Abstract
Objectives:
To investigate the protective effects of L-carnitine (LC) on lungs in an experimental obstructive jaundice (OJ) model.
Methods:
This was conducted for 2 months between May 2011 and July 2011 at Suleyman Demirel University School of Medicine Experimental and Clinical Research Center, Isparta, Turkey. Thirty-eight Wistar-Albino rats with an average weight of 250-300 g were divided into 3 groups of control, OJ, and OJ + L-carnitine treatment (LCT). L-carnitine was injected intravenously into the tail vein at a dose of 50 mg/kg/day for 10 days to the LCT group. Animals were sacrificed 10 days later. Enzyme levels were measured in the lung tissue; malondialdehyde, myeloperoxidase (MPO), glutathione peroxidase (GSH-Px), catalase, and superoxide dismutase. Tumor necrosis factor-alfa, interleukin 6 (IL-6), IL-8, and C-reactive protein levels were studied in plasma samples. Histopathological changes in the lungs were examined.
Results:
There was a decreased in GSH-Px, MPO, and IL-8 levels (p<0.05) in the LCT group. The histopathological examination showed that neutrophil leukocyte infiltration and edema formation decreased and destruction of lung parenchyma disappeared following the treatment with LC (p<0.05).
Conclusion:
L-carnitine has a protective effect against lung damage due to experimental obstructive jaundice, possibly by altering anticytokine and antioxidant activity, and by decreasing the neutrophil migration.
Obstructive jaundice (OJ) is one of the most important problems in gastroenterology and general surgery due to negative effects on many systems, high incidence and high morbidity, and mortality rates despite advances in operative techniques, and the development of broad-spectrum antibiotics.1 Obstructive jaundice can be created experimentally by ligation of the main bile duct in rats.2 Endotoxemia is a major complication, which aggravates the inflammatory response.3 Thus, the increased level of free oxygen radicals may cause oxidative damage and multiple organ dysfunctions, including dysfunction of the lungs.4,5 There is also a decrease in antioxidant activity.6 Lipid peroxidation, formed by free oxygen radicals, and protein oxidation causes structural and functional cell damage.7 The accumulation of stimulated inflammatory cells in the small airways and interstitial region of the lung form alveolar epithelial injury, microcirculatory damage, and alveolar interstitial edema. The continuation of this process results in acute respiratory distress syndrome. There have been many studies, which have identified OJ damage to organs such as the liver, kidneys, and the heart, but studies on “lung injury” are few with only 2 articles identified from scanning PubMed literature in English.8 L-carnitine (LC) is a basic transporter that functions in the energy production of mammalian metabolism, detoxification of organic acids, and mitochondrial membrane transport of long-chain fatty acids. It is known that LC has a detoxifying effect, inhibiting tissue damage, and increasing antioxidant enzyme activities.9 The aim of this study was to determine whether LC prevents lung injury that occurs in OJ.
Methods
This experimental study was performed at Suleyman Demirel University School of Medicine Experimental and Clinical Research Center, Suleyman Demirel University, Isparta, Turkey. The duration of the study was 2 months from May 2011 to July 2011.
Thirty-eight male Wistar-Albino rats with an average weight of 250-300 g were used in the study. The rats were kept in standard plastic animal cages in groups and maintained on a 12-hour light-dark cycle at 25±2°C relative humidity 50%±15%. The rats were allowed free access to standard rat chow and water ad libitum. All experiments described in this study were approved by the Süleyman Demirel University Animal Care and Use Committee, and followed the Guide for the Care and use of laboratory animals. The rats were randomly divided into 3 groups: control group (Group I, n=8): the common bile duct was mobilized after laparotomy. Obstructive jaundice group (Group II, n=15): at laparotomy, the common bile duct was ligated then cut to create OJ. The OJ+L-carnitine group (LCT) (Group III; n=15): at laparotomy, the common bile duct was ligated then cut to create obstructive jaundice. L-carnitine (Levocarnitine® 1gr/5ml American Regent, Inc. Shirley, NY, USA) was injected intravenously into the tail vein at a dose of 50 mg/kg/day for 10 days.10
Surgical technique
All the procedures were performed aseptically. The rats were anesthetized using intramuscular injections of 25 mg/kg ketamine hydrochloride (Ketalar®, Parke-Davis, Eczacibasi, Istanbul, Turkey) and 10 mg/kg xylazine (Rompun®, Bayer, Leverkusen, Germany). After shaving the abdomen and preparing the site with 10% povidone-iodine solution, the abdomen was opened through a small upper midline incision. The common biliary duct was identified, mobilized, doubly ligated using 4-0 silk and divided. The control group animals had a similar incision followed by mobilization of the common biliary duct without ligation or division. All abdominal incisions were closed in 2 layers using 3-0 polyglactine (Vicryl®; Ethicon, Woluwe, Belgium) and 4-0 polypropylene (Prolene®; Ethicon, Woluwe, Belgium). After peritoneal saline (2cc) was injected for fluid resuscitation, the abdominal incisions were closed. All groups had access to unlimited consumption of rat chow and tap water until the sampling process.
