PvD1 was able to inhibit the proliferation of Leishmania amazonensis promastigotes; PvD1 caused cell membrane permeabilization and alterations in the cytoplasmic contents of these cells; PvD1 was internalized in these cells, what suggests a possible intracellular target.
Keywords: antimicrobial activity, Leishmania amazonensis, Phaseolus vulgaris, plant antimicrobial peptides, plant defensin
Abstract
Plant defensins are small cysteine-rich peptides and exhibit antimicrobial activity against a variety of both plant and human pathogens. Despite the broad inhibitory activity that plant defensins exhibit against different micro-organisms, little is known about their activity against protozoa. In a previous study, we isolated a plant defensin named PvD1 from Phaseolus vulgaris (cv. Pérola) seeds, which was seen to be deleterious against different yeast cells and filamentous fungi. It exerted its effects by causing an increase in the endogenous production of ROS (reactive oxygen species) and NO (nitric oxide), plasma membrane permeabilization and the inhibition of medium acidification. In the present study, we investigated whether PvD1 could act against the protozoan Leishmania amazonensis. Our results show that, besides inhibiting the proliferation of L. amazonensis promastigotes, the PvD1 defensin was able to cause cytoplasmic fragmentation, formation of multiple cytoplasmic vacuoles and membrane permeabilization in the cells of this organism. Furthermore, we show, for the first time, that PvD1 defensin was located within the L. amazonensis cells, suggesting the existence of a possible intracellular target.
INTRODUCTION
Leishmaniasis is an infectious disease that is prevalent worldwide, particularly in tropical and subtropical regions, killing thousands and debilitating millions of people each year. With 2 million new cases reported annually, infection by the Leishmania parasite represents an important global health problem for which there is no vaccine and few effective drugs [1].
The selection of an increasing number of antibiotic-resistant micro-organisms and other antimicrobial agents has attracted the attention of many researchers in an attempt to develop new therapeutic agents [2]. The therapeutic potential of AMPs (antimicrobial peptides) is enhanced owing to the ability of these compounds to rapidly kill a large number of micro-organisms such as bacteria, viruses, fungi and parasites that are multidrug-resistant [3,4]. There is an increasing interest in the study of AMPs because of their capacity to interact with certain cellular membranes, and the resulting antimicrobial activity that they display against pathogens [5].
Promising AMPs include the plant-derived defensins, a family of basic peptides which consists of 45–54 amino acids, arranged in three-dimensional structures formed by three antiparallel β-strands and one α-helix. This structure is stabilized by four disulfide bonds, which form a cysteine-stabilized α-helix β-strands motif, commonly found in these peptides [5,6]. The antimicrobial activity of plant defensins is mainly observed against fungi. However, some bacteria, particularly Gram-positive species, are also inhibited, although the activity is less pronounced than that against fungi. The growth of several fungal species, including several filamentous fungi and yeast cells, was inhibited when incubated with these peptides [7–21]. Despite the broad inhibitory activity that plant defensins exhibit against different micro-organisms, scientists know little about their activity against protozoa.
In a previous study, we isolated a plant defensin named PvD1 from Phaseolus vulgaris (cv. Pérola) seeds. This protein was able to negatively affect different yeast cells and filamentous fungi [22], causing an increase in the endogenous production of ROS (reactive oxygen species) and NO (nitric oxide), plasma membrane permeabilization and the inhibition of medium acidification [23]. In the present study, we investigated the action of PvD1 against the protozoan Leishmania amazonensis. We were also interested to understand the mechanism by which PvD1 affects L. amazonensis promastigotes.
EXPERIMENTAL
Biological material
P. vulgaris L. seeds were supplied by the Empresa de Pesquisa Agropecuária do Estado do Rio de Janeiro (Pesagro), Campos dos Goytacazes, Rio de Janeiro, Brazil.
Promastigote-stage L. amazonensis (Josefa strain) were supplied by Laboratório de Biologia Tecidual, Centro de Ciências e Biotecnologia, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, Rio de Janeiro, Brazil. The protozoa were cultivated in 5 ml of Warren's medium [90% brain heart broth (Fluka) containing 10% (v/v) heat-inactivated FBS], enriched with 0.01% folic acid and 0.4% haemin at 28°C. The protozoa were transferred to new medium every 3 days.
