Abstract
Objectives
The increasing number of clinical strains resistant to one or more of the front-line TB drugs complicates the management of this disease. To develop next-generation benzimidazole-based FtsZ inhibitors with improved efficacy, we employed iterative optimization strategies based on whole bacteria potency, bactericidal activity, plasma and metabolic stability and in vivo efficacy studies.
Methods
Candidate benzimidazoles were evaluated for potency against Mycobacterium tuberculosis H37Rv and select clinical strains, toxicity against Vero cells and compound stability in plasma and liver microsomes. The efficacy of lead compounds was assessed in the acute murine M. tuberculosis infection model via intraperitoneal and oral routes.
Results
MICs of SB-P17G-A33, SB-P17G-A38 and SB-P17G-A42 for M. tuberculosis H37Rv and select clinical strains were 0.18–0.39 mg/L. SB-P17G-A38 and SB-P17G-A42 delivered at 50 mg/kg twice daily intraperitoneally or orally demonstrated efficacy in reducing the bacterial load by 5.7–6.3 log10 cfu in the lungs and 3.9–5.0 log10 cfu in the spleen. SB-P17G-A33 delivered at 50 mg/kg twice daily intraperitoneally or orally also reduced the bacterial load by 1.7–2.1 log10 cfu in the lungs and 2.5–3.4 log10 cfu in the spleen.
Conclusions
Next-generation benzimidazoles with excellent potency and efficacy against M. tuberculosis have been developed. This is the first report on benzimidazole-based FtsZ inhibitors showing an equivalent level of efficacy to isoniazid in an acute murine M. tuberculosis infection model.
Introduction
Globally, TB is the leading cause of death from bacterial infection and latent infections hinder disease management. Bedaquiline (TMC207) is the most recent chemotherapeutic drug developed and is available for use as part of the combinatorial treatment options for TB.1–3 Importantly, bedaquiline is an example that novel drugs with unique modes of action can be used effectively to augment current therapeutic regimens and substantiates the larger effort of novel drug discovery.
The bacterial cell division protein filamentous temperature-sensitive protein Z (FtsZ), which is an essential bacterial cytokinesis protein and homologue of tubulin/microtubule, is a valid yet underexploited molecular target for TB therapeutic discovery.4–8 Early drug discovery efforts to target FtsZ in Mycobacterium tuberculosis started with tubulin inhibitors that were shown to inhibit the FtsZ polymerization/depolymerization balance.4,6,7,9,10 Taking into account the structural similarity of pyridopyrazine, pteridine, albendazole and thiabendazole skeletons,9,11–13 and based on previous studies, we selected and designed the trisubstituted benzimidazole scaffold for the development of novel M. tuberculosis FtsZ inhibitors.14
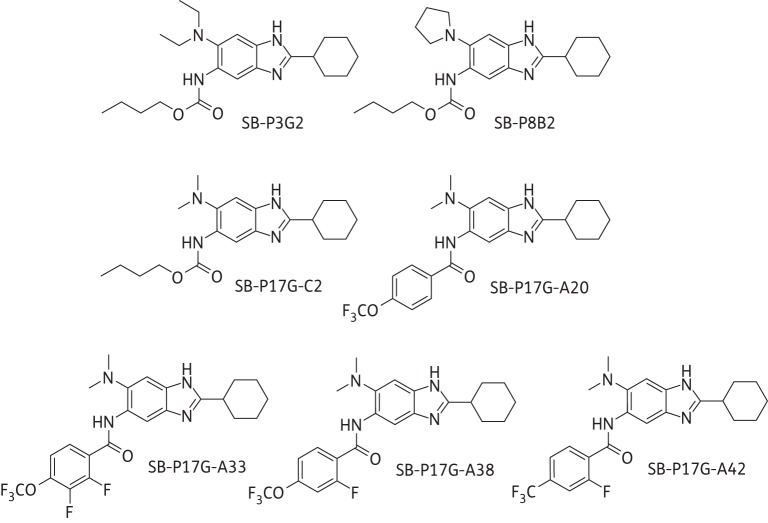
Accordingly, a library of trisubstituted benzimidazoles was created and screened for potency, which resulted in the identification of first-generation lead compounds that included SB-P3G2 and SB-P8B2 (Figure 1).14 SB-P3G2 and SB-P8B2 had potency against drug-resistant and -susceptible strains and SB-P3G2 exhibited efficacy in a murine M. tuberculosis infection model.14,15 Structure–activity relationship (SAR)-based modifications of these benzimidazoles led to the development of second-generation compounds with high potency, including SB-P17G-C2 (MIC 0.06 mg/L; Figure 1).16 However, examination of the plasma and metabolic stability of these compounds revealed that the carbamate groups at C5 were labile in plasma and metabolized by murine microsomes.16,17 Fluorine-containing benzamide groups were introduced at C5 to address the lability issue, which indeed substantially improved the plasma and metabolic stability. One of the compounds in this series, SB-P17G-A20 (MIC 0.16 mg/L; Figure 1) bearing a 4-trifluoromethoxybenzamido group at C5, exhibited improved plasma and metabolic stability as well as improved efficacy in the acute murine M. tuberculosis infection model when compared with the first-generation lead compounds.17
Figure 1.