In all groups, 10 days after the laparotomy, sternotomy was performed under general anesthesia. For biochemical analysis, 5 cc of blood was taken from the aorta before the sacrifice of each rat. The rats were sacrificed by inducing cardiac arrest through bloodletting from the inferior vena cava. After sacrifice, the lungs were extracted. The lung tissue samples taken for pathology were fixed with 10% formalin and the lung tissue for biochemical analysis was put in phosphate buffer.
Biochemical evaluation. Preparation of tissue homogenates
After the procedure, the lung tissues were put in glass tubes and filled with pH 7.4 phosphate buffers. The lung tissues were stored at -80°C, then removed and homogenized before testing (PCV Kinematice Status Homogenizator).
Determination of malondialdehyde (MDA) levels
The analysis of lipid peroxidation was carried out as described with a minor modification.11 The reaction mixture was prepared by adding 250 µL homogenate into 2 mL reaction solution (15% trichloroacetic acid: 0.375% thiobarbituric acid: 0.25 N HCl, 1:1:1, w/v) and heated at 100°C for 15 minute. The mixture was cooled to room temperature, centrifuged (10.000 g for 10 min) and the absorbance of the supernatant was recorded at 532 nm. 1,1,3,3-tetramethoxypropane was used as MDA standard. Malondialdehyde results were expressed as nmol/mg protein in the homogenate.
Determination of glutathione peroxidase (GSH-Px) levels
The formation of 5-thio-2-nitrobenzoate (TNB) was followed spectrophotometrically at 412 nm.12 The amount of GSH in the extract was determined as nmol/mg protein utilizing a commercial GSH-Px as the standard.
Measurement of catalase (CAT) activity
Catalase activity was measured at 37°C by following the rate of disappearance of hydrogen peroxide (H2O2 at 240 nm [e240 = 40 M−1 cm−1]).13 One unit of catalase activity was defined as the amount of enzyme catalyzing the degradation of 1 µmol of H2O2 permin at 37°C and specific activity corresponding to transformation of substrate (in µmol) (H2O2) per min per mg protein.
Measurement of superoxide dismutase (SOD) activity
The activity of SOD was assessed according to the method of Minami and Yoshikawa.14 In brief, this method depends on computing the difference between auto-oxidation of pyrogallol alone and in the presence of a cytosolic fraction that contains the enzyme. Enzymatic activity was expressed as ug/gm of tissue.
Measurement of myeloperoxidase (MPO) activity
Measurement of myeloperoxidase activity in the rat lungs was measured according to a previously described method. Lung samples were homogenized with a homogenizer using 6 mL of a homogenization buffer (50 mmol/L phosphate, pH 6.0). The homogenate was then sonicated and centrifuged at 4.4.500g for 30 minutes at 4°C. Measurement of myeloperoxidase activity in the supernatants was reacted with H2O2 in the presence of o-dianisidine (Sigma). The change in optical density at 460 nm was determined over one minute at 25°C in a spectrophotometer (DU-54; Beckman, Irvine, CA, USA).15
The rat C-reactive protein (CRP) enzyme-linked immunosorbent assay (ELISA) is based on a solid phase ELISA. The rat CRP of serum was performed using a commercial kit (Life Diagnostics, Inc., Westchester, PA, USA), according to the manufacturer’s instructions. Total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were measured as parameters indicative of hepatic function with an autoanalyser (Olympus AU640, Tokyo, Japan).