Purification of the P. vulgaris defensin PvD1
The purification of the defensin from P. vulgaris (cv. Pérola) seeds was conducted as described by Games et al. [22].
Gel electrophoresis
PvD1 defensin purification was monitored by SDS/Tricine gel electrophoresis performed according to the method previously described by Schägger and Von Jagow [24].
Cell proliferation assay
The effect of PvD1 on the proliferation of L. amazonensis promastigotes was determined by incubating the parasites (1.5×106 parasites/ml) with the PvD1 defensin (300 and 600 μg/ml) for 48 h at 28°C in 200 μl microplate wells. The peptide was diluted in DMSO (1.5% final concentration) and Warren's medium (100 μl) and incubated with the parasites in Warren's medium (100 μl). The proliferation of the parasites was monitored at 24 and 48 h by cell counting in Neubauer chamber. The proliferation of L. amazonensis promastigote controls (without the addition of peptides) was also assessed. The experiments were performed in triplicate, and S.E.M. were calculated.
Plasma membrane permeabilization assay
Membrane permeabilization of L. amazonensis promastigotes was assessed by measuring Sytox Green uptake as described previously, with some modifications, by Thevissen et al. [25]. Sytox Green is a dye that only penetrates cells when the plasma membrane is structurally compromised. Once inside the parasitic cytoplasm, it binds to nucleic acids, resulting in a fluorescent complex. The L. amazonensis promastigotes (1.5×106 parasites/ml) were incubated with PvD1 at a concentration of 300 μg/ml for 24 h at 28°C in 200 μl microplate wells. Aliquots (100 μl) of the parasite cell suspension were incubated with 0.2 μM Sytox Green in 1.5 ml microcentrifuge tubes for 30 min at 25°C with periodic agitation. After incubation, the parasites were fixed in 2% (w/v) paraformaldehyde. The cells were examined using a DIC (differential interference contrast) microscope (Axiophoto, Zeiss) equipped with a fluorescence filter set for the detection of fluorescein (excitation wavelengths, 450–490 nm; emission wavelength, 500 nm). Negative (no PvD1 added) controls were also run to evaluate baseline membrane permeability.
TEM (transmission electron microscopy)
For ultrastructural analyses, untreated or treated L. amazonensis promastigotes were washed with PBS at 37°C and fixed at room temperature in a solution containing 1% (w/v) glutaraldehyde, 4% (w/v) paraformaldehyde, 5 mM CaCl2 and 5% (w/v) sucrose in 0.1 M cacodylate buffer (pH 7.2). The cells were post-fixed for 1 h in a solution containing 2% OsO4, 0.8% potassium ferrocyanide and 5 mM CaCl2 in 0.1 M cacodylate buffer (pH 7.2), rinsed with 0.1 M cacodylate buffer (pH 7.2), dehydrated in acetone and embedded in PolyBed (Polysciences). Thin sections were stained with uranyl acetate and lead citrate and subsequently examined using a Zeiss 900 Transmission Electron Microscope at 80 kV acceleration.
Intracellular localization of FITC-conjugated PvD1
PvD1 was conjugated to FITC (Sigma), according to the manufacturer's instructions. The L. amazonensis promastigotes (1.5×106 parasites/ml) were incubated with PvD1–FITC at a concentration of 150 μg/ml for 24 h at 28°C in 200 μl microplate wells. The cells were examined using a DIC microscope equipped with a fluorescence filter set for fluorescein detection (excitation wavelengths, 450–490 nm; emission wavelength, 500 nm).