Chemical structures of the 2,5,6-trisubstituted benzimidazoles. Generation 1 lead compounds include SB-P3G2 (MIC 0.78–1.56 mg/L) and SB-P8B2 (MIC 0.39–0.78 mg/L). Generation 2 lead compounds include SB-P17G-C2 (MIC 0.06 mg/L) and SB-P17G-A20 (MIC 0.16 mg/L). Generation 3 lead compounds include SB-P17G-A33 (MIC 0.39 mg/L), SB-P17G-A38 (MIC 0.31 mg/L) and SB-P17G-A42 (MIC 0.18 mg/L).
In this report, we highlight the continued optimization of the second-generation benzimidazoles that has led to the development of the highly potent and efficacious next-generation lead compounds. A fluorine substituent was strategically introduced into the 4-trifluoromethoxy- or 4-trifluoromethylbenzamide moiety at C5 to further improve plasma and metabolic stability. These next-generation benzimidazoles demonstrate improved in vivo efficacy compared with the first- and second-generation compounds and, more importantly, their activity is equal to the activity of a front-line drug in the acute murine M. tuberculosis infection model.
Methods
MICs, cytotoxicity, metabolism and efficacy
The MICs of SB-P17G-A33, SB-P17G-A38 and SB-P17G-A42 for M. tuberculosis H37Rv and clinical isolates TN587, W210, NHN382 and NHN20 were determined using the microplate Alamar blue assay.15,17 The cytotoxicity in Vero cells, growth inhibition response in M. tuberculosis H37Rv, plasma stability and metabolic lability assays were performed as described previously.15,17
Efficacy was assessed using the acute murine M. tuberculosis infection model as described previously.15,17 Benzimidazoles delivered intraperitoneally were solubilized as described previously.17 The benzimidazoles delivered orally were solubilized using a formulation of 40% captex 200, 40% Solutol HS 15 and 20% capmul.mcn, and diluted with sterile deionized water. Benzimidazoles were delivered at 50 mg/kg twice daily and isoniazid was delivered at 25 mg/kg daily as a control. Animals were treated for 10 consecutive days. Bacterial burden in the lungs and spleen was determined by plating and outgrowth on solid medium.
Ethics statement
Use of vertebrate animals at Colorado State University is conducted under AAALAC approval OLAW number A3572-01 under file with NIH. Animals are housed in an ABL-3 facility supervised by full-time veterinarians per American Veterinary Medical Association guidelines.
Results and discussion
In order to improve the efficacy of the lead compound SB-P17G-A20, arising from the second-generation benzimidazoles (vide supra), we strategically introduced a fluorine at the 2-position of the 4-trifluoromethoxy- or 4-trifluoromethylbenzamido group at C5. The introduction of the fluorine adjacent to the carbonyl group of an amide linkage was anticipated to block or mitigate hydrolysis by esterases in blood plasma, as well as hydroxylation of the phenyl moiety of the benzamido group by cytochrome P-450 enzymes.18 The lead benzimidazoles studied in this report bear 2,3-difluoro-4-trifluoromethoxybenzamido (SB-P17G-A33), 2-fluoro-4-trifluoromethoxybenzamido (SB-P17G-A38) and 2-fluoro-4-trifluoromethylbenzamido (SB-P17G-A42) groups at C5 of the 2-cyclohexyl-6-dimethylaminobenzimidazole skeleton.