Histopathological evaluation
The lung tissues fixed in 10% formalin for 24 hours were embedded in paraffin. Tissue sections cut at 5 µm were stained with hematoxylin & eosin (H&E) and Masson’s trichrome methods. Samples were examined using a Leica DFC280 light microscope and a Leica Q Win Image Analysis system (Leica Micros Imaging Solutions Ltd., Cambridge, UK). Assessment of tissue alterations in 20 different fields for each section was conducted by an experienced histologist who was unaware of the treatment. The results were classified into 4 grades that were described by Özdülger et al.16 Grade 1: Normal lung histology, Grade 2: Mild neutrophil infiltration of leukocytes, Grade 3: Moderate neutrophil leukocyte infiltration, perivascular edema formation, the partial destruction of lung structure, and Grade 4: Intensive neutrophil leukocyte infiltration, which defines complete destruction in the pulmonary structure.
Statistical analysis
The data was then entered and analyzed using the Statistical Package for Social Sciences version 18 (SPSS Inc, Chicago, IL, USA). The Kruskal-Wallis test was used for the comparison of the biochemical values of TNF-α, IL-6, IL-8, MDA, MPO, GSH-Px, and CRP with independent samples. As a result of the Kruskal-Wallis test, the Mann-Whitney U test was used to examine the differences between groups, and the Chi-square test was used for comparisons of the histopathological examination of lung tissue on day 10. A value of p<0.05 was accepted as statistically significant.
Results
Biochemical results
The mean TNF-a levels of the control group was 14.88±3.15pg/mL, OJ was 15.21±3.70pg/mL, and LC was 17.56±6.31pg/mL. The difference between the control and the other groups was not significant (p=0.455).
The mean IL-6 levels of the control group was 13.81±3.07pg/mL, OJ 15.46±3.49pg/mL, and LC was 13.75±3.32pg/mL. The difference between the control and the other groups was not significant (p=0.867). The mean IL-8 levels of the control group was 81.86±33.98 pg/mL, OJ was 461.07±201.14 pg/mL, and LC was 153.98±50.72 pg/mL.
The IL-8 levels in the OJ group were significantly elevated in comparison with those in the control and LC groups (p<0.05, Figure 1).
Figure 1.
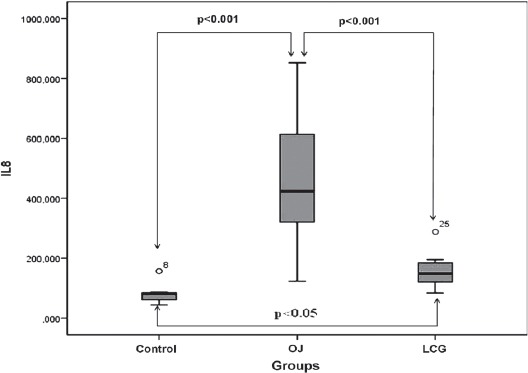
Distribution of serum IL-8 levels among groups. OJ - obstructive jaundice, LCG - L-carnitine group
The mean MDA level of the control group was 1.17± 0.82 nmol/g protein, OJ was 0.83±0.81 nmol/g protein, and the LCT was 1.17±0.89 nmol/g protein. The difference between the control and the other groups was not significant (p=0.136).
The mean MPO levels of the control group was 14056.71±4486.13 nmol/g protein; OJ was 82529.98±40997.88 nmol/g protein, and the LCT was 38305.97±16778.43nmol/g protein. The MPO levels in the OJ group were significantly elevated in comparison with those in the control and LCT groups (p<0.05, Figure 2). The OJ group produced a significant increase in levels as compared with the control group (p<0.05). The MPO levels also increased significantly in LCT compared with the control (p<0.001, Figure 2).
Figure 2.
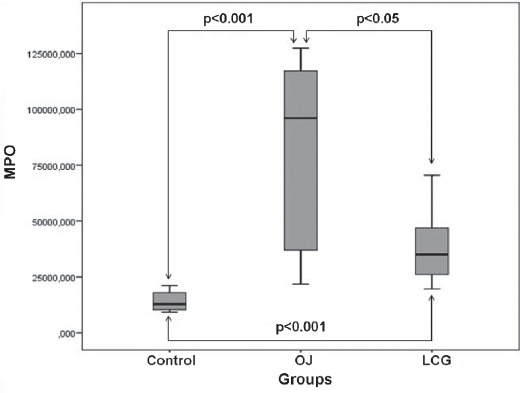
Distribution of pulmonary tissue homogenate myeloperoxidase (MPO) values, as antioxidative parameters for the groups. OJ - obstructive jaundice, LCG - L-carnitine group
The mean GSH-Px level of the control group was 1506.39±127.84 U/g protein, OJ was 1824.05±410.15 U/g protein, and the LCT was 1336.72±203.34 U/g protein. The GSH-Px levels in the OJ group were significantly elevated in comparison with those in the control and LCG groups (p<0.05, Figure 3). The OJ group produced a significant increase in GSH-Px levels compared with the control group (p<0.05). The LCT group had significantly lower GSH-Px levels compared with the control group (p=0.043, Figure 3).