RESULTS
Cell proliferation assay
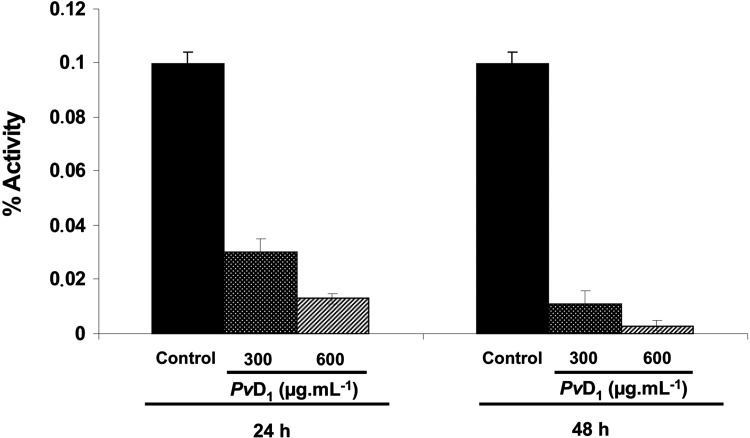
The effect of PvD1 on the proliferation of L. amazonensis promastigotes was evaluated. Figure 1 shows the inhibitory effects of PvD1 (at concentrations of 300 and 600 μg/ml) towards the proliferation of parasites for periods of 24 h and 48 h. When compared with the control parasites grown in the absence of PvD1, inhibitory rates of 70 and 89% were observed when testing PvD1 at a concentration of 300 μg/ml at 24 h and 48 h respectively, whereas at a concentration of 600 μg/ml, inhibition rates of 87% and 96.5% were observed at 24 h and 48 h respectively.
Figure 1. Inhibition of the proliferation of L. amazonensis promastigotes in the presence of PvD1.
Proliferation was observed for a 48 h period. The proliferation of L. amazonensis promastigote controls (without the addition of peptide) was also assessed. The experiments were performed in triplicate, and results are means±S.E.M.
Plasma membrane permeabilization assay
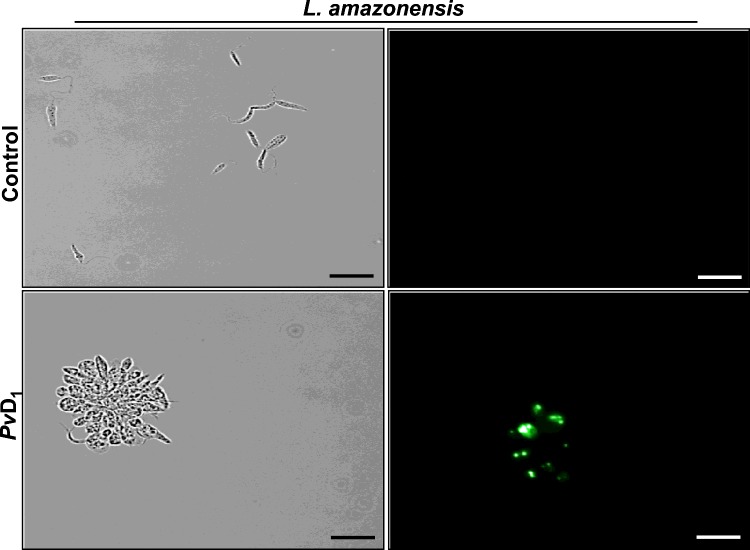
In order to investigate further the negative effects of PvD1 on L. amazonensis promastigote proliferation, we analysed whether cell membranes had been disrupted by the defensin. At 24 h of the proliferation inhibition assay, L. amazonensis promastigotes were treated with the fluorescent dye Sytox Green which only penetrates cells with structurally compromised plasma membranes. The observation of these cells by fluorescence microscopy showed that the L. amazonensis promastigotes were labelled by Sytox Green when treated with 300 μg/ml PvD1 (Figure 2). In control cells, when parasites were grown in the absence of PvD1, no fluorescence was observed.
Figure 2. Plasma membrane permeabilization assay.
L. amazonensis promastigotes treated for 24 h with PvD1 (300 μg/ml) and incubated with Sytox Green. The proliferation of L. amazonensis promastigote controls (without the addition of peptide) was also assessed. Scale bars, 20 μm.