Whole bacteria cell activity
SB-P17G-A33, SB-P17G-A38 and SB-P17G-A42 were equally potent against M. tuberculosis H37Rv and the M. tuberculosis clinical strains as evident from MIC values of 0.39 ± 0.16, 0.31 ± 0.22 and 0.18 ± 0.1 mg/L, respectively, and were not cytotoxic to Vero cells at 200 mg/L. Growth curves of M. tuberculosis in the presence of each of the analogues revealed sigmoidal inhibition curves and concentration-dependent inhibition, both features that have been shown to be indicative of efficacy.15,17 SB-P17G-A33 demonstrated less bactericidal activity than SB-P17G-A38 and SB-P17G-A42, requiring 3–6× MIC to reduce the bacterial viability by 3.6–4.4 log10 cfu, which is an equivalent level of reduction as achieved with 1× MIC of the other two lead compounds. The results indicate that the introduction of only one fluorine at the 2-position of the benzamido moiety is well tolerated for bactericidal activity, but that the introduction of two fluorine substituents is rather counterproductive.
Plasma stability and metabolic lability
After 4 h of incubation, SB-P17G-A33 and SB-P17G-A38 were stable in human (1% and 2% hydrolysis) and murine (10.7% and 11.5% hydrolysis) plasma. SB-P17G-A33, SB-P17G-A38 and SB-P17G-A42 exhibited limited lability in the presence of liver microsomes with 5%, 13% and 12% conversion in human liver microsomes, respectively, and 17%, 4% and 13% conversion in mouse liver microsomes, respectively. The results indicate that the introduction of one or two fluorine substituents into the benzamido moiety substantially improved the human and mouse plasma and metabolic stability when compared with the previous lead compound SB-P17G-A20.17
Efficacy results in an acute murine M. tuberculosis infection model
SB-P17G-A33, SB-P17G-A38 and SB-P17G-A42 reduced the bacterial load in the lungs when delivered orally or intraperitoneally in an acute murine M. tuberculosis infection model (Table 1). SBP17G-A38 and SB-P17G-A42 reduced the bacterial load in the lungs by 6.1–6.3 and 5.7–5.8 log10 cfu, respectively. Moreover, no bacteria were detected in the spleens of the groups treated with SB-P17G-A38 and SB-P17G-A42 (intraperitoneally or orally). Significantly, SB-P17G-A38 and SB-P17G-A42 demonstrated efficacy comparable to the front-line M. tuberculosis drug isoniazid. SB-P17G-A33 showed lesser efficacy (bacterial load in lungs reduced by 1.7–2.1 log10 cfu). As expected based on the in vitro assessments, the introduction of two fluorine substituents into the benzamido moiety at C5 did not improve in vivo efficacy.
Table 1.
Efficacy results from SB-P17G-A33, SB-P17G-A38 and SB-P17G-A42 in the acute murine M. tuberculosis infection model
| Route | Log10 cfu lungs | Difference in log10 cfu lungs between the means from the test and control groups | Log10 cfu spleen | Difference in log10 cfu spleen between the means from the test and control groups | |
|---|---|---|---|---|---|
| Control | ip | 6.69 ± 0.05, n = 2 | NA | 4.96 ± 0.43 | NA |
| po | 6.31 ± 0.04, n = 5 | NA | 3.89 ± 0.56 | NA | |
| SB-P17G-A33 | ip | 4.59 ± 0.71, n = 3 | −2.10** | 1.60 ± 0 | −3.36*** |
| po | 4.58 ± 0.21, n = 5 | −1.72*** | 1.40 ± 1.24 | −2.49*** | |
| SB-P17G-A38 | ip | 0.39 ± 0.63, n = 4 | −6.30*** | 0 ± 0 | −4.96*** |
| po | 0.22 ± 0.34, n = 5 | −6.10*** | 0 ± 0 | −3.89*** | |
| SB-P17G-A42 | ip | 0.91 ± 0.64, n = 4 | −5.78*** | 0 ± 0 | −4.96*** |
| po | 0.66 ± 0.43, n = 5 | −5.65*** | 0 ± 0 | −3.89*** | |
| Isoniazid | ip | 0.12 ± 0.16, n = 5 | −6.57*** | 0 ± 0 | −4.96*** |
| po | 0 ± 0, n = 5 | −6.31*** | 0 ± 0 | −3.89*** |
ip, intraperitoneal; po, oral; NA, not applicable.
**<0.01 and ***<0.001 significance from Tukey's test.
Spontaneous resistance during treatment with both SB-P17G-A38 and SB-P17G-A42 was assessed by plating bacteria isolated from mouse tissue on plates with and without the appropriate compound at a concentration to avoid false positives. No colonies grew on medium containing 1.6 mg/L drug, thus demonstrating that no drug resistance developed during drug treatment in the animals. The improved efficacy in the acute murine M. tuberculosis infection model and no observable resistance clearly indicate that these novel benzimidazoles have excellent potential as new-generation chemotherapeutic agents against TB.
