Abstract
The cortisol rise after awakening (cortisol awakening response, CAR) is a core biomarker of hypothalamic-pituitary-adrenal (HPA) axis regulation related to psychosocial stress and stress-related psychiatric disorders. However, the neural regulation of the CAR has not been examined in humans. Here, we studied neural regulation related to the CAR in a sample of 25 healthy human participants using an established psychosocial stress paradigm together with multimodal functional and structural (voxel-based morphometry) magnetic resonance imaging. Across subjects, a smaller CAR was associated with reduced grey matter volume and increased stress-related brain activity in the perigenual ACC, a region which inhibits HPA axis activity during stress that is implicated in risk mechanisms and pathophysiology of stress-related mental diseases. Moreover, functional connectivity between the perigenual ACC and the hypothalamus, the primary controller of HPA axis activity, was associated with the CAR. Our findings provide support for a role of the perigenual ACC in regulating the CAR in humans and may aid future research on the pathophysiology of stress-related illnesses, such as depression, and environmental risk for illnesses such as schizophrenia.
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is the organism’s most important neuroendocrine stress system (de Kloet et al, 2005) and plays a crucial role in the pathophysiology of stress-related mental illnesses, including major depressive disorder (MDD) (Pariante and Lightman, 2008) and posttraumatic stress disorder (PTSD) (de Kloet et al, 2006). It has also been implicated in the pathophysiology of environmental risk factors for a broader range of severe mental disorders including schizophrenia and MDD, such as urban upbringing, childhood abuse, and migration status (Meyer-Lindenberg and Tost, 2012). HPA axis function is typically quantified using proxy measurements, such as the cortisol increase within the first hour after awakening, also known as the cortisol awakening response (CAR) (Pruessner et al, 1997; Wilhelm et al, 2007). This measure of HPA axis reactivity is a longitudinally relatively stable readout (Wust et al, 2000b) for which a significant heritability was consistently reported (Wust et al, 2000a; Bartels et al, 2003; Kupper et al, 2005). The CAR is associated with psychosocial stress (Chida and Steptoe, 2009) and predicts current and future incidence of depression (Pruessner et al, 2003; Huber et al, 2006; Mannie et al, 2007; Vreeburg et al, 2009; Adam et al, 2010; Dedovic et al, 2010; Lamers et al, 2013; Vrshek-Schallhorn et al, 2013). It is also altered in patients with PTSD (Wessa et al, 2006) and those with first-episode psychosis (Mondelli et al, 2010), and has been associated with childhood trauma (Heim et al, 2009; Mangold et al, 2010). Interestingly, the CAR is a stronger prospective predictor of MDD than other readouts of HPA axis activity (Adam et al, 2010), suggesting that this parameter mirrors aspects of HPA axis function particularly implicated in the risk for psychiatric disorders.
Preclinical work has demonstrated that a key region for neural control of HPA axis function is the anterior cingulate cortex (ACC), which has dense anatomical connections to downstream visceral and emotional sites, such as the amygdala, the hippocampus, and the hypothalamus (Herman et al, 2005; Ulrich-Lai and Herman, 2009; Etkin et al, 2011). In rodents, the ACC inhibits HPA axis activity through trans-synaptic connections to the paraventricular nucleus of the hypothalamus (Diorio et al, 1993), the primary promoter of HPA axis activity (Herman et al, 2005), and plays a role in glucocorticoid-mediated feedback control of stress-related HPA axis activity (Diorio et al, 1993). In line with this animal work, the ACC has been implicated in neural control of stress-related HPA axis activity in a human functional neuroimaging study (Pruessner et al, 2008), but the mechanistic basis of this involvement remains to be elucidated. In humans, the perigenual division of the ACC (pACC) has also been implicated in emotion processing, gene–environment interactions (Pezawas et al, 2005; Meyer-Lindenberg et al, 2006), the pathophysiology of depression (Pezawas et al, 2005; Hamani et al, 2011), PTSD (Karl et al, 2006), schizophrenia (Fusar-Poli et al, 2012), and risk factors relevant to these disorders (Lederbogen et al, 2011; Meyer-Lindenberg and Tost, 2012; Tost and Meyer-Lindenberg, 2012; Akdeniz et al, 2014).
Here, we hypothesized that the pACC regulates HPA axis function as measured with the CAR. We combined multimodal structural (using voxel-based morphometry, VBM) and functional magnetic resonance imaging (fMRI) with an established psychosocial stress protocol (Pruessner et al, 2008) in healthy human participants to test this hypothesis.
Materials and Methods
Subjects
Functional and structural MRI data from 25 healthy participants (mean age±SD 41.9±15.2 years, 14 women (mean age±SD 40.6±15.7, range 21–68 years) and 11 men (mean age±SD 43.5±15.3, range 21–67 years)) who took part in a previously reported study including 36 participants with a different focus (Lederbogen et al, 2011) and for whom the CAR has been assessed were reanalyzed to identify neural correlates of the CAR. One participant from the foregoing study showed a CAR>3 SD above the group mean and was therefore excluded from the present analyses as an outlier. All participants were examined by a trained physician who also obtained a medical history of current or past treatment of a psychiatric disorder. Exclusion criteria included left-handedness, a lifetime history of significant general medical, psychiatric, or neurological illness, prior psychiatric, psychological, or psychotropic pharmacological treatment, and head trauma. Current clinically relevant depression was assessed using the Hamilton Depression Rating Scale with 21 Items (HAMD-21, Hamilton, 1960). No participant had a HAMD-21 score above 4 (mean±SD 0.88±1.2). All participants gave written informed consent to a study protocol approved by the ethics committee of the University of Heidelberg.
CAR Assessment and Quantification
For quantification of basal HPA axis activity, participants were asked to collect four saliva samples on a regular weekday (awakening (t0), as well as 30 min (t30), 8 h (t8), and 14 h (t14) after awakening). The mean±SD temporal distance of the saliva collection from the stress test was 23±34 days (before or after the stress test). Subjects were carefully instructed to refrain from food, drinks other than water, or brushing their teeth before completion of saliva sampling. Sampling times and adherence to the sampling procedure was documented by the participants in a written protocol. Upon receipt, samples were frozen and stored at −80 °C. For cortisol analysis, a time-resolved immunoassay with fluorescence detection was used with coefficients of intra- and interassay variation of <8% (Dressendorfer et al, 1992). Subsequently, the CAR was calculated as the increase in cortisol from the first to the second saliva sample in nmol/l (t30–t0).
Stress Paradigm
Upon arrival, participants gave written informed consent and were allowed to get acquainted to the test hardware outside the scanner. Afterwards, they spend 30 min in a quiet room to acclimatize. Brain function during social evaluative stress was studied using fMRI and the Montreal Imaging Stress Task (MIST) (Dedovic et al, 2005) as detailed by us previously (Lederbogen et al, 2011). Briefly, the task consisted of three different experimental conditions that were repeated twice in each of the three scan runs, lasting 7 min each. In the stress condition, participants were asked to solve cognitively demanding arithmetic problems displayed on a screen. Time pressure was induced by a countdown timer that was continuously adapted to the subjects’ performance levels to ensure error rates in the range of 60–75%. After each incorrect response, a negative visual feedback was displayed. Social-evaluative threat was imposed through negative verbal feedback given by the experimenters between experimental runs. In the control condition, the arithmetic problems were presented and solved without time pressure and any feedback. In the resting condition, subjects observed a blank screen. A T1-weighted structural MRI scan was acquired immediately before the start of the stress test.
Saliva cortisol was measured at seven time points throughout the experimental session (after rest (Cort1), before entering the scanner (Cort2), after the anatomical scan (Cort3), after MIST runs 1 to 3 (Cort4 to 6), after leaving the scanner (Cort7)). The increase in cortisol was calculated as the difference between cortisol samples taken before entering the scanner (Cort2) and at the group maximum cortisol level at the end of the scanning session (Cort7, see Table 1). We used this measure of cortisol reactivity instead of the area under the curve because cortisol samples during MRI scanning were not fully available for two participants. Heart rate was measured continuously during the course of the stress task using an MR-compatible fingertip pulse oximeter. Subjective responses to stress were quantified prior to and after stress induction using an 11-point rating analogue scale ranging from 0 (absence of any subjective stress) to 10 (maximum subjective stress intensity).
Table 1. Saliva Cortisol (Cort1 to Cort7), Heart Rate Systolic and Diastolic Blood Pressure (BP), and Perceived Stress (Visual Analogue Scale) at Seven Time Points throughout the Experimental Session as well as in Response to the Stress Task.
|
Stress task (MIST) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Rest | Pre MRI | After anatomical scan | After MIST Run1 | After MIST Run2 | After MIST Run3 | Post MRI | Stress reactivity and CAR | |
| Cort1 to Cort7 (nmol/l) | 9.0±4.1 | 6.6±3.1 | 7.8±5.3 | 8.2±5.5 | 9.8±7.5 | 12.0±8.7 | 14.8±11.2 | 7.7±10.6 |
| Heart rate (bpm) | 61.2±5.6 | 66.8±5.2 | 66.9±10.2 | 82.1±20.2 | 83.6±21.4 | 83.8±19.6 | 72.5±9.6 | 15.9±19.0 |
| Systolic BP (mm Hg) | 123.7±13.5 | 135.4±15.8 | 11.1±10.4 | |||||
| Diastolic BP (mm Hg) | 79.2±7.9 | 87.8±8.5 | 8.4±8.6 | |||||
| Perceived stress | 2.5±1.2 | 7.2±1.7 | 4.7±2.0 | |||||
| CAR (nmol/l) | 7.2±8.3 | |||||||
The Cortisol awakening response (CAR) was measured on a separate day as the increase in saliva cortisol within the first 30 min after awakening, Cort1 to Cort7: Cort1=after the initial resting period, Cort2=before entering the scanner, Cort3=after the anatomical MRI scan, Cort4 to Cort6=after MIST runs 1 to 3, Cort7=after leaving the MRI scanner, MIST=Montreal Imaging Stress Task, MRI=Magnetic resonance imaging.
