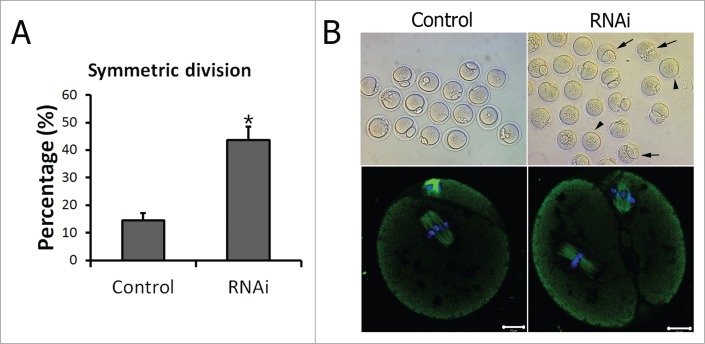
TGN38 depletion caused failure of asymmetric cell division in mouse oocytes. (A) Percentages of symmetric division in oocytes when cultured for 12 h after TGN38 RNAi. Data are represented as mean ± s.e.m. of 3 independent experiments. *, P < 0.05. (B) Representative images of oocytes at 12 h of incubation. Upper lane: images from an optical microscope. Arrows: oocytes that divided symmetrically. Arrow heads: oocytes without a polar body. Lower lane: Oocytes were co-stained with anti-α-tubulin (Green) antibody and Hoechst 33342 (Blue), and examined under a confocal microscope. Bars, 10 μm.
Peripheral spindle migration at MI stage was impaired after TGN38 depletion in mouse oocytes
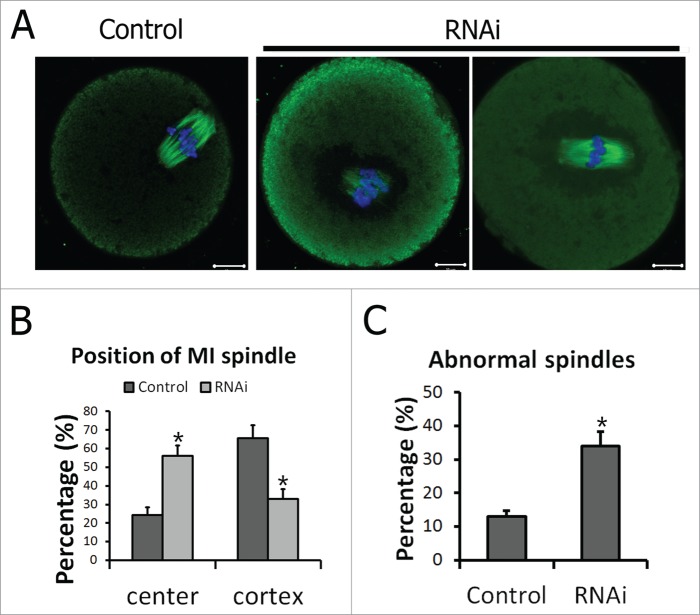
Spindle migration from the center of cytoplasm to the cell cortex is a prerequisite step for asymmetric cell division. After 8.5 h in culture, when most oocytes had reached MI stage, spindles of the oocytes in the control group migrated to the cell cortex. While in the TGN38 RNAi group, the spindles stayed near the center of the cell, with abnormal morphologies (Fig. 5A). Further statistics showed that in the control group the percentage of oocytes with central located spindles (24.29 ± 4.26%, n = 140) was significantly lower than that in the TGN38RNAi group (56.10 ± 5.60%, n = 164, P < 0.05, Fig. 5B). And considerably less oocytes were with cortex localized spindles in TGN38 RNAi group than that in the control group (32.92 ± 5.31% vs 65.71 ± 6.87%, P < 0.05, Fig. 5B). In addition, significantly more oocytes were detected with aberrant spindles than that in the control group (34.03 ± 4.33% vs. 12.99 ± 1.83%, P < 0.05, Fig. 5C).
Figure 5.
Peripheral spindle migration at MI stage was impaired after TGN38 depletion in mouse oocytes. (A) Representative images of oocytes in different siRNAs injected groups. After cultured for 8.5 h following TGN38 RNAi, oocytes were fixed and co-stained with anti-α-tubulin (Green) antibody and Hoechst 33342 (Blue). Bars, 10 μm. (B) Percentages of position of MI spindle in oocytes. *, P < 0.05. (C) Percentages of oocytes with abnormal spindles. *, P < 0.05.