Ultrastructural analysis of L. amazonensis promastigotes treated with PvD1
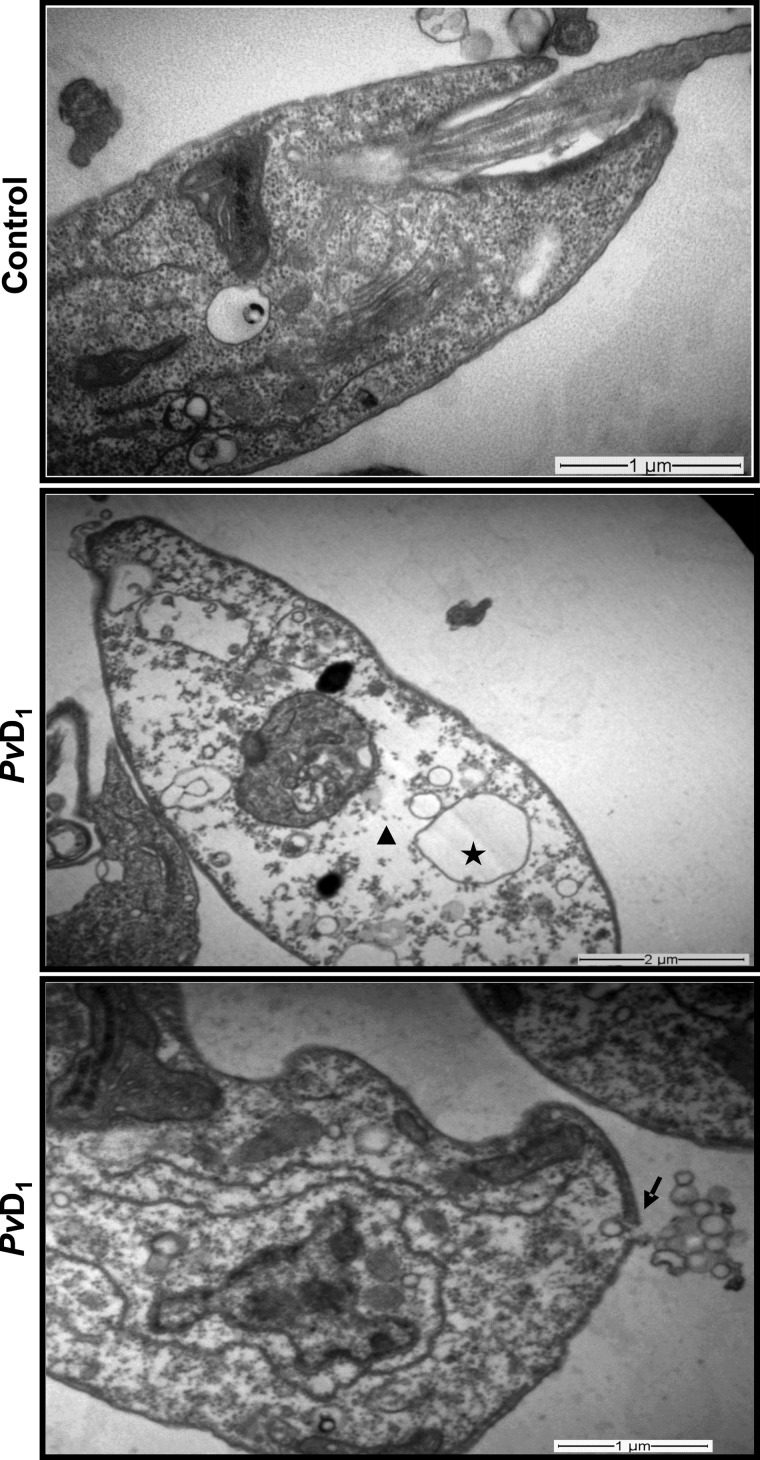
To confirm the effect of PvD1 defensin on membrane permeabilization of L. amazonensis promastigotes, these cells were analysed by TEM. The ultrastructure analysis of promastigotes treated with 300 μg/ml PvD1 revealed that this defensin was able to cause plasma membrane disruption followed by leakage of cytoplasmic material. It also caused cytoplasmic fragmentation and vacuolization. In contrast, control cells exhibited membrane integrity and normal intracellular organization (Figure 3).
Figure 3. Ultrastructural analysis of L. amazonensis promastigotes treated with PvD1.