Comparability of Baseline Cortisol Values
As CAR assessment and MIST exposure took place on separate days, we tested whether basal saliva cortisol levels on the day of the CAR assessment and the day of the MIST exposure were comparable. To this end, we used the cortisol data measured on the day of the CAR assessment to predict the cortisol level at the time of Cort2 (pre-scanning baseline) acquisition on the day of the MIST exposure and tested whether there was a significant correlation between the predicted and the measured cortisol level at Cort2. In more detail, we fitted a quadratic function to the cortisol data measured on the day of the CAR assessment with time of measurement (time of t0, t8, t14, acquisition) as predictor and cortisol level at these time points as dependent variable. The cortisol value measured 30 min after awakening (t30) was excluded from this analysis because the CAR reflects an increase in cortisol that is superimposed on the overall diurnal pattern of cortisol secretion (Wilhelm et al, 2007). The parameters from this function were then used to predict the cortisol level at the time of Cort2 assessment on the day of the MIST. We found a significant correlation between the predicted cortisol level at the time of Cort2 acquisition and the actually measured Cort2 cortisol level on the day of the MIST (r=0.42, p<0.05), indicating that the baseline values on both days of measurement (CAR assessment vs MIST exposure) were indeed comparable.
fMRI Data Acquisition and Analysis
Blood-oxygen-level-dependent fMRI was performed on a 3.0 Tesla Siemens Trio scanner using a gradient-echo echo-planar-imaging sequence with the following specifications: repetition time=2000 ms, echo time=30 ms, flip angle=80°, 64 × 64 matrix, 192 mm field of view, 32 axial slices, 4 mm slice thickness, 1 mm gap. In addition, we used a 3D magnetization-prepared rapid gradient echo sequence (TR=1300 ms; TE=3.93 ms) to acquire a T1-weighted scan of the entire brain (alpha=10° sagittal orientation; spatial resolution=1 × 1 × 1.5 mm).
Functional image processing followed previously published procedures using standard processing routines in SPM8 (Wellcome Department of Imaging Neuroscience Group, London, UK, http://www.fil.ion.icl.ac.uk/spm). Briefly, all images were realigned, spatially normalized to the Montreal Neurologic Institute (MNI) template, and smoothed with a 9 mm full-width at half-maximum Gaussian kernel. For functional connectivity analyses, data preprocessing included slice-time correction. For functional activation analysis, separate general linear models were defined for each subject by modeling the alternating fMRI task conditions by convolving a box-car reference vector with the canonical hemodynamic response function implemented in SPM8. To account for residual motion artifacts, the convolved motion regressors from the realignment step were included as nuisance covariates. At the model estimation stage, the data were high-pass filtered with a cutoff of 128 s. Contrast images were calculated for each subject to identify brain regions with greater activation during the stress conditions relative to the control conditions (stress>control).
These individual first-level contrast images were subsequently subjected to group-level statistical inference using multiple regression models with CAR values as covariate of interest, and age, sex, and time of awakening as nuisance covariates. To reflect our a priori hypothesis, significance level for the activation analysis was set to P<0.05 family-wise error (FWE) corrected for multiple comparisons over an a priori defined anatomical mask of the pACC derived from the Harvard Oxford Atlas as available in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) that has previously been used by our group (Lederbogen et al, 2011). We used the FWE correction implemented in SPM8, which is based on Gaussian Random fields theory (Worsley et al, 1996). Our ACC mask covered the pACC (including BA 24 a-c, BA 25, BA 32, and BA 33, see Supplementary Figure S2) as defined by Bush et al, (2000).
Functional Connectivity Analysis
Seed-based functional connectivity between the pACC and the hypothalamus was assessed using the software package Lipsia 2.0 (www.cbs.mpg.de/institute/software/lipsia/index.html). We focused our analysis on low-frequency fluctuations following previously published procedures (Lohmann et al, 2010). First, the preprocessed functional images were band-pass filtered between 0.1 and 0.01 Hz and baseline drifts were removed. Moreover, despiking as implemented in Lipsia 2.0 was used to remove values in a voxel’s blood-oxygen-level-dependent time series above or below 4 standard deviations of the mean value of this voxel’s time series. Next, variance related to the stress task, and several nuisance variables (blood-oxygen-level-dependent time series for white matter, cerebrospinal fluid, and six motion parameters) was removed from every voxel’s time series using a general linear model. Blood-oxygen-level-dependent time series for white matter and CSF were derived from 4 mm spherical masks centered in the deep white matter and the lateral ventricles in MNI152 space. Next, the mean residual time series from voxels within a 4 mm spherical mask around the peak of the CAR effect in the pACC was extracted and Pearson’s correlation coefficients of the averaged time course with all other voxels in the brain were calculated. The resulting correlations were normalized using Fisher’s r-to-z transformation and subjected to a second level analysis as described above. Significance was measured at P<0.05 FWE-corrected for multiple comparisons in a probabilistic mask covering bilateral hypothalamus taken from the WFU pickatlas (Maldjian et al, 2003).
Voxel-Based Morphometry (VBM)
Structural MRI data were preprocessed using SPM8 and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm). Images were corrected for bias-field inhomogeneities and tissue-classified into grey matter (GM), white matter, and cerebrospinal fluid (see the VBM8 user guide for a more detailed description). Resulting GM segments were normalized using DARTEL, and multiplied by the nonlinear components derived from the normalization matrix to preserve local GM values, while accounting for individual differences in brain size. Finally, spatial smoothing with a Gaussian kernel of 10 mm full-width at half-maximum was applied. Preprocessed GM images were then subjected to a multiple regression analysis with CAR values as covariate of interest, and age and sex as nuisance covariates. Significance was defined as detailed for the fMRI activation analysis.
Control for Effects of Participants’ Sex and the Temporal Difference between the CAR Assessment and the Time of the MIST Exposure
In the present study, data on history of menses and history of oral contraceptives use were not available for our female participants. As previous work showed that female menopausal status can affect individual differences in the CAR (Pruessner et al, 1997), we tested whether participants’ sex affected our imaging results. To this end, we extracted contrast estimates and GM volume (GMV) scores for all participants from peak voxels identified in our imaging analyses and used these as dependent variables in a general linear model including sex, the CAR, and the interaction term between these variables as parameters of interest. Moreover, as described above, we statistically controlled for sex effects in all voxel-wise imaging analyses.
As the temporal distance between the day of the CAR assessment and the day of the MIST exposure may have affected our results, we included the temporal difference between both days as an additional nuisance variable in the general linear model described above and re-run all imaging analyses. Notably, none of the reported neuroimaging results was affected by including the temporal distance as a nuisance variable into the models.
Results
Response to Stress Induction
Across all participants, performance of the fMRI experiment resulted in significant increases in salivary cortisol (P<0.001), heart rate (P<0.001), systolic and diastolic blood pressure (P<0.001), and subjective feelings of stress (visual analogue scale, P<0.001), indicating that stress was successfully induced in the scanner. Mean endocrine, cardiovascular, and subjective stress responses as well as mean magnitude of the CAR are presented in Table 1. Individual differences in the CAR were neither significantly associated with stress-induced cortisol (r=0.10, P=0.63) nor the group peak cortisol level (Cort7, r=0.19, P=0.37).
At the neural level, corresponding to our previously reported work (Lederbogen et al, 2011), the stress condition resulted in significant differential activation in a distributed network of brain regions including core regions of the extended limbic circuitry such as pACC, hippocampus, amygdala, and hypothalamus (all P-values<0.01, FWE corrected for the whole brain, see Supplementary Figure S1).
CAR and Brain Function
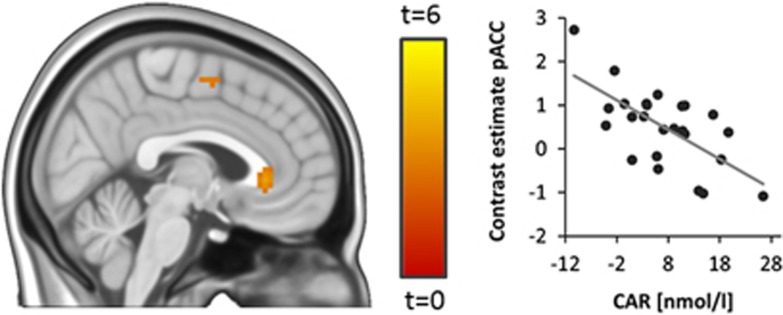
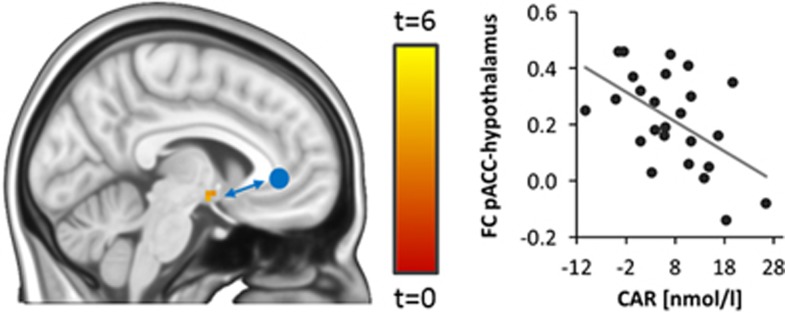
Investigating associations between the CAR (defined as the increase in saliva cortisol within the first 30 min after awakening, t30–t0) and stress-related brain activity, we found a significant inverse relationship between the CAR and stress-related activity in a pregenually located area within our pACC region of interest (MNI 0 30 0, T1,20=4.02, p<0.05, FWE-corrected for bilateral pACC; Figure 1, see Table 2 for results of an exploratory whole-brain analysis). Overall, brain activity in this pACC region increased during stress (MNI 0 33 3, T1,20=4,81, p=0.01, FWE corrected for bilateral pACC). Participants’ sex did not affect this relationship (sex by CAR interaction: F1,21=0.24, P=0.63) We reasoned that, if the pACC region showing the association with the CAR was directly involved in neural control of the HPA axis, then it should functionally interact with the hypothalamus, the final controller of HPA axis activity. Moreover, individual differences in the magnitude of the CAR should be associated with the strength of these interactions. We measured functional connectivity between the pACC and the hypothalamus to test these hypotheses and found that both regions were significantly positively functionally connected during stress (MNI −6 0 −9, T1,20=11.42, P<0.001, FWE-corrected for hypothalamus). Moreover, individual differences in the CAR were inversely associated with the strength of functional connectivity between the pregenual pACC and the hypothalamus (MNI −6 −3 −12, T1,20=4.39, P<0.05, FWE-corrected for hypothalamus, Figure 2). Again, participants’ sex did not affect this relationship (sex by CAR interaction: F1,21=0.98, P=0.33)
Figure 1.
CAR and stress-related activity. Individual differences in the CAR (assessed as the increase in cortisol within the first 30 min after awakening, t30–t0) predict stress-related brain activity in a pregenual area of the pACC (T1,21=4.04, p<0.05, FWE-corrected for bilateral pACC, shown at P<0.005 uncorrected for display purposes).
Table 2. Brain Regions Showing Associations Between Stress-Related Activity and Individual Differences in the CAR (voxel P<0.001 uncorrected).
|
MNI coordinate (mm) |
||||
|---|---|---|---|---|
| Region | Peak T value (df=21) | x | y | z |
| Insula right | 6.14 | 45 | −12 | 0 |
| Insula left | 5.48 | −51 | −9 | 0 |
| Middle temporal gyrus | 4.49 | 39 | −3 | −39 |
| Post central gyrus | 4.38 | −51 | −18 | 45 |
| pACC | 4.04 | 0 | 30 | 0 |
| Inferior frontal gyrus | 3.96 | 60 | 12 | 21 |
Figure 2.
CAR and pACC functional connectivity (FC). FC between the pregenual pACC (seed region indicated as blue circle) and the hypothalamus was associated with individual differences in the CAR (assessed as the increase in cortisol within the first 30 min after awakening, t30–t0, T1,21=4.39, P<0.05, FWE-corrected for hypothalamus, shown at P<0.005 uncorrected for display purposes).
In order to investigate the specificity of our functional imaging finding for the CAR, we tested whether individual differences in stress-induced cortisol predicted stress-related brain activity in the pACC. At a liberal uncorrected level of p<0.01, stress-induced cortisol predicted stress-related brain activity in the anterior pACC (MNI 6 54 0, Tdf=21=2.65). This effect did not survive correction for multiple testing (p>0.30 FWE-corrected for bilateral pACC) and did not overlap with the pACC area showing the association with the CAR.
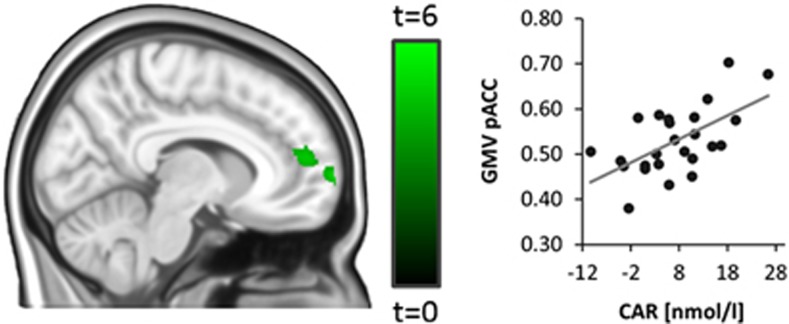
CAR and Brain Structure
We conducted a VBM analysis to identify associations between the CAR and GMV in the pACC. Individual differences in the CAR showed a significant positive association with local GMV in the dorsal anterior pACC (MNI 8 51 13, T1,20=4.10, p<0.05 FWE-corrected for bilateral pACC, Figure 3). This effect extended into the adjacent medial prefrontal cortex and did not overlap with the pregenual pACC area identified in our functional imaging analysis. The relationship between pACC GMV and the CAR was not affected by participants’ sex (sex by CAR interaction: F1,21=0.07, P=0.79). No voxels in the pACC showed a significant association with stress-induced cortisol even at a liberal uncorrected threshold of p<0.01.
Figure 3.