Knockdown of TGN38 disrupted actin cap formation in mouse oocytes
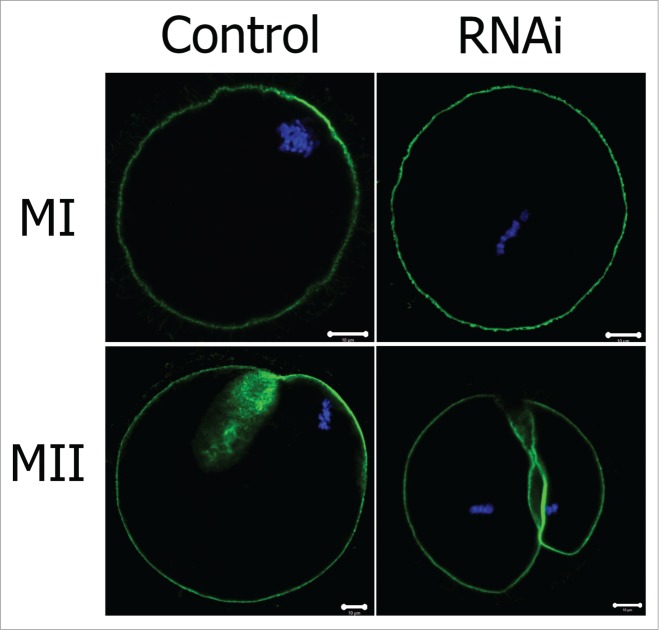
Actin cap formation is another feature of oocyte polarization and is required for peripheral spindle migration and anchoring. We then examined formation of actin cap after TGN38 knockdown in oocytes. At later MI stage, the chromosomes (and thus the spindle) had already migrated to the cell cortex and an actin cap assembled above the chromosomes (Fig. 6). But in the TGN38 RNAi group, the spindle stayed near the center of the cell and no actin cap was detected. In the MII oocyte of the control group, one small polar body was extruded and the MII spindle was found at the cortex below the polar body, under the actin cap (Fig. 6). Whereas in the TGN38 RNAi group, the oocyte underwent symmetric cytokinesis and formed 2 half-size eggs, without typical actin cap structure monitored (Fig. 6).
Figure 6.
Effect of TGN38 depletion on actin cap formation in mouse oocytes. Oocytes were co-stained with Phalloidin-FITC (green) and Hoechst 33342 (Blue). Bars, 10 μm.
Discussion
The cellular functions of the trans-Golgi network protein TGN38 was unknown previously. In this research, we investigated the expression, localization and possible roles of TGN38 during mouse oocyte meiotic maturation. Knockdown of TGN38 induced 2 different phenotypes in mouse oocytes. In a proportion of oocytes, SAC was activated and the extrusion of first polar body was inhibited. Whereas in the oocytes that reached MII stage, depletion of TGN38 caused the formation of 2 half-size cells rather than a large MII egg and a small polar body.
Two isoforms of TGN38 were identified in ICR mouse.22 The TGN38 siRNAs and primers were designed to be included in the conserved domains of both isoforms. Indeed, the siRNAs depleted the expression of TGN38 efficiently, as indicated by the results of quantitative RT-PCR with the TGN38 primers (Fig. 2A). The expression and localization pattern of TGN38 in mouse oocyte maturation was similar with that of GM130.17
In NRK cells, centrosomes associated with the Golgi resident proteins TGN38 and Golgin-97 throughout the cell cycle.23 Moreover, this association was stable after the cells were treated with brefeldin A or nocodazole, while GM130 was not associated with centrosomes in mitosis and its localization was disrupted with the drugs.23 In mouse oocytes, which lack centrosomes,24 TGN38 colocalized with γ-tubulin during oocyte maturation (Fig. 1C). The result implies that TGN38 may employ an alternative mechanism to interact with MTOCs in oocytes. Treatment of the oocytes with spindle perturbing drugs nocodazole and taxol did not disrupt the colocalization of TGN38 and γ-tubulin (Fig. 2), suggesting the association between TGN38 and MTOSs is independent on microtubule assembly. However, nocodazole treatment of oocytes lead to dispersal of the cis-Golgi protein GM130.17 Thus, tans-Golgi protein TGN38 may be more involved in the functions of MTOCs than cis-Golgi protein GM130 during oocyte meiotic maturation. In mouse oocytes, spindle assembly originates from MTOCs which contain proteins normally present in centrosomes.25,26 The constant colocalization of TGN38 and γ-tubulin suggests that TGN38 may be a component of MTOCs and participates in meiotic spindle formation.