L. amazonensis promastigotes treated for 24 h with PvD1 (300 μg/ml). The star indicates vacuolization of the cytoplasm; the triangle indicates fragmentation of the cytoplasm; the arrow indicates disruption in the plasma membrane of promastigotes leading to loss of cytoplasmic material.
Intracellular localization of PvD1
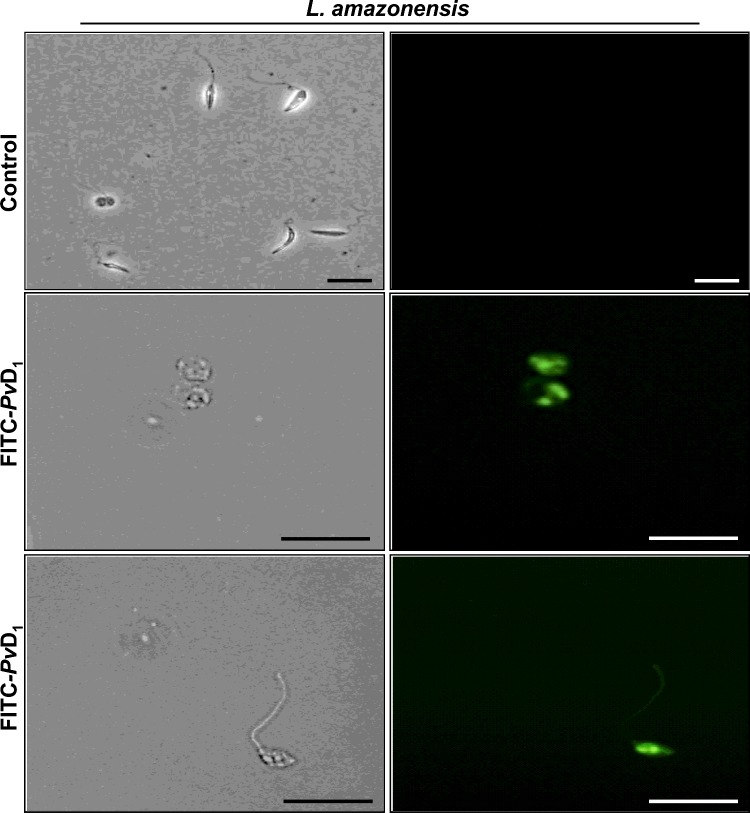
In order to investigate further whether the PvD1 defensin could be internalized in L. amazonensis promastigotes, its ability to penetrate and accumulate inside the cell was evaluated by coupling the peptide with FITC. After the L. amazonensis promastigote proliferation inhibition assay using 150 μg/ml PvD1–FITC, we observed the presence of PvD1–FITC in intracellular spaces of these cells (Figure 4), suggesting a possible intracellular target of PvD1 in L. amazonensis promastigotes. In control cells, no fluorescence was observed.
Figure 4. localization of FITC-conjugated PvD1.
L. amazonensis promastigotes were treated for 24 h with FITC–PvD1 (150 μg/ml). The L. amazonensis promastigote control (without the addition of FITC–PvD1) was also assessed. Scale bars, 20 μm.
DISCUSSION
Plant defensins are capable of inhibiting the growth of a wide variety of filamentous fungi and yeast [5,6,23,22,26,27]. Despite the wide inhibitory activity that plant defensins have against different micro-organisms, there is a gap in our knowledge regarding the mechanism of action of plant defensins against protozoa. Only two studies to date show plant defensin activity against protozoan parasites of the genus Leishmania [28,29]. In the present study, we analysed the activity of a plant defensin, PvD1, against L. amazonensis promastigotes and characterized some aspects of this plant defensin's action against this parasite.
We observed that at 300 μg/ml, PvD1 was able to inhibit 70% of the protozoan proliferation, after 24 h of incubation (Figure 1). Berrocal-Lobo et al. [30] observed the activity of a PTH1 defensin from Solanum tuberosum and a thionin from Triticum aestivum against L. donovani; however, these authors did not test these peptides against L. amazonensis. More recently, Souza et al. [29] showed that the natural defensin from Vigna unguiculata seeds (Vu-Def), as well as its recombinant homologues (Vu-Defr), both at a concentration of 100 μg/ml, were also able to inhibit the proliferation of the parasite L. amazonensis by approximately 50% after 48 h incubation. Nonetheless, it is still unknown whether the plant defensins would affect other species of protozoa with similar strength.