CAR and grey matter volume (GMV). GMV in the dorsal anterior pACC is associated with individual differences in the CAR (assessed as the increase in cortisol within the first 30 min after awakening, t30–t0, T1,21=4.27, p<0.05 FWE-corrected for bilateral pACC, shown at P<0.001 uncorrected for display purposes). This effect extends into the adjacent medial prefrontal cortex and is located anterior to the pregenual pACC region identified in our functional imaging analysis (see Figure 1).
In order to test whether there was a relationship between GMV in the dorsal anterior pACC and stress-related activity in the pregenual pACC, we extracted GMV and stress-related activity from peak voxels identified in our VBM and brain activity analyses for all subjects and calculated the partial correlation between these extracted values controlling for age and sex. We found a significant negative relationship between GMV in the dorsal anterior pACC and stress-related activity in the pregenual pACC (partial r=−0.52, p<0.05).
Discussion
The current study provides evidence for a link between pACC structure, function, and connectivity with the CAR that supports a role of this brain region in the neural control of the HPA axis in humans.
Consistent with the established role of the ACC in HPA axis inhibition, we found lower stress-related brain activity in the pACC in individuals with a higher CAR. One mechanism through which the ACC inhibits HPA axis activity is glucocorticoid receptor-mediated feedback control (Ahima and Harlan, 1990; Diorio et al, 1993; Boyle et al, 2005). As cortisol increased in response to the present stress task, there is a possibility that the inverse association between the CAR and pACC activity is affected by such feedback-related glucocorticoid receptor signaling in response to stress-induced cortisol. However, pACC activity was not associated with the task-induced cortisol increase and appears therefore not to reflect neural processes directly involved in glucocorticoid receptor-mediated feedback signaling.
In line with previous work (Schmidt-Reinwald et al, 1999), individual differences in the CAR were not associated with stress-induced cortisol, suggesting that the psychosocial stress-induced increase in HPA axis activity and the CAR represent different aspects of HPA axis functioning. The CAR is a measure of basal HPA axis regulation and might as such more strongly be affected by brain mechanism involved in the tonic control of HPA axis activity than the stress-induced change in saliva cortisol. The brain mineralocorticoid receptor binds cortisol with high affinity and is essential for the maintenance of the basal circadian rhythm and tonic regulation of the HPA axis (Joels et al, 2008). Mineralocorticoid receptors are expressed with high density in the hippocampus and, to a lesser extent, in the cerebral cortex including the medial prefrontal cortex (Patel et al, 2000). It has recently been shown that genetic variation in the mineralocorticoid receptor gene affects the magnitude of the CAR after ingestion of a low dose of dexamethasone, a synthetic glucocorticoid, supporting a role of the mineralocorticoid receptor in the CAR (van Leeuwen et al, 2010). On the basis of these data, we speculate that individual differences in mineralocorticoid receptor signaling in the pACC might play a role in the CAR. Future studies are needed to test this hypothesis directly.
Consistent with our brain activity findings, the CAR was positively associated with GMV in the dorsal anterior pACC. This structural phenotype is in good agreement with the previous literature linking the CAR to psychosocial risk. Childhood trauma, a major environmental risk factor for important mental illnesses, has been associated with a lower CAR and reduced GMV in the ACC in most (Cohen et al, 2006; Heim et al, 2009; Mangold et al, 2010) but not all studies (Lu et al, 2013). The latter study also reported a negative association between cingulate gyrus volume and the CAR. However, this effect was restricted to the middle cingulate gyrus, which was not in the focus of the present study. A lower CAR and reduced pACC GMV have also been found in patients with PTSD (Karl et al, 2006; Wessa et al, 2006). The link to major depression is less clear as it has typically been found associated with reduced pACC GMV (Bora et al, 2012) but linked to an increase in the CAR in most (Vreeburg et al, 2009; Lamers et al, 2013) but not all studies (Stetler and Miller, 2005). However, subclinical depressive symptoms in a community sample have been linked to a lower CAR (Dedovic et al, 2010). Given that we studied healthy participants, who were carefully screened for depression, the directionality of our structural imaging data is in line with this latter finding. The present finding of a relationship between pACC GMV and the CAR in healthy individuals support a speculation that alterations in pACC GM structure may mediate changes in HPA axis function, which may in turn contribute to the development of psychiatric disorders, such as MDD, schizophrenia, and PTSD in traumatized individuals. Although the present study cannot address whether changes in pACC GMV are primary or secondary to alterations in HPA axis function, it appears plausible that stress-induced HPA axis alterations could cause changes in pACC GMV as the ACC is a well-known target of glucocorticoid action.
The negative relationship between GMV in the dorsal anterior pACC and stress-related activity in the pregenual pACC suggest that both pACC regions participate in a neural system involved in control of the CAR. Given that stress-related activity in the dorsal anterior pACC was not associated with the CAR, higher GMV in this pACC region could reflect a compensatory increase in GMV associated with lower reactivity of the pregenual pACC to stress. However, future studies, including longitudinal approaches, are necessary to examine the relationship between pACC GMV and stress-related activity with respect to the CAR in more detail.
Owing to its dense connections to downstream limbic structures including the hypothalamus (Etkin et al, 2011), and supported by functional imaging and electrophysiological studies, the pACC is considered to stand at the top of the response hierarchy that regulates stress-related HPA axis activation (Rosenkranz et al, 2003; Pezawas et al, 2005; Ulrich-Lai and Herman, 2009). Moreover, individual differences in fiber bundle asymmetry in the cingulate cortex have been linked to the CAR in a recent MRI study using diffusion tensor imaging (Madsen et al, 2012). Our finding of lower functional connectivity between the pACC and the hypothalamus in individuals with a higher CAR indicates that individual differences in pACC–subcortical interactions also play a role in the CAR and thus provides a neural mechanism through which altered pACC functioning may affect the CAR.
Here, we focused our analyses on the pACC, a brain region known to be involved in neural control of the HPA axis and the pathophysiology of various stress-related mental disorders. An exploratory whole-brain analysis did not reveal associations between stress-related brain activity and the CAR in other known regulators of the HPA axis, importantly the amygdala and the hippocampus, even at an uncorrected level. Although this finding does not exclude a role of the amygdala and the hippocampus in the CAR, it suggests that pACC networks involved in psychosocial stress play a specific role in this measure of HPA axis reactivity.
This first study of the neural regulation of the CAR has several limitations. Owing to the correlational and cross-sectional nature of our data, we cannot infer directionality or causality of our links between pACC structure and function and HPA axis reactivity. However, as we have discussed, our observations fit with preclinical evidence and clinical observation in at-risk and patient populations. Secondly, as our participants were healthy, any implications related to psychiatric risk are suggested by previous literature, not the data presented here. Thirdly, although the CAR has been shown to be a longitudinally stable readout of HPA axis activity (Wust et al, 2000b), it is affected by several situational factors, such as chronic stress, weekday to weekend differences, and failure of adherence to the sampling protocol which affect day-to-day variability. In addition, the degree of intra-individual variability of the CAR has been reported to be associated with subject age and sex (Almeida et al, 2009) and seasonality, ie, the tendency to show seasonal variation in mood, is associated with the CAR (Thorn et al, 2009). As we measured the CAR with a substantial temporal distance from the stress test, individual differences in seasonality may have affected our results. Here, we aimed at minimizing these state effects on our imaging results by conducting the CAR assessment as well as the stress test on a regular weekday and instructing the participants to adhere closely to the sampling protocol and to report the exact time point of awakening and saliva sampling. Moreover, we statistically controlled for age and sex in all imaging analyses and tested whether controlling for the temporal distance between the CAR assessment and the MIST exposure affected our findings, which was not the case. Nevertheless, we cannot exclude the possibility that some of the inter-individual variability in the CAR in the present sample is explained by such situational factors that may have affected our imaging results. As it has been reported that aggregation of CAR measurements from different days increases its independence from situational factors (Almeida et al, 2009) and thus reduces intra-individual variability, we recommend this strategy for future studies. Fourth, we measured saliva cortisol at two time points after awakening only. Measuring cortisol more often would allow a more fine-grained examination of the relationship between morning HPA axis activity and stress-related brain activity as well as brain structure. Finally, it must be borne in mind that functional and structural MRI parameters only indirectly measure neuronal function and integrity.
With these caveats, our data provide initial evidence for a role of a stress-related pACC-hypothalamic circuit in neural control of the CAR in humans. Given that the pACC has been implicated strongly in the pathophysiology of major psychiatric disorders including MDD and PTSD, as well as environmental risk for mental illness, these findings may aid future research on the pathophysiology of stress-related mental disorders.