Knockdown of TGN38 lead to spindle abnormality, activation of SAC and MI arrest in mouse oocytes (Fig. 3). As microtubule nucleation at trans-Golgi network and was previous reported,27 we propose that TGN38 depletion may partially impair the function of MTOCs. Defective MTOCs then induced aberrant spindle assembly, which resulted in improper attachments of microtubules to chromosomes and thus incorrect tension at kinetochores, activating the SAC.28-31 And the oocytes were arrested at MI stage (Fig. 3).
A portion of TGN38 depleted oocytes overrode MI arrest and progressed to MII, but the percentage of oocytes with symmetric cell division was significantly higher (Fig. 4). Impaired peripheral spindle migration may be crucial cause of this phenotype (Fig. 5). Spindle migration is microtubule-independent but actin-dependent.4 Recently, the actin nucleators Formin-2, Spire 1/2 and Arp2/3 were shown to be essential for spindle positioning in mouse oocytes.32-37 TGN38 was reported to interact with the F-actin binding protein neurobin both in vitro and in vivo, which provided a direct association between TGN38 and the actin cytoskeleton.38 Further studies are needed to elucidate whether TGN38 interacts with other actin binding proteins or action nucleators and how the interaction affects the actin cytoskeleton dynamics, such as the action cap formation we detected in this study (Fig. 6).
In conclusion, we investigated the possible cellular functions of the trans-Golgi network protein TGN38 during oocyte meiotic maturation for the first time. Our results indicated that TGN38 might function as a component of MTOCs to coordinate spindle assembly checkpoint in mouse oocyte meiotic maturation. Additionally, TGN38 could regulate asymmetric cell division of mouse oocytes through affecting peripheral spindle migration and actin cap formation.
Materials and methods
Antibodies and reagents
Sheep polyclonal anti-TGN38 antibody was from Novus Biologicals (Littleton, CO). Sheep polyclonal anti-BubR1 antibody was obtained from Abcam (Cambridge, MA). Rabbit polyclonal anti-γ-tubulin antibody was from Santa Cruz Biotechnology. Mouse monoclonal FITC-conjugated anti-α-tubulin antibody, Phalloidin-FITC and M2 medium were purchased from Sigma (St Louis, MO). FITC-conjugated donkey anti-sheep IgG was from Jackson Immuno Research Laboratories (West Grove, PA).
Oocyte collection and culture
Care and handling of ICR mouse (6 to 8 weeks old) were performed according to the guidelines from Animal Research Committee of the Institute of Zoology, Chinese Academy of Sciences. Immature oocytes with intact germinal vesicles were collected in M2 medium. Then oocytes cultured under paraffin oil in a 5% CO2 incubator. The oocytes were collected for further analysis after 0 h, 8 h or 12 h in culture, corresponding to GV, MI or MII stages.
Real time quantitative PCR analysis
Each sample contained 50 oocytes, and total RNA was extracted with RNeasy micro purification kit (Qiagen). PrimeScript™ 1st strand cDNA synthesis kit (Takara) was employed to generate 1st strand DNA. The primers used for the amplification of TGN38 fragment are listed as follows.
Forward: 5′-GCAGCCACTTCTTTGCATATCTAG-3′;
Reverse: 5′-AGTGACTTTGGATCTTTTCCCTTC-3′.
GAPDH was chosen as the reference gene. We utilized UltraSYBR Mixture (CoWin Biotech, Beijing) on a Rotor Gene Q (Qiagen). The program for real time quantitative PCR was 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min.
Immunofluorescence and confocal microscopy
Oocytes were fixed in 4% paraformaldehyde in PBS for 30 min and permeabilized with 0.5% Triton X-100 for 20 min at room temperature. After 1 h blocking in 1% BSA at room temperature, oocytes were incubated with sheep polyclonal anti-TGN38 antibody (1:50), sheep polyclonal anti-BubR1 antibody (1:30), Phalloidin-FITC (10 μg/ml) or mouse monoclonal FITC-conjugated anti-tubulin antibody (1:200) overnight at 4 °C. Three times (5 min each) of washing with PBS supplemented with 0.1% Tween 20 and 0.01% Triton X-100 were followed. For staining of TGN38 and BubR1, the oocytes were then incubated with FITC-conjugated donkey anti-sheep IgG (1:200) for 2 h at room temperature, followed by 3 times of washing. DNA was stained with Hoechst 33342 and the oocytes were mounted on glass slides for microscopy. The slides were examined under a confocal microscope (Carl Zeiss LSM780, Germany).
Western blot
Samples (each contains 200 oocytes) were collected in SDS loading buffer and boiled for 5 min. Proteins were separated on SDS-PAGE and electrically transferred to polyvinylidene difluoride (PVDF) membranes. TBST containing 5% skimmed milk was used to block the membranes for 1 h. The membranes were then incubated with sheep polyclonal anti-TGN38 antibody (1:1000) at 4 °C overnight. After 3 times of washing with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated donkey anti-sheep IgG (1:10000) at 37 °C for 2 h. Finally, the membranes were washed 3 times with TBST and examined with the enhanced chemiluminescence (ECL) detection system (Amersham, Piscataway, NJ).
Treatment of oocytes with nocodazole or taxol
After 8 h in culture, oocytes were transferred to pre-warmed M2 medium containing nocodazole (20 μg/ml) or taxol (10 μM). The oocytes were then incubated in a 5% CO2 incubator. Incubation time was 10 min for nocodazole treatment and 45 min for taxol treatment. Next, the oocytes were fixed for immunofluorescent staining.
Microinjection of TGN38 siRNAs
Microinjection of siRNAs was performed with Narishige MM0–202N hydraulic 3-dimensional micromanipulators (Narishige Inc., Sea Cliff, NY) under a Nikon Diaphot ECLIPSE TE 300 (Nikon UK Ltd., Kingston upon Thames, Surrey, UK) and finished in 30 min. The sequences of TGN38 siRNAs were:
TGN38 siRNA-1: 5′-GCCACUUCUUUGCAUAUCUtt-3′
TGN38 siRNA-2: 5′-GUGACUACCAACGUUUGAAtt-3′
TGN38 siRNA-3: 5′ GGCCACAGAAGAUGAUUCUtt-3′.
And concentration of each siRNA was 25 μM. The same amount of scrambled siRNAs were injected as control. Oocytes were arrested at GV stage in M2 medium containing 2.5 μM milrinone for 24 h to allow the depletion of TGN38. Then the oocytes were transferred to milrinone free medium.
Statistical Analysis
At least 3 replications were performed for all experiments. Data are presented as mean ± s.e.m. and analyzed with Student t-test of Microsoft Excel. P < 0.05 was regarded as significant difference.
Funding Statement
This study was supported by grants from National Natural Science Foundation of China (81300480 and 31271605).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We are grateful to Shi-Wen Li and Hua Qin for technical assistance.
References
- 1. Brunet S, Verlhac MH. Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update 2011; 17:68-75; PMID: 20833637; http://dx.doi.org/ 10.1093/humupd/dmq044 [DOI] [PubMed] [Google Scholar]
- 2. Chaigne A, Verlhac MH, Terret ME. Spindle positioning in mammalian oocytes. Exp Cell Res 2012; 318:1442-7; PMID: 22406266; http://dx.doi.org/ 10.1016/j.yexcr.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 3. Sun QY, Schatten H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 2006; 131:193-205; PMID: 16452714; http://dx.doi.org/ 10.1530/rep.1.00847 [DOI] [PubMed] [Google Scholar]
- 4. Longo FJ, Chen DY. Development of cortical polarity in mouse eggs–involvement of the meiotic apparatus. Dev Biol 1985; 107:382-94; PMID: 4038667; http://dx.doi.org/ 10.1016/0012-1606(85)90320-3 [DOI] [PubMed] [Google Scholar]