After treatment of L. amazonensis promastigotes with PvD1, we used the Sytox Green fluorescent dye which binds to nucleic acids when the cell plasma membranes are structurally compromised. We observed that PvD1 was capable of causing damage to the plasma membrane (Figure 2), which was confirmed by the effects of PvD1 on membrane permeabilization, seen using TEM. Using ultrastructural analysis, we showed that the cells of protozoa treated with the defensin exhibited a disruption of the promastigote plasma membrane leading to the loss of cytoplasmic material as well as cytoplasmic fragmentation and formation of multiple cytoplasmic vacuoles (Figure 3). Bera et al. [28] examined the effects of the AMP indolicidin (derived from bovine neutrophils) and two other peptides, 27RP and SPFK (derived from bovine seminal plasma), on Leishmania donovani. Interestingly, cells treated with these peptides exhibited extensive degeneration of intracellular organization and the formation of multiple cytoplasmic vacuoles without disruption of the plasma membrane. In contrast with the effect caused by AMPs, cells treated with amphotericin B exhibited no vacuolarization, although amphotericin B causes significant disruption of the plasma membrane. In the present study, we found both effects for PvD1, which is the first reported plant AMP capable of causing cytoplasm fragmentation, formation of multiple cytoplasmic vacuoles and plasma membrane disruption of L. amazonensis promastigotes.
After this primary membrane-permeabilizing event, we analysed whether PvD1 was able to internalize L. amazonensis promastigotes. For this, FITC-tagged PvD1 was observed by fluorescence microscopy (Figure 4). The present study is the first to show that a plant defensin was able to enter L. amazonensis cells. On the basis of these data, we suggest the existence of a possible intracellular target for this defensin as a part of the mechanism responsible for leading to the protozoan's death.
In the present paper, we report the activity of a plant defensin against L. amazonensis and provide information on its mechanism of action. We demonstrate that, in addition to inhibiting the proliferation of L. amazonensis promastigotes, the PvD1 defensin was able to cause cytoplasmic fragmentation, formation of multiple cytoplasmic vacuoles and membrane permeabilization in these cells. Furthermore, we show for the first time that PvD1 defensin located within these cells may be acting on a possible intracellular target. These results open new perspectives regarding the antimicrobial mechanism of plant defensins as it suggests that the toxicity of these peptides may not be restricted to the plasma membrane.
Acknowledgments
This study comprises part of the postdoctoral research of Dr Viviane Veiga do Nascimento and part of the D.Sc. degree thesis of Erica O. Mello. This work was performed at the Universidade Estadual do Norte Fluminense Darcy Ribeiro. We are grateful to L.C.D. Souza and Kokis V.M. for general laboratory technical support.
Abbreviations
- AMP
antimicrobial peptide
- DIC
differential interference contrast
AUTHOR CONTRIBUTION
The study was conceived by Valdirene M. Gomes and Edésio J.T. de Melo. Experimental procedures were carried out by Érica de O. Mello, Viviane V. do Nascimento and Laís P. Carvalho. Data analyses were performed by Érica de O. Mello, Viviane V. do Nascimento, Laís P. Carvalho, André de O. Carvalho, Valdirene M. Gomes and Edésio J.T. de Melo. The paper was written by Érica de O. Mello, Viviane V. do Nascimento, André de O. Carvalho, Katia V.S. Fernandes and Valdirene M. Gomes.
FUNDING
We acknowledge the financial support of the Brazilian agencies Programa Nacional de Pós Doutorado (PNPD)/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), CAPES/Toxinology, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
References
- 1.Peacock C.S., Seeger K., Harris D., Murphy L., Ruiz J.C., Quail M.A., Peters N., Adlem E., Tivey A., Aslett M., et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat. Genet. 2007;39:839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallo R.L., Murakami M., Ohtake T., Zaiou M. Biology and clinical relevance of naturally occurring antimicrobial peptides. J. Allergy Clin. Immunol. 2002;110:823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- 3.Guaní-Guerra E., Santos-Mendoza T., Lugo-Reyes S.O., Terán L.M. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin. Immunol. 2010;135:1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira V., Feio M.J., Bastos M. Role of lipids in the interaction of antimicrobial peptides with membranes. Prog. Lipid Res. 2012;51:149–177. doi: 10.1016/j.plipres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho A.O., Gomes V.M. Plant defensins and defensin-like peptides: biological activities and biotechnological applications. Curr. Pharm. Des. 2011;17:4270–4293. doi: 10.2174/138161211798999447. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho A.O., Gomes V.M. Plant defensins: prospects for the biological functions and biotechnological properties. Peptides. 2009;30:1007–1020. doi: 10.1016/j.peptides.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Anaya-López J.L., López-Meza J.E., Baizabal-Aguirre V.M., Cano-Camacho H., Ochoa-Zarzosa A. Fungicidal and cytotoxic activity of a Capsicum chinense defensin expressed by endothelial cells. Biotechnol. Lett. 2006;28:1101–1108. doi: 10.1007/s10529-006-9060-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen G.-H., Hsu M.-P., Tan C.-H., Sung H.-Y., Kuo C.G., Fan M.-J., Chen H.-M., Chen S., Chen C.-S. Cloning and characterization of a plant defensin VaD1 from azuki bean. J. Agric. Food Chem. 2005;53:982–988. doi: 10.1021/jf0402227. [DOI] [PubMed] [Google Scholar]
- 9.Finkina E.I., Shramova E.I., Tagaev A.A., Ovchinnikova T.V. A novel defensin from the lentil Lens culinaris seeds. Biochem. Biophys. Res. Commun. 2008;371:860–865. doi: 10.1016/j.bbrc.2008.04.161. [DOI] [PubMed] [Google Scholar]
- 10.Odintsova T.I., Rogozhin E.A., Baranov Y., Musolyamov A.K., Nasser Y., Egorov T.A., Grishin E.V. Seed defensins of barnyard grass Echinochloa crusgalli (L.) Beauv. Biochimie. 2008;90:1667–1673. doi: 10.1016/j.biochi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Olli S., Kirti P.B. Cloning, characterization and antifungal activity of defensin Tfgd1 from Trigonella foenum-graecum L. J. Biochem. Mol. Biol. 2006;39:278–283. doi: 10.5483/BMBRep.2006.39.3.278. [DOI] [PubMed] [Google Scholar]
- 12.Osborn R.W., De Samblanx G.W., Thevissen K., Goderis I., Torrekens S., Van Leuven F., Attenborough S., Rees S.B., Broekaert W.F. Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett. 1995;368:257–262. doi: 10.1016/0014-5793(95)00666-W. [DOI] [PubMed] [Google Scholar]
- 13.Solis J., Medrano G., Ghislain M. Inhibitory effect of a defensin gene from the Andean crop maca (Lepidium meyenii) against Phytophthora infestans. J. Plant Physiol. 2007;164:1071–1082. doi: 10.1016/j.jplph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Terras F.R.G., Torrekens S., Van Leuven F., Osborn R.W., Vanderleyden J., Cammue B.P.A., Broekaert W.F. A new family of basic cysteine-rich plant antifungal proteins from Brassicaceae species. FEBS Lett. 1993;316:233–240. doi: 10.1016/0014-5793(93)81299-F. [DOI] [PubMed] [Google Scholar]
- 15.Thevissen K., Ferket K.K.A., François I.E.J.A., Cammue B.P.A. Interactions of antifungal plant defensins with fungal membrane components. Peptides. 2003;24:1705–1712. doi: 10.1016/j.peptides.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Thevissen K., François I.E.J.A., Takemoto J.Y., Ferket K.K.A., Meert E.M.K., Cammue B.P.A. DmAMP1, an antifungal plant defensin from dahlia (Dahlia merckii), interacts with sphingolipids from Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2003;226:169–173. doi: 10.1016/S0378-1097(03)00590-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang H.X., Ng T.B. An antifungal peptide from baby lima bean. Appl. Microbiol. Biotechnol. 2006;73:576–581. doi: 10.1007/s00253-006-0504-5. [DOI] [PubMed] [Google Scholar]
- 18.Wisniewski M.E., Bassett C.L., Artlip T.S., Webb R.P., Janisiewicz W.J., Norelli J.L., Goldway M., Droby S. Characterization of a defensin in bark and fruit tissues of peach and antimicrobial activity of a recombinant defensin in the yeast, Pichia pastoris. Physiol. Plant. 2003;119:563–572. doi: 10.1046/j.1399-3054.2003.00204.x. [DOI] [Google Scholar]
- 19.Wong J.H., Ng T.B. Sesquin, a potent defensin-like antimicrobial peptide from ground beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase. Peptides. 2005;26:1120–1126. doi: 10.1016/j.peptides.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Wong J.H., Ng T.B. Vulgarinin, a broad-spectrum antifungal peptide from haricot beans (Phaseolus vulgaris) Int. J. Biochem. Cell Biol. 2005;37:1626–1632. doi: 10.1016/j.biocel.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Ye X.Y., Ng T.B. A new antifungal peptide from rice beans. J. Pept. Res. 2002;60:81–87. doi: 10.1034/j.1399-3011.2002.20962.x. [DOI] [PubMed] [Google Scholar]
- 22.Games P.D., Santos I.S., Mello E.O., Diz M.S.S., Carvalho A.O., Souza-Filho G.A., Da Cunha M., Vasconcelos I.M., Ferreira B.S., Gomes V.M. Isolation, characterization and cloning of a cDNA encoding a new antifungal defensin from Phaseolus vulgaris L. seeds. Peptides. 2008;29:2090–2100. doi: 10.1016/j.peptides.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Mello E.O., Ribeiro S.F.F., Carvalho A.O., Da Cunha M., Santa-Catarina C., Gomes V.M. Antifungal activity of PvD1 defensin involves plasma membrane permeabilization, inhibition of medium acidification and induction of ROS in fungi cells. Curr. Microbiol. 2011;62:1209–1217. doi: 10.1007/s00284-010-9847-3. [DOI] [PubMed] [Google Scholar]
- 24.Schägger H., Von Jagow G. Tricine-sodium dodecylsulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 25.Thevissen K., Terras F.R.G., Broekaert W.F. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl. Environ. Microbiol. 1999;65:5451–5458. doi: 10.1128/aem.65.12.5451-5458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broekaert W.F., Cammue B.P.A., De Bolle M.F.C., Thevissen K., De Samblanx G., Osborn R.W. Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 1997;16:297–323. doi: 10.1080/07352689709701952. [DOI] [Google Scholar]
- 27.Thevissen K., de Mello Tavares P., Xu D., Blankenship J., Vandenbosch D., Idkowiak-Baldys J., Govaert G., Bink A., Rozental S., de Groot P.W., et al. The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans. Mol. Microbiol. 2012;84:166–180. doi: 10.1111/j.1365-2958.2012.08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bera A., Singh S., Nagaraj R., Vaidya T. Induction of autophagic cell death in Leishmania donovani by antimicrobial peptides. Mol. Biochem. Parasitol. 2003;127:23–35. doi: 10.1016/S0166-6851(02)00300-6. [DOI] [PubMed] [Google Scholar]
- 29.Souza G.S., Nascimento V.V., Carvalho L.P., Melo E.J.T., Fernandes K.V., Machado O.L.T., Retamal C.A., Gomes V.M., Carvalho A.O. Activity of recombinant and natural defensins from Vigna unguiculata seeds against Leishmania amazonensis. Exp. Parasitol. 2013;135:116–125. doi: 10.1016/j.exppara.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Berrocal-Lobo M., Molina A., Rodriguez-Palenzuela P., Garcia-Olmeda F., Rivas L. Leishmania donovani: thionins, plant antimicrobial peptides with leishmanicidal activity. Exp. Parasitol. 2009;122:247–249. doi: 10.1016/j.exppara.2009.03.019. [DOI] [PubMed] [Google Scholar]