Funding
This work was supported by grants from the National Institutes of Health [AI078251 (I. O.) and U01 AI082164 (R. A. S.)], and the New York State Office of Science, Technology and Academic Research (NYSTAR).
Transparency declarations
A. C., L. G., S. L. and H. V. are all Sanofi-Aventis R&D employees. All other authors: none to declare.
Author contributions
The chemistry and enzyme studies were performed at Stony Brook University by D. A., K. K. and I. O. The plasma and microsome studies were performed at Sanofi-Aventis R&D by A. C., L. G., S. L. and H. V. The in vitro and efficacy studies were performed at Colorado State University by S. E. K. and R. A. S.
References
- 1.Andries K, Verhasselt P, Guillemont J et al. . A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005; 307: 223–7. [DOI] [PubMed] [Google Scholar]
- 2.Diacon AH, Pym A, Grobusch M et al. . The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009; 360: 2397–405. [DOI] [PubMed] [Google Scholar]
- 3.Matteelli A, Carvalho AC, Dooley KE et al. . TMC207: the first compound of a new class of potent anti-tuberculosis drugs. Future Microbiol 2010; 5: 849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vollmer W. The prokaryotic cytoskeleton: a putative target for inhibitors and antibiotics? Appl Microbiol Biotechnol 2006; 73: 37–47. [DOI] [PubMed] [Google Scholar]
- 5.Margalit DN, Romberg L, Mets RB et al. . Targeting cell division: small-molecule inhibitors of FtsZ GTPase perturb cytokinetic ring assembly and induce bacterial lethality. Proc Natl Acad Sci USA 2004; 101: 11821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Q, Kirikae F, Kirikae T et al. . Targeting FtsZ for antituberculosis drug discovery: noncytotoxic taxanes as novel antituberculosis agents. J Med Chem 2006; 49: 463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Q, Tonge PJ, Slayden RA et al. . FtsZ: a novel target for tuberculosis drug discovery. Curr Top Med Chem 2007; 7: 527–43. [DOI] [PubMed] [Google Scholar]
- 8.Respicio L, Nair PA, Huang Q et al. . Characterizing septum inhibition in Mycobacterium tuberculosis for novel drug discovery. Tuberculosis 2008; 88: 420–9. [DOI] [PubMed] [Google Scholar]
- 9.Slayden RA, Knudson DL, Belisle JT. Identification of cell cycle regulators in Mycobacterium tuberculosis by inhibition of septum formation and global transcriptional analysis. Microbiology 2006; 152: 1789–97. [DOI] [PubMed] [Google Scholar]
- 10.Mathew B, Srivastava S, Ross LJ et al. . Novel pyridopyrazine and pyrimidothiazine derivatives as FtsZ inhibitors. Bioorg Med Chem 2011; 19: 7120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White EL, Ross LJ, Reynolds RC et al. . Slow polymerization of Mycobacterium tuberculosis FtsZ. J Bacteriol 2000; 182: 4028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White EL, Suling WJ, Ross LJ et al. . 2-Alkoxycarbonylaminopyridines: inhibitors of Mycobacterium tuberculosis FtsZ. J Antimicrob Chemother 2002; 50: 111–4. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds RC, Srivastava S, Ross LJ et al. . A new 2-carbamoyl pteridine that inhibits mycobacterial FtsZ. Bioorg Med Chem Lett 2004; 14: 3161–4. [DOI] [PubMed] [Google Scholar]
- 14.Kumar K, Awasthi D, Lee S-Y et al. . Novel trisubstituted benzimidazoles, targeting Mtb FtsZ, as a new class of antitubercular agents. J Med Chem 2011; 54: 374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudson SE, Kumar K, Awasthi D et al. . In vitro-in vivo activity relationship of substituted benzimidazole cell division inhibitors with activity against Mycobacterium tuberculosis. Tuberculosis 2014; 94: 271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awasthi D, Kumar K, Knudson SE et al. . SAR studies on trisubstituted benzimidazoles as inhibitors of Mtb FtsZ for the development of novel antitubercular agents. J Med Chem 2013; 56: 9756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudson SE, Awasthi D, Kumar K et al. . A trisubstituted benzimidazole cell division inhibitor with efficacy against Mycobacterium tuberculosis. PLoS One 2014; 9: e93953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamazaki T, Taguchi T, Ojima I. Unique properties of fluorine and their relevance to bioorganic and medicinal chemistry. In: Ojima I, ed. Fluorine in Medicinal Chemistry and Chemical Biology. Chichester: Wiley-Blackwell, 2009; 3–46. [Google Scholar]