- 5. Li R. The art of choreographing asymmetric cell division. Dev Cell 2013; 25:439-50; PMID: 23763946; http://dx.doi.org/ 10.1016/j.devcel.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 6. Almonacid M, Terret ME, Verlhac MH. Actin-based spindle positioning: new insights from female gametes. J Cell Sci 2014; 127:477-83; PMID: 24413163; http://dx.doi.org/ 10.1242/jcs.142711 [DOI] [PubMed] [Google Scholar]
- 7. Sun SC, Kim NH. Molecular mechanisms of asymmetric division in oocytes. Microsc Microanal 2013; 19:883-97; PMID: 23764118; http://dx.doi.org/ 10.1017/S1431927613001566 [DOI] [PubMed] [Google Scholar]
- 8. Dehapiot B, Halet G. Ran GTPase promotes oocyte polarization by regulating ERM (Ezrin/Radixin/Moesin) inactivation. Cell Cycle 2013; 12:1672-8; PMID: 23656777; http://dx.doi.org/ 10.4161/cc.24901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klute MJ, Melancon P, Dacks JB. Evolution and diversity of the Golgi. Cold Spring Harb Perspect Biol 2011; 3:a007849; PMID: 21646379; http://dx.doi.org/ 10.1101/cshperspect.a007849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Seemann J. Golgi biogenesis. Cold Spring Harb Perspect Biol 2011; 3:a005330; PMID: 21690214; http://dx.doi.org/ 10.1101/cshperspect.a005330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson C, Venditti R, Rega LR, Colanzi A, D'Angelo G, De Matteis MA. The Golgi apparatus: an organelle with multiple complex functions. Biochem J 2011; 433:1-9; PMID: 21158737; http://dx.doi.org/ 10.1042/BJ20101058 [DOI] [PubMed] [Google Scholar]
- 12. Sutterlin C, Colanzi A. The Golgi and the centrosome: building a functional partnership. J Cell Biol 2010; 188:621-8; PMID: 20212314; http://dx.doi.org/ 10.1083/jcb.200910001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sutterlin C, Hsu P, Mallabiabarrena A, Malhotra V. Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell 2002; 109:359-69; PMID: 12015985; http://dx.doi.org/ 10.1016/S0092-8674(02)00720-1 [DOI] [PubMed] [Google Scholar]
- 14. Moreno RD, Schatten G, Ramalho-Santos J. Golgi apparatus dynamics during mouse oocyte in vitro maturation: effect of the membrane trafficking inhibitor brefeldin A. Biol Reprod 2002; 66:1259-66; PMID: 11967185; http://dx.doi.org/ 10.1095/biolreprod66.5.1259 [DOI] [PubMed] [Google Scholar]
- 15. Wang L, Wang Z-B, Zhang X, FitzHarris G, Baltz JM, Sun Q-Y, Liu XJ. Brefeldin A disrupts asymmetric spindle positioning in mouse oocytes. Dev Biol 2008; 313:155-66; PMID: 18053978; http://dx.doi.org/ 10.1016/j.ydbio.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 16. Wang S, Hu J, Guo X, Liu JX, Gao S. ADP-ribosylation factor 1 regulates asymmetric cell division in female meiosis in the mouse. Biol Reprod 2009; 80:555-62; PMID: 19005166; http://dx.doi.org/ 10.1095/biolreprod.108.073197 [DOI] [PubMed] [Google Scholar]
- 17. Zhang CH, Wang ZB, Quan S, Huang X, Tong JS, Ma JY, Guo L, Wei YC, Ouyang YC, Hou Y, et al. GM130, a cis-Golgi protein, regulates meiotic spindle assembly and asymmetric division in mouse oocyte. Cell Cycle 2011; 10:1861-70; PMID: 21552007; http://dx.doi.org/ 10.4161/cc.10.11.15797 [DOI] [PubMed] [Google Scholar]
- 18. Reaves B, Wilde A, Banting G. Identification, molecular characterization and immunolocalization of an isoform of the trans-Golgi-network (TGN)-specific integral membrane protein TGN38. Biochem J 1992; 283(Pt 2):313-6.; PMID: 1575675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luzio JP, Brake B, Banting G, Howell KE, Braghetta P, Stanley KK. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38). Biochem J 1990; 270:97-102.; PMID: 2204342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell 2008; 135:1175-87; PMID: 19109890; http://dx.doi.org/ 10.1016/j.cell.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 21. Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol 2006; 7:568-79; PMID: 16936697; http://dx.doi.org/ 10.1038/nrm1985 [DOI] [PubMed] [Google Scholar]
