Abstract
Purpose
To examine whether prenatal iron deficiency delays auditory brainstem response (ABR) maturation in infancy.
Methods
One hundred and fifteen full-term healthy Chinese infants with maternal and cord blood haemoglobin and serum ferritin determinations were recruited into this study. Forty-eