Abstract
Objectives:
To evaluate the relationship between alpha epithelial sodium channel (alpha-ENaC) T663A polymorphism and the risk of essential hypertension.
Methods:
This meta-analysis was conducted between November 2014 and February 2015 in Shanghai Medical Instrumentation College, Shanghai, China. We collected all published available case-control data (N=12) identified through PubMed, Web of Science, Scopus, and Chinese National Knowledge Infrastructure (CNKI) up to December 2014. The pooled odds ratio (OR) with 95% confidence interval (CI) was calculated using the fixed- or random-effect model.
Results:
Although subgroup analysis showed that alpha-ENaC T663A polymorphism was associated with essential hypertension in North American individuals (OR=1.55, 95% CI=1.22-1.98, p=0.0003), our meta-analysis results did not confirm such association overall (OR=1.03, 95% CI=0.92-1.15, p=0.62). The lack of association was further confirmed by the non-superiority test (p<0.0001).
Conclusion:
Alpha-ENaC T663A polymorphism might not be a risk factor for essential hypertension.
Hypertension is one of the most important risk factor for cardiovascular disease.1-3 However, its etiology in the vast majority of cases (~90%) is unknown, and thus the essential hypertension term is employed to describe such situations.4 Nowadays, essential hypertension is considered as a multifactorial disease resulting from the interplay of many genetic, environmental, and behavioral factors.5 Among them, sodium has long been deemed as one of the pivotal environmental factors due to its direct regulatory effect on blood pressure.5,6 The amiloride-sensitive epithelial sodium channel (ENaC) lies in the collecting duct of the kidney, and regulates sodium reabsorption. This channel is composed of 3 homologous subunits: alpha, beta, and gamma. Several studies have reported that mutations in beta-ENaC or gamma-ENaC can result in constitutive sodium reabsorption, thus leading to the development of an autosomal-dominant Mendelian hypertensive disorder, Liddle syndrome.7-11 Therefore, some “milder” mutations or functional polymorphisms were assumed to play some etiological roles in essential hypertension. Following this hypothesis, recent studies have reported some potential associated variants.12 Among them, T663A polymorphism in the alpha-ENaC gene has attracted some attention due to its reported ability to influence the channel activity.13,14 The A allele of T663A polymorphism could reduce the surface expression of ENaC, and the T allele of T663A polymorphism could increase ENaC activity.13,14 Accordingly, Ambrosius et al15 observed that the A allele of T663A polymorphism was associated with being normotensive in Blacks and Caucasians. However, a similar study performed in a Japanese population indicated that the A allele of T663A polymorphism was enriched in essential hypertensive patients,16 and Wang et al17 found that there was a lack of association between the T663A polymorphism and essential hypertension in 2 ethnic groups in China. Therefore, there is still a controversy over the association between T663A polymorphism in the alpha-ENaC gene and essential hypertension. Therefore, we performed a meta-analysis to investigate the relationship between alpha-ENaC T663A polymorphism and essential hypertension.
Methods
Literature search
A systematic literature search of PubMed, Web of Science, Scopus, and Chinese National Knowledge Infrastructure (CNKI) databases was conducted by 2 researchers independently for all relevant articles published before December 2014. The research keywords included “primary hypertension,” “essential hypertension,” “ENaC,” “T663A,” “variant,” “genotype,” “SNP,” “mutation” and “polymorphism”. We also manually searched the reference lists of the included studies to find additional eligible studies.
Inclusion and exclusion criteria
The criteria of the inclusion of studies for this meta-analysis should meet the following: 1) case-control studies or cohort studies focusing on the association between T663A polymorphism and essential hypertension; 2) hypertension was defined as systolic blood pressure (SBP) >140 mm Hg and/or diastolic blood pressure (DBP) >90 mm Hg, or taking antihypertensive medications; and 3) the numbers of patients and normotensive people with different genotypes were available for data extraction. The exclusion criteria of the meta-analysis were: 1) studies involving animals; 2) meta-analyses/systematic reviews, reviews/mini-reviews, meeting abstracts/conference abstracts, or editorials; and 3) studies with incomplete or duplicate data.
Data extraction
Information was extracted from all eligible studies and checked. The collected information included the first author’s name, publication date, population, cases’ and controls’ genotypes, method for genotyping, and the numbers of cases and controls.
Statistical analysis
We used Review Manager 5.2 (Cochrane Collaboration, Oxford, United Kingdom) and Stata version 12.0 (Stata Corporation, Lakeway Drive College Station, Texas, USA) for all the statistical analysis. The association was evaluated in the allelic model (mutation allele versus wild allele). We calculated the OR and 95% CI for each study, as well as the combined OR and corresponding 95% CI for all the included studies. The heterogeneity between individual studies was assessed using Chi-square-based Q-tests with the significance level set at p<0.1. If the heterogeneity existed among the included studies, we calculated the pooled OR using the random-effect model (the DerSimonian and Laird method). Otherwise, we adopted the fixed-effect model (the Mantel-Haenszel method). The significance of the pooled OR was assessed by Z-test with p<0.05 considered significant. Sensitivity analysis with a single individual study omitted each time was also performed to check the stability of the meta-analysis results. The non-superiority test was conducted to confirm the absence of the association between alpha-ENaC T663A polymorphism and essential hypertension as described.18,19 For each study, the Hardy-Weinberg equilibrium (HWE) was assessed by Fisher’s exact test with p<0.05 considered statistically significant.20 In the pooled analysis, when studies with controls not in HWE were involved, pooled ORs with these studies included and excluded were calculated to investigate the influence of these studies. The potential publication bias was checked by Begg’s funnel plot, and the funnel plot asymmetry was assessed by Egger’s linear regression test with the significance level set at p<0.05.
Results
Characteristics of the studies
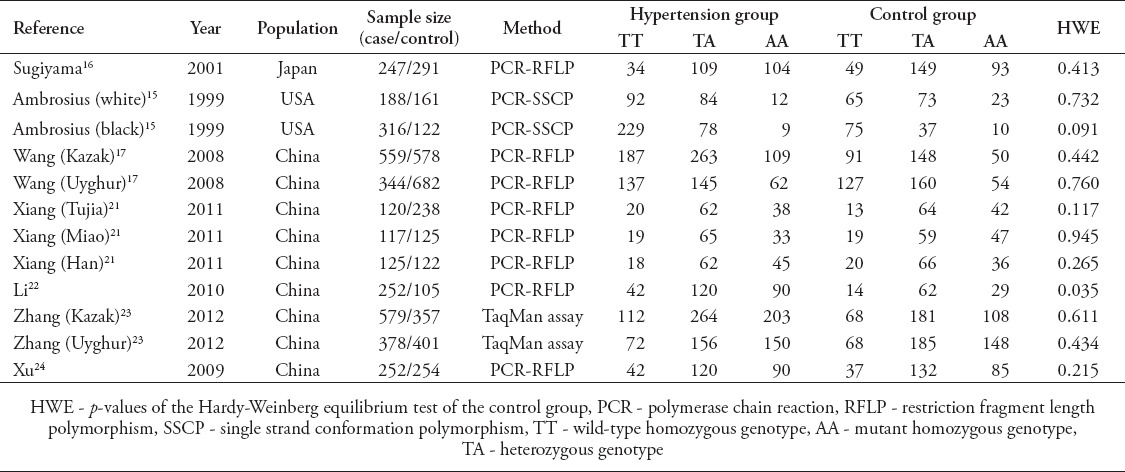
We found a total of 463 articles after an initial search from PubMed, Web of Science, Scopus, and CNKI databases. By reviewing the titles and abstracts, 449 of them were excluded due to no relevance to the association of alpha-ENaC T663A polymorphism with essential hypertension. After excluding reviews/mini-reviews, meta-analyses, studies with incomplete or duplicate data, 7 eligible studies were included for the following pooled analysis.15-17,21-24 Among all eligible studies, 4 articles by Ambrosius et al,15 Wang et al,17 Xiang et al,21 and Zhang et al,23 contained more than one study group, and these different groups were treated separately in the following pooled analysis. Each of the other 3 articles contained only one study group.16,22,24 Thus, in total, 12 case-control studies/groups involving 3,477 cases and 2,687 controls were included in this meta-analysis to evaluate the relationship between alpha-ENaC T663A polymorphism and essential hypertension. Table 1 summarizes the main characteristics of these included studies, including sample size, genotype distribution, and HWE of controls.
Table 1.
Characteristics of the 7 eligible studies included for the investigation of alpha-epithelial sodium channel T663A polymorphism’s association with essential hypertension.
Pooled analysis
Overall, we recruited a total sample size of 6,164 subjects with 3,477 cases and 2,687 controls included for the evaluation of the relationship between alpha-ENaC T663A polymorphism and essential hypertension. Under the allelic model (T allele versus A allele), significant heterogeneity existed, and therefore the random-effect model was adopted to pool the results (Pheterogeneity=0.02, I2=52%). The corresponding meta-analysis result showed that alpha-ENaC T663A polymorphism was not associated with the risk of essential hypertension (OR=1.03, 95% CI=0.92-1.15, p=0.62; Figure 1). To explore the source of the heterogeneity, we performed the subsequent subgroup analysis stratified by continents. We observed that the heterogeneity was significantly lower in the Asia (Pheterogeneity=0.49, I2=0%) and North America (Pheterogeneity=0.53, I2=0%) groups than in the whole population (Pheterogeneity=0.02, I2=52%; Figure 2). Therefore, the regions where the individual studies were performed could explain the source of heterogeneity, which probably reflected the difference in ethnicity. There was still no evident association of alpha-ENaC T663A polymorphism with essential hypertension in the Asia subgroup under the allelic model (T allele versus A allele; OR=0.96, 95% CI=0.89-1.04, p=0.30). However, there was a significant association between alpha-ENaC T663A polymorphism and essential hypertension in the North America subgroup under the allelic model (T allele versus A allele; OR=1.55, 95% CI=1.22-1.98, p=0.0003).
Figure 1.
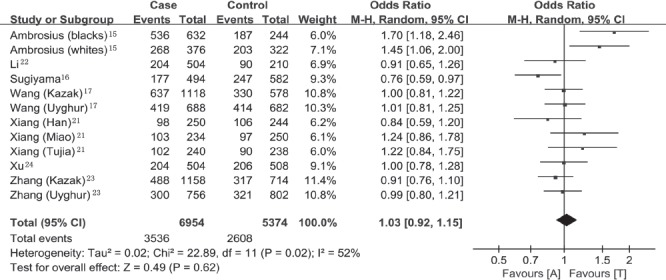
Forest plot of essential hypertension associated with alpha-epithelial sodium channel T663A polymorphism under the allelic model (T allele versus A allele). 95% CI - 95% confidence interval, M-H - Mantel-Haenszel method, df - degrees of freedom
Figure 2.
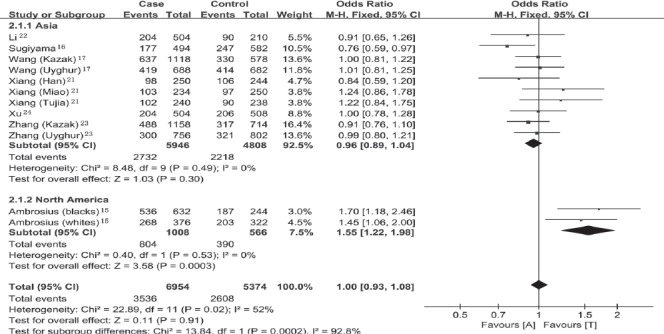
Forest plot of essential hypertension associated with alpha-epithelial sodium channel T663A polymorphism stratified by continents under the allelic model (T allele versus A allele). CI - confidence interval, M-H - Mantel-Haenszel method, df - degrees of freedom
Sensitivity analysis
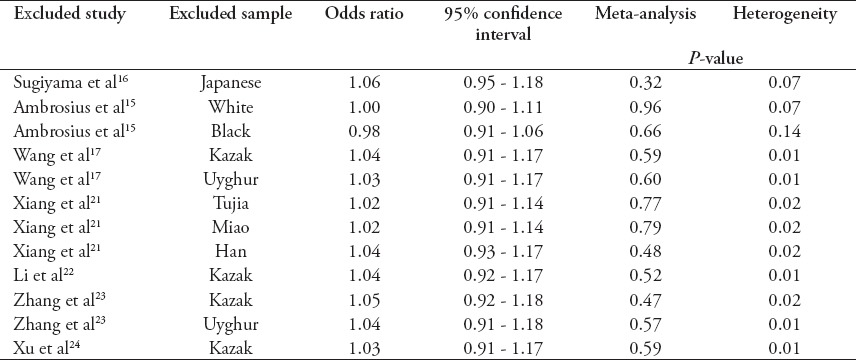
Since there was one study with controls not in HWE,22 we conducted another pooled analysis with this study excluded. The result still indicated that there was a lack of association between alpha-ENaC T663A polymorphism and the risk of essential hypertension under the allelic model (T allele versus A allele; OR=1.04, 95% CI=0.92-1.17, p=0.52). We removed the included studies one at a time and calculated the pooled ORs for the remaining studies to check the stability of our meta-analysis results. As Table 2 indicates, removal of any included studies still led to the nonsignificant association of alpha-ENaC T663A polymorphism with the risk of essential hypertension. Therefore, the meta-analysis results illustrated by Figure 1 were reliable and stable.
Table 2.
Sensitivity analysis of the association of alpha-epithelial sodium channel T663A polymorphism with the risk of essential hypertension under the allelic model (T allele versus A allele).
Equivalence-based analysis
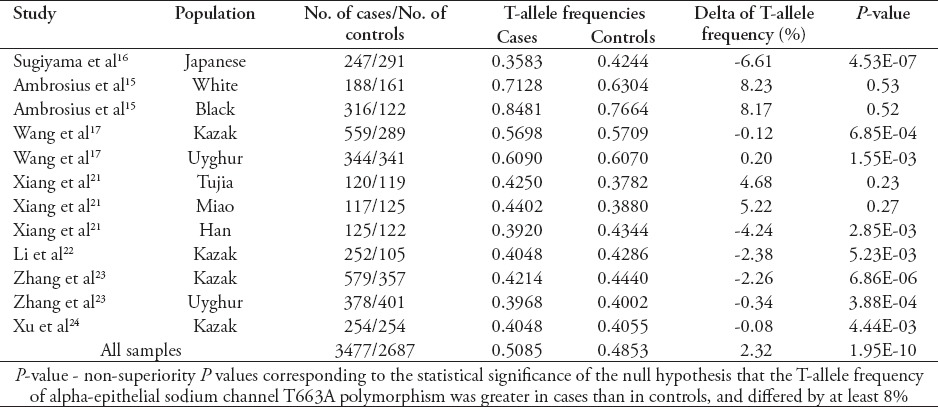
We conducted a non-superiority test to confirm the absence of the association between alpha-ENaC T663A polymorphism and essential hypertension. The null hypothesis was that the frequency of T-allele of alpha-ENaC T663A polymorphism in patients with essential hypertension was greater by 8% than in controls. The 8% excess was set as the lower bound of previous estimations according to the reported difference of this allele between essential hypertension patients and healthy controls in Caucasians (8.3%) and Blacks (8.2%).15 The non-superiority p-values are presented in Table 3. As a whole, the result of the combined case-control samples suggested that the excess of the T allele of alpha-ENaC T663A polymorphism in the cases was lower than 8% (p<0.0001). This result supported the absence of the association between alpha-ENaC T663A polymorphism and the risk of essential hypertension.
Table 3.
Non-superiority test of alpha-epithelial sodium channel T663A polymorphism with essential hypertension.
Publication bias
We used Begg’s funnel plot and Egger’s linear regression test to evaluate the potential publication bias of the included studies. We did not observe obvious asymmetry of the funnel plot under the allelic model (p=0.086). Egger’s linear regression test did not show any significant statistical evidence of publication bias under the allelic model (p=0.098), either. Therefore, no evident publication bias existed in this meta-analysis.
Discussion
The ENaC gene has attracted some attention in the pathogenesis of essential hypertension due to its recognized significance in promotion of sodium reabsorption.13 Therefore, any polymorphisms able to affect the channel activity of ENaC have become appropriate candidates for the study of the etiological mechanism of essential hypertension. Among them, alpha-ENaC T663A polymorphism has been investigated in several reports with regard to its association with essential hypertension.15-17,21-24
In the current meta-analysis, there was a lack of association between alpha-ENaC T663A polymorphism and the risk of essential hypertension. Subsequent sensitivity analysis and non-superiority test supported this result. This is in accordance with Wang et al’s17 former report that there was a lack of association between T663A polymorphism and essential hypertension. As there was significant between-study heterogeneity, we performed the subgroup analysis to explore the source. There was no obvious heterogeneity in the Asia (pheterogeneity=0.49, I2=0%) or North America (pheterogeneity=0.53, I2=0%) groups in our meta-analysis. The reduction of heterogeneity in the subgroup analysis stratified by continents probably reflected the difference in ethnicity, and was consistent with the former report that pointed out that the A allele frequency of the alpha-ENaC T663A polymorphism in the Chinese population (Tujia, Miao, and Han) was not significantly different from that in the Japanese population, but was significantly higher than those in Blacks and Caucasians.21 Besides, although alpha-ENaC T663A polymorphism was not associated with the risk of essential hypertension in the Asia subgroup (T allele versus A allele; OR=0.96, 95% CI=0.89-1.04, p=0.30), it was associated with essential hypertension in the North America subgroup (T allele versus A allele; OR=1.55, 95% CI=1.22-1.98, p=0.0003). However, although alpha-ENaC T663A polymorphism was found to be associated with essential hypertension in the North American population, there is only one relevant report available to date.15 More related studies in the same population are still needed to confirm such association. Collectively, this meta-analysis indicated that different ethnicity or geographical regions might influence the effect of alpha-ENaC T663A polymorphism on the risk of essential hypertension, and published studies did not support the association between alpha-ENaC T663A polymorphism and essential hypertension on the whole. Ethnicity or geographical regions should be matched in future studies to better investigate the relationship between alpha-ENaC T663A polymorphism and essential hypertension.
Some limitations existed in this meta-analysis. The total number of the included studies was small and more relevant studies are called upon to confirm the above meta-analysis results. A similar problem could also be found in the corresponding subgroup analysis stratified by continents. More studies are needed to investigate the role of geographical regions or ethnicity in determining the relationship between alpha-ENaC T663A polymorphism and essential hypertension. Moreover, other clinical factors like age, gender, body mass index, family history, and environment may result in bias. Further investigation is called upon to determine if these factors affect the results of our meta-analysis.
In conclusion, our meta-analysis results did not support the speculation that alpha-ENaC T663A polymorphism was a risk factor for essential hypertension. However, further studies are necessary to verify the above conclusions.
Footnotes
Related Articles.
Alkahtani SA. Pediatric hypertension in the Eastern Province of Saudi Arabia. Saudi Med J 2015; 36: 713-719.
Akbar S, Alorainy MS. The current status of beta blockers’ use in the management of hypertension. Saudi Med J 2014; 35: 1307-1317.
Soudani NY, Fakhoury RM, Kaissi SS, Zgheib NK. The role of genetic polymorphisms in endothelial nitric oxide synthase and beta2-adrenergic receptors with risk of hypertension in a sample of Lebanese people. Saudi Med J 2014; 35: 255-260.
References
- 1.Gonzalez J, Valls N, Brito R, Rodrigo R. Essential hypertension and oxidative stress: New insights. World J Cardiol. 2014;6:353–366. doi: 10.4330/wjc.v6.i6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li YY. Lack of association of A-6G polymorphism of AGT gene with essential hypertension in the Chinese population. J Cardiovasc Med (Hagerstown) 2012;13:505–510. doi: 10.2459/JCM.0b013e328355a726. [DOI] [PubMed] [Google Scholar]
- 3.Oliveras A, de la Sierra A. Resistant hypertension: patient characteristics, risk factors, co-morbidities and outcomes. J Hum Hypertens. 2014;28:213–217. doi: 10.1038/jhh.2013.77. [DOI] [PubMed] [Google Scholar]
- 4.Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- 5.Bolivar JJ. Essential hypertension: an approach to its etiology and neurogenic pathophysiology. Int J Hypertens. 2013;2013:547809. doi: 10.1155/2013/547809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adrogue HJ, Madias NE. Sodium surfeit and potassium deficit: keys to the pathogenesis of hypertension. J Am Soc Hypertens. 2014;8:203–213. doi: 10.1016/j.jash.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Ellison DH. Ubiquitylation and the pathogenesis of hypertension. J Clin Invest. 2013;123:546–548. doi: 10.1172/JCI66882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bubien JK. Epithelial Na+channel (ENaC), hormones, and hypertension. J Biol Chem. 2010;285:23527–23531. doi: 10.1074/jbc.R109.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelheit O, Hanukoglu I, Shriki Y, Tfilin M, Dascal N, Gillis D, et al. Truncated beta epithelial sodium channel (ENaC) subunits responsible for multi-system pseudohypoaldosteronism support partial activity of ENaC. J Steroid Biochem Mol Biol. 2010;119:84–88. doi: 10.1016/j.jsbmb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busst CJ, Bloomer LD, Scurrah KJ, Ellis JA, Barnes TA, Charchar FJ, et al. The epithelial sodium channel gamma-subunit gene and blood pressure: family based association, renal gene expression, and physiological analyses. Hypertension. 2011;58:1073–1078. doi: 10.1161/HYPERTENSIONAHA.111.176370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LP, Gao LG, Zhou XL, Wu HY, Zhang L, Wen D, et al. Genetic diagnosis of Liddle’s syndrome by mutation analysis of SCNN1B and SCNN1G in a Chinese family. Chin Med J (Engl) 2012;125:1401–1404. [PubMed] [Google Scholar]
- 13.Jin HS, Hong KW, Lim JE, Hwang SY, Lee SH, Shin C, et al. Genetic variations in the sodium balance-regulating genes ENaC, NEDD4L, NDFIP2 and USP2 influence blood pressure and hypertension. Kidney Blood Press Res. 2010;33:15–23. doi: 10.1159/000275706. [DOI] [PubMed] [Google Scholar]
- 14.Mueller GM, Yan W, Copelovitch L, Jarman S, Wang Z, Kinlough CL, et al. Multiple residues in the distal C terminus of the alpha-subunit have roles in modulating human epithelial sodium channel activity. Am J Physiol Renal Physiol. 2012;303:F220–F228. doi: 10.1152/ajprenal.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosius WT, Bloem LJ, Zhou L, Rebhun JF, Snyder PM, Wagner MA, et al. Genetic variants in the epithelial sodium channel in relation to aldosterone and potassium excretion and risk for hypertension. Hypertension. 1999;34:631–637. doi: 10.1161/01.hyp.34.4.631. [DOI] [PubMed] [Google Scholar]
- 16.Sugiyama T, Kato N, Ishinaga Y, Yamori Y, Yazaki Y. Evaluation of selected polymorphisms of the Mendelian hypertensive disease genes in the Japanese population. Hypertens Res. 2001;24:515–521. doi: 10.1291/hypres.24.515. [DOI] [PubMed] [Google Scholar]
- 17.Wang XF, Lu XM, Lin RY, Wang SZ, Zhang LP, Qian J, et al. Lack of association of functional variants in alpha-ENaC gene and essential hypertension in two ethnic groups in China. Kidney Blood Press Res. 2008;31:268–273. doi: 10.1159/000151286. [DOI] [PubMed] [Google Scholar]
- 18.Zhong R, Tian Y, Liu L, Qiu Q, Wang Y, Rui R, et al. HBV-related hepatocellular carcinoma susceptibility gene KIF1B is not associated with development of chronic hepatitis B. PLoS One. 2012;7:e28839. doi: 10.1371/journal.pone.0028839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourraud PA. International Multiple Sclerosis Genetics Consortium (IMSGC). When is the absence of evidence, evidence of absence? Use of equivalence-based analyses in genetic epidemiology and a conclusion for the KIF1B rs10492972*C allelic association in multiple sclerosis. Genet Epidemiol. 2011;35:568–571. doi: 10.1002/gepi.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Zhu Z, Wang J, Ye W, Ding Y. Evaluation of association of maternal IL-10 polymorphisms with risk of preeclampsia by a meta-analysis. J Cell Mol Med. 2014;18:2466–2477. doi: 10.1111/jcmm.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang B, Wang XC, Zhou MH, Zhang YJ, Zhong F, Chen ZY, et al. Correlation of the SCNN1A gene polymorphism and the essential hypertension in Tujia, Miao and Han Chinese. J Clin Cardiol. 2011;27:783–786. [Google Scholar]
- 22.Li NF, Xu H, Zhou L, Yang J, Luo WL. The relationship between alpha-ENaC gene T663A and T3593C polymorphisms and blood pressure and serum electrolyte of Kazakhs in Xinjiang. J Med Res. 2010;39:45–49. [Google Scholar]
- 23.Zhang LP, Chen HJ, Yang M, Chen Y, Li H, Ma R. The association between alpha-ENaC gene polymorphism and essential hypertension in Xinjiang Kazaks and Uygurs. J Xinjiang Med Univ. 2012;35:99–1003. [Google Scholar]
- 24.Xu H, Li NF, Hong J, Zhang L, Zhou L, Li T, et al. Relationship between four single nucleotide polymorphisms of epithelial sodium channel alpha subunit gene and essential hypertension of Kazakhs in Xinjiang. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009;31:740–745. doi: 10.3881/j.issn.1000-503X.2009.06.018. Chinese. [DOI] [PubMed] [Google Scholar]