- 22. Kasai K, Takahashi S, Murakami K, Nakayama K. Strain-specific presence of two TGN38 isoforms and absence of TGN41 in mouse. J Biol Chem 1995; 270:14471-6; PMID: 7540170; http://dx.doi.org/ 10.1074/jbc.270.24.14471 [DOI] [PubMed] [Google Scholar]
- 23. Takatsuki A, Nakamura M, Kono Y. Possible implication of Golgi-nucleating function for the centrosome. Biochem Biophys Res Commun 2002; 291:494-500; PMID: 11855815; http://dx.doi.org/ 10.1006/bbrc.2002.6433 [DOI] [PubMed] [Google Scholar]
- 24. Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 2007; 130:484-98; PMID: 17693257; http://dx.doi.org/ 10.1016/j.cell.2007.06.025 [DOI] [PubMed] [Google Scholar]
- 25. Dumont J, Desai A. Acentrosomal spindle assembly and chromosome segregation during oocyte meiosis. Trends Cell Biol 2012; 22:241-9; PMID: 22480579; http://dx.doi.org/ 10.1016/j.tcb.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schatten H, Sun QY. Centrosome dynamics during mammalian oocyte maturation with a focus on meiotic spindle formation. Mol Reprod Dev 2011; 78:757-68; PMID: 21887720; http://dx.doi.org/ 10.1002/mrd.21380 [DOI] [PubMed] [Google Scholar]
- 27. Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia AR, McLeod IX, et al. Asymmetric CL ASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell 2007; 12:917-30; PMID: 17543864; http://dx.doi.org/ 10.1016/j.devcel.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U. Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res 2008; 651:14-29; PMID: 18096427; http://dx.doi.org/ 10.1016/j.mrgentox.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 29. Yin S, Sun XF, Schatten H, Sun QY. Molecular insights into mechanisms regulating faithful chromosome separation in female meiosis. Cell Cycle 2008; 7:2997-3005; PMID: 18802407; http://dx.doi.org/ 10.4161/cc.7.19.6809 [DOI] [PubMed] [Google Scholar]
- 30. Meyer RE, Dawson DS. Attaching to spindles before they form Do early incorrect chromosome-microtubule attachments promote meiotic segregation fidelity? Cell Cycle 2013; 12:2011-5; PMID: 23759585; http://dx.doi.org/ 10.4161/cc.25252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang SW, Gao C, Chen L, Song YL, Zhu JL, Qi ST, Jiang ZZ, Wang ZW, Lin F, Huang H, et al. Nek9 regulates spindle organization and cell cycle progression during mouse oocyte meiosis and its location in early embryo mitosis. Cell Cycle 2012; 11:4366-77; PMID: 23159858; http://dx.doi.org/ 10.4161/cc.22690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac M-H. Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol 2008; 18:1514-9; PMID: 18848445; http://dx.doi.org/ 10.1016/j.cub.2008.08.044 [DOI] [PubMed] [Google Scholar]
- 33. Dumont J, Million K, Sunderland K, Rassinier P, Lim H, Leader B, Verlhac MH. Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev Biol 2007; 301:254-65; PMID: 16989804; http://dx.doi.org/ 10.1016/j.ydbio.2006.08.044 [DOI] [PubMed] [Google Scholar]
- 34. Leader B, Lim H, Carabatsos MJ, Harrington A, Ecsedy J, Pellman D, Maas R, Leder P. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol 2002; 4:921-8; PMID: 12447394; http://dx.doi.org/ 10.1038/ncb880 [DOI] [PubMed] [Google Scholar]
- 35. Pfender S, Kuznetsov V, Pleiser S, Kerkhoff E, Schuh M. Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr Biol 2011; 21:955-60; PMID: 21620703; http://dx.doi.org/ 10.1016/j.cub.2011.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, Li R. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol 2011; 13:1252-8; PMID: 21874009; http://dx.doi.org/ 10.1038/ncb2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun SC, Wang ZB, Xu YN, Lee SE, Cui XS, Kim NH. Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PloS one 2011; 6:e18392; PMID: 21494665; http://dx.doi.org/ 10.1371/journal.pone.0018392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stephens DJ, Banting G. Direct interaction of the trans-Golgi network membrane protein, TGN38, with the F-actin binding protein, neurabin. J Biol Chem 1999; 274:30080-6; PMID: 10514494; http://dx.doi.org/ 10.1074/jbc.274.42.30080 [DOI] [PubMed] [Google Scholar]