Funding and Disclosure
The research leading to these results has received funding from the European Community’s Seventh Framework Program under grant agreement No. HEALTH-F2-2010-241909 (Project EU-GEI), German Research Foundation (Deutsche Forschungsgemeinschaft SFB 636-B7) and Federal Ministry of Education and Research (MooDS) to A.M.L. EU-GEI is an acronym for the project ‘‘European network of National Schizophrenia Networks Studying Gene–Environment Interactions’’. Dr Meyer-Lindenberg has received consultant fees and travel expenses from Alexza Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Defined Health, Decision Resources, Desitin Arzneimittel, Elsevier, F. Hoffmann–La Roche, Gerson Lehrman Group, Grupo Ferrer, Les Laboratoires Servier, Lilly Deutschland, Lundbeck Foundation, Outcome Sciences, Outcome Europe, PriceSpective, and Roche Pharma and has received speaker’s fees from Abbott, AstraZeneca, BASF, Bristol-Myers Squibb, GlaxoSmithKline, Janssen- Cilag, Lundbeck, Pfizer Pharma, and Servier Deutschland. No other disclosures were reported.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology 35: 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE (1990). Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience 39: 579–604. [DOI] [PubMed] [Google Scholar]
- Akdeniz C, Tost H, Streit F, Haddad L, Wust S, Schafer A et al (2014). Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry 71: 672–680. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS (2009). Interindividual differences and intraindividual variability in the cortisol awakening response: an examination of age and gender. Psychol Aging 24: 819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJ (2003). Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology 28: 121–137. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yucel M (2012). Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord 138: 9–18. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y et al (2005). Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA 102: 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A (2009). Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol 80: 265–278. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D et al (2006). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry 59: 975–982. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG (2006). Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res 40: 550–567. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F (2005). Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6: 463–475. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Engert V, Duchesne A, Lue SD, Andrews J, Efanov SI et al (2010). Cortisol awakening response and hippocampal volume: vulnerability for major depressive disorder? Biol Psychiatry 68: 847–853. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC (2005). The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci 30: 319–325. [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 13: 3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ (1992). Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol 43: 683–692. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Radua J, McGuire P, Borgwardt S (2012). Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull 38: 1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM (2011). The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry 69: 301–308. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nater UM, Maloney E, Boneva R, Jones JF, Reeves WC (2009). Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Arch Gen Psychiatry 66: 72–80. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29: 1201–1213. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Issa K, Schik G, Wolf OT (2006). The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology 31: 900–904. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H, DeRijk R, de Kloet ER (2008). The coming out of the brain mineralocorticoid receptor. Trends Neurosci 31: 1–7. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A (2006). A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 30: 1004–1031. [DOI] [PubMed] [Google Scholar]
- Kupper N, de Geus EJ, van den Berg M, Kirschbaum C, Boomsma DI, Willemsen G (2005). Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology 30: 857–868. [DOI] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW (2013). Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 18: 692–699. [DOI] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P et al (2011). City living and urban upbringing affect neural social stress processing in humans. Nature 474: 498–501. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Hoehl S, Brauer J, Danielmeier C, Bornkessel-Schlesewsky I, Bahlmann J et al (2010). Setting the frame: the human brain activates a basic low-frequency network for language processing. Cereb Cortex 20: 1286–1292. [DOI] [PubMed] [Google Scholar]
- Lu S, Gao W, Wei Z, Wu W, Liao M, Ding Y et al (2013). Reduced cingulate gyrus volume associated with enhanced cortisol awakening response in young healthy adults reporting childhood trauma. PloS One 8: e69350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KS, Jernigan TL, Iversen P, Frokjaer VG, Mortensen EL, Knudsen GM et al (2012). Cortisol awakening response and negative emotionality linked to asymmetry in major limbic fibre bundle architecture. Psychiatry Res 201: 63–72. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mangold D, Wand G, Javors M, Mintz J (2010). Acculturation, childhood trauma and the cortisol awakening response in Mexican-American adults. Horm Behav 58: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannie ZN, Harmer CJ, Cowen PJ (2007). Increased waking salivary cortisol levels in young people at familial risk of depression. Am J Psychiatry 164: 617–621. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, R Hariri A, Pezawas L, Blasi G et al (2006). Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 103: 6269–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Tost H (2012). Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci 15: 663–668. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Dazzan P, Hepgul N, Di Forti M, Aas M, D'Albenzio A et al (2010). Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res 116: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL (2008). The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31: 464–468. [DOI] [PubMed] [Google Scholar]
- Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF (2000). Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res 34: 383–392. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS et al (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8: 828–834. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C et al (2008). Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry 63: 234–240. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S et al (1997). Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61: 2539–2549. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ (2003). Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom Med 65: 92–99. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA (2003). The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci 23: 11054–11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurmeyer TH et al (1999). The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci 64: 1653–1660. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE (2005). Blunted cortisol response to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contacts. J Abnorm Psychol 114: 697–705. [DOI] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, Evans P, Clow A (2009). The cortisol awakening response, seasonality, stress and arousal: a study of trait and state influences. Psychoneuroendocrinology 34: 299–306. [DOI] [PubMed] [Google Scholar]
- Tost H, Meyer-Lindenberg A (2012). Puzzling over schizophrenia: schizophrenia, social environment and the brain. Nat Med 18: 211–213. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP (2009). Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen N, Kumsta R, Entringer S, de Kloet ER, Zitman FG, DeRijk RH et al (2010). Functional mineralocorticoid receptor (MR) gene variation influences the cortisol awakening response after dexamethasone. Psychoneuroendocrinology 35: 339–349. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R et al (2009). Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 66: 617–626. [DOI] [PubMed] [Google Scholar]
- Vrshek-Schallhorn S, Doane LD, Mineka S, Zinbarg RE, Craske MG, Adam EK (2013). The cortisol awakening response predicts major depression: predictive stability over a 4-year follow-up and effect of depression history. Psychol Med 43: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, Flor H (2006). Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology 31: 209–215. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S (2007). Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology 32: 358–366. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C (2000. a). Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology 25: 707–720. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C (2000. b). The cortisol awakening response—normal values and confounds. Noise Health 2: 79–88. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.