Figure 3.
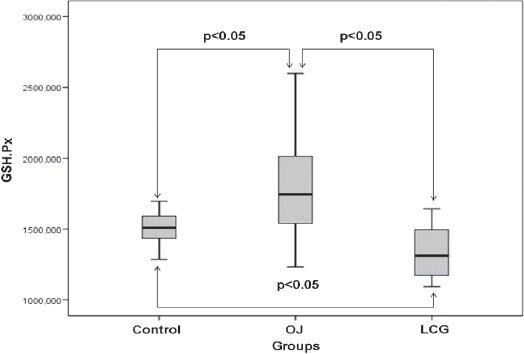
Distribution of pulmonary tissue homogenate GSH-Px values, as antioxidative parameters for the groups. OJ - obstructive jaundice, LCG - L-carnitine group
The mean SOD level of the control group was 208.18±159.98 U/g protein, OJ was 509.20± 587.90 U/g protein, and the LCT was 192.28± 43.11 U/g protein. The difference between the control and the other groups was not significant (p=0.102).
The mean CAT levels of the control group was 682.15±101.79 Uk/g protein, OJ was 677.14±555.74 Uk/g protein, and the LCT was 589.98±363.86 Uk/g protein. The difference between the control and the other groups was not significant (p=0.429).
The mean C-reactive protein (CRP) level of the control group was 514.93±399.50 mg/L, OJ was 868.20±769.07 mg/L, and the LCT was 682.70±661.25 mg/L. The difference between the control and the other groups was not significant (p=0.413).
Histopathological results
In the control group, histopathological examinations of the lung tissue were normal. The specimens from this group were classified as Grade 1 according to the Ozdulger classification.16 The mean lung damage score was (2.50±0.53) in the LCT group, and this score was significantly lower than the score in the OJ group (3.42±0.51) (p<0.05) (Figure 4). Lung damage after OJ injury in the groups is shown in Figure 5. In the histopathological comparison of lung damage, a statistically significant difference was determined between the groups (p<0.05) (Figure 4 & Figure 5).
Figure 4.
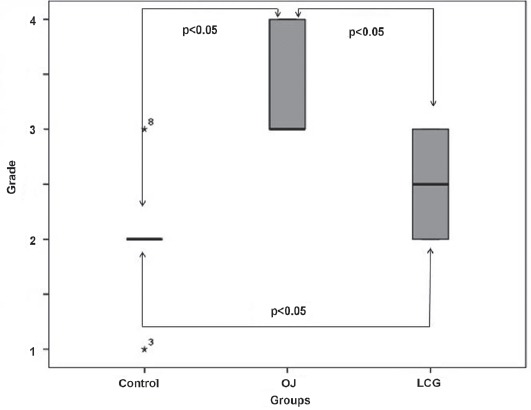
Histopathological scores of pulmonary tissue in the groups. OJ - obstructive jaundice, LCG - L-carnitine group
Figure 5.
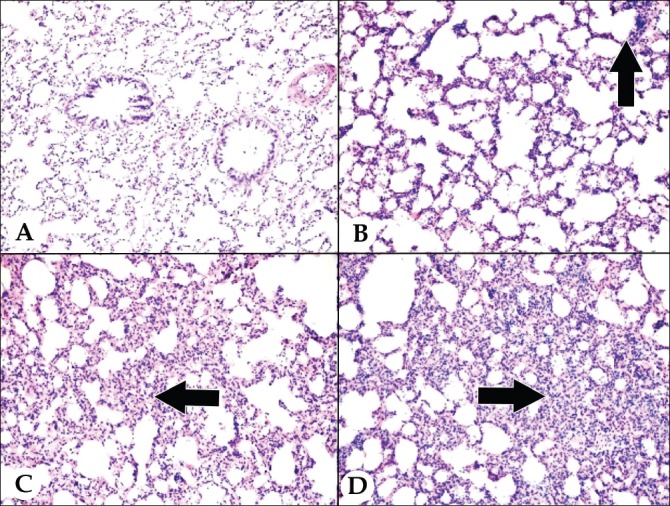
Histopathological image of lung damage; A) Grade 1 lung injury: normal lung histology, B) Grade 2 lung injury: mild neutrophil infiltration of leukocytes (arrow), C) Grade 3 lung injury: moderate neutrophil leukocyte infiltration, perivascular edema formation, the partial destruction of lung structure (arrow), D) Grade 4 lung injury: intensive neutrophil leukocyte infiltration, which defines complete destruction in the pulmonary structure (arrow), (x200; hematoxylin & eosin.
A statistically significant difference was determined between the control group and the OJ group (p<0.05) in respect with grade of the lung damage, with much more severe lung damage in the OJ group. Levels of lung damage in the control group and the LCT group were similar (p=0.085) with no significant difference determined. A statistically significant difference was determined between the OJ group and the LCT group (p<0.05) with less damage in the LCT group (Figure 4).
Discussion
L-carnitine reduces acute lung damage in experimental OJ. Biliary obstruction is associated with an intense state of oxidative stress. Antioxidant defence (as demonstrated by CAT and SOD activities) is diminished and lipid peroxidation (as demonstrated by MDA levels) is increased during extrahepatic OJ in rat models.17,18 Tissue damage associated with OJ may be caused by accelerated generation of hydroxyl radicals. Many clinical observations and experimental studies point to the frequent occurrence of different organ complications in patients with OJ.19 The oxidative stress known to occur as a systemic response to cholestasis could give rise to the involvement of organs other than liver, such as the lung.3,20
Recent in vivo and in vitro studies have demonstrated that LC can prevent oxidative injury including reducing lipid peroxidation, scavenging hydrogen peroxide and superoxide radicals, chelating transition metal ions,21,22 and up-regulating the endogenous antioxidant defence system, by increasing CAT, SOD, and GPx activities.23,24 Due to the obstructive jaundice, there is an excessive release of a number of pro-inflammatory cytokines, such as TNFa, IL-6, and IL-8.25
In this study, when compared with the control group, TNF-a was determined at a higher level in the OJ group, although the difference was not significant. L-carnitine treatment had no effect on the TNF-a level. In the current study, an increase was determined in the IL-6 and IL-8 levels of the rats with OJ. Although LC treatment decreased the IL-6 levels, there was no significant difference between the 2 groups. A statistically significant decrease in the level of IL-8 indicated that LC has a reducing effect on IL-8 levels in lung damage through anticytokine properties. Lipid peroxidation is one of the major causes of the damage and destruction of the cell membrane. Malondialdehyde is the final product of lipid peroxidation, which is a major indicator of oxidative stress.10 The MDA level increases in OJ.26 In the current study, the tissue MDA levels were not increased in the OJ group. L-carnitine administration did not result in any statistically significant differences of tissue levels of MDA between the groups.
It has been reported that in OJ, neutrophil infiltration is observed in the liver and distant internal organs, and active PMN leukocytes in tissue expose enzymes such as MPO. This enzyme both increases tissue damage and causes increased expression of free radicals.27,28 The present study also demonstrated that the serum MPO level decreased significantly because of LC treatment. This also shows that in OJ, LC exhibits a protective effect against neutrophil infiltration. Glutathione peroxidase, CAT, and SOD which remove free oxygen radicals from the medium have declining activity on experimental animals, whose bile duct has been ligated, and so have increased susceptibility to tissue damage from free oxygen radicals.7 It has been reported that neutrophil adhesion constitutes a significant mechanism in the development of chronic pulmonary inflammation, and that this is related to the disturbance of the glutathione redox equilibrium. Furthermore, the correlation of decreased GSH-Px levels with apoptosis possibly linked to the pathogenesis of inflammatory pulmonary diseases has been demonstrated. Similarly, in the present study, it was ascertained that LC increased GSH-Px levels in the pulmonary tissue significantly against pulmonary damage caused by OJ. In the present study, while GSH-Px levels increased in the OJ group, there was no statistically significant reduction in SOD and CAT levels in the LCT group. Therefore, it can be considered that the minimizing effect of LC on lung damage in obstructive jaundice is brought about through antioxidant activity from the GSH-Px pathway.
C-reactive protein serum level is a well-known parameter showing inflammatory activation.29 It provides an increase in inflammation precursors such as IL-6, IL-8 and TNF-a in many cell types. In a study by Barut et al it was determined that in obstructive jaundice, CRP level is predictive for bacterial translocation.9 In the current study, LC was observed to have no effect on decreasing the CRP level, which suggests that LC does not prevent bacterial translocation.
According to Rahman and MacNee, inflammatory cells, which are activated and accumulated in small airways, not only increase the formation of reactive oxygen radicals, but also decrease the factors for the removal of these radicals.30 In the present study, the histopathological examination showed that neutrophil leukocyte infiltration and edema formation decreased and destruction of lung parenchyma disappeared following the treatment with LC. Nevertheless, the limitation of this study is that more studies need to be conducted to establish the beneficial clinical effects of LC therapy.
The results of this experimental study have shown that LC reduced lung damage associated with obstructive jaundice and decreased the levels of IL-8, MPO, and GSH-Px. Further studies are required to strengthen and clarify these results for the clinical treatment of OJ using L- carnitine.
Acknowledgment
The authors would like to thank Selçuk Kaya, Department of Microbiology, Suleyman Demirel University Medical School, for his assistance. We would like to thank Mrs. Caroline J. Walker for assisting with the preparation and editing of the manuscript.
Footnotes
References
- 1.Su CH, P’eng FK, Lui WY. Factors affecting morbidity and mortality in biliary tract surgery. World J Surg. 1992;16:536–540. doi: 10.1007/BF02104465. [DOI] [PubMed] [Google Scholar]
- 2.Assimakopoulos SF, Vagianos CE. Bile duct ligation in rats: a reliable model of hepatorenal syndrome? World J Gastroenterol. 2009;15:121. doi: 10.3748/wjg.15.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papakostas C, Bezirtzoglou E, Pitiakoudis M, Polychronidis A, Simopoulos C. Endotoxinemia in the portal and the systemic circulation in obstructive jaundice. Clin Exp Med. 2003;3:124–128. doi: 10.1007/s10238-003-0015-y. [DOI] [PubMed] [Google Scholar]
- 4.Sakrak O, Akpinar M, Bedirli A, Akyurek N, Aritas Y. Short and long-term effects of bacterial translocation due to obstructive jaundice on liver damage. Hepatogastroenterology. 2003;50:1542–1546. [PubMed] [Google Scholar]
- 5.Kucuk C, Ok E, Yilmaz Z, Sozuer E, Muhtaroglu S, Arar M. The effects of dimethylsulfoxide in experimental obstructive jaundice. Acta Chir Belg. 2003;103:392–395. doi: 10.1080/00015458.2003.11679450. [DOI] [PubMed] [Google Scholar]
- 6.Ogetman Z, Dirlik M, Caglikulekci M, Canbaz H, Karabacak T, Yaylak F, et al. The effect of aminoguanidine on blood and tissue lipid peroxidation in jaundiced rats with endotoxemia induced with LPS. J Invest Surg. 2006;19:19–30. doi: 10.1080/08941930500444396. [DOI] [PubMed] [Google Scholar]
- 7.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 8.Akca T, Canbaz H, Tataroglu C, Caglikulekci M, Tamer L, Colak T, et al. The effect of N-acetylcysteine on pulmonary lipid peroxidation and tissue damage. J Surg Res. 2005;129:38–45. doi: 10.1016/j.jss.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Barut I, Kaya S. The diagnostic value of C-reactive protein in bacterial translocation in experimental biliary obstruction. Adv Clin Exp Med. 2014;23:197–203. doi: 10.17219/acem/37054. [DOI] [PubMed] [Google Scholar]
- 10.Wilson AD, Hart A, Wiberg M, Terenghi G. Acetyl-l-carnitine increases nerve regeneration and target organ reinnervation - a morphological study. J Plast Reconstr Aesthet Surg. 2010;63:1186–1195. doi: 10.1016/j.bjps.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;12:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 12.Theodorus P, Akerboom M, Sies H. Assay of glutathione, glutathione disulfide and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–383. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- 13.Luck H. Catalase. In: Bergmeyer HU, editor. Methods in enzymatic analysis. New York (NY): Academic Press; 1971. p. 885. [Google Scholar]
- 14.Minami M, Yoshikawa H. A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta. 1979;92:337–342. doi: 10.1016/0009-8981(79)90211-0. [DOI] [PubMed] [Google Scholar]
- 15.Warren JS, Yabroff KR, Remick DG, Kunkel SL, Chensue SW, Kunkel RG, et al. Tumor necrosis factor participates in the pathogenesis of acute immune complex alveolitis in the rat. J Clin Invest. 1989;84:1873. doi: 10.1172/JCI114374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozdulger A, Cinel I, Koksel O, Cinel L, Avlan D, Unlu A, et al. The protective effect of N-Acetylcysteine on apoptotic lung injury in cecal ligation and puncture-induced sepsis model. Shock. 2003;19:336–372. doi: 10.1097/00024382-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Jiang WG, Puntis MC, Hallett MB. Neutrophil priming by cytokines in patients with obstructive jaundice. HPB Surg. 1994;7:281–289. doi: 10.1155/1994/74202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yildiz H, Coskuner I, Bulbuloglu E, Silay E, Kurutas EB, Dogan Z, et al. The protective effects of ketamine and propofol in obstructive jaundice: an experimental study. Bratisl Lek Listy. 2012;113:139–144. doi: 10.4149/bll_2012_034. [DOI] [PubMed] [Google Scholar]
- 19.Hatipoğlu AR, Oğuz S, Gürcan S, Yalta T, Albayrak D, Erenoğlu C, et al. Combined effects of tauroursodeoxycholic Acid and glutamine on bacterial translocation in obstructive jaundiced rats. Balkan Med J. 2013;30:362–368. doi: 10.5152/balkanmedj.2013.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White JS, Hoper M, Parks RW, Clements WD, Diamond T. Patterns of bacterial translocation in experimental biliary obstruction. J Surg Res. 2006;132:80–84. doi: 10.1016/j.jss.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Kolodziejczyk J, Saluk-Juszczak J, Wachowicz B. L-carnitine protects plasma components against oxidative alterations. Nutrition. 2011;27:693–699. doi: 10.1016/j.nut.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Mingorance C, Rodrıguez-Rodrıguez R, Justo ML, Alvarez de Sotomayor M, Herrera MD. Critical update for the clinical use of L-carnitine analogs in cardiometabolic disorders. Vasc Health Risk Manag. 2011;7:169–176. doi: 10.2147/VHRM.S14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Augustyniak A, Skrzydlewska E. The influence of L-carnitine supplementation on the antioxidative abilities of serum and the central nervous system of ethanol-induced rats. Metab Brain Dis. 2010;25:381–389. doi: 10.1007/s11011-010-9217-7. [DOI] [PubMed] [Google Scholar]
- 24.Miguel-Carrasco JL, Monserrat MT, Mate A, Vazquez CM. Comparative effects of captopril and l-carnitine on blood pressure and antioxidant enzyme gene expression in the heart of spontaneously hypertensive rats. Eur J Pharmacol. 2010;632:65–72. doi: 10.1016/j.ejphar.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Badger SA, Jones C, McCaigue M, Clements BW, Parks RW, Diamond T, et al. Cytokine response to portal endotoxaemia and neutrophil stimulation in obstructive jaundice. Eur J Gastroenterol Hepatol. 2012;24:25–32. doi: 10.1097/MEG.0b013e32834b0dd3. [DOI] [PubMed] [Google Scholar]
- 26.Dirlik M, Karahan A, Canbaz H, Caglikulekci M, Polat A, Tamer L, et al. Effects of sulfasalazine on lipid peroxidation and histologic liver damage in a rat model of obstructive jaundice and obstructive jaundice with lipopoly-saccharide-induced sepsis. Curr Ther Res. 2009;70:299–315. doi: 10.1016/j.curtheres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 28.Adam M, Gajdova S, Kolarova H, Kubala L, Lau D, Geisler A, et al. Red blood cells serve as intravascular carriers of myeloperoxidase. J Mol Cell Cardiol. 2014;74:353–363. doi: 10.1016/j.yjmcc.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Ramage L, Proudfoot L, Guy K. Expression of C-reactive protein in human lungepithelial cells and upregulation by cytokines and carbon particles. Inhal Toxicol. 2004;16:607–613. doi: 10.1080/08958370490464599. [DOI] [PubMed] [Google Scholar]
- 30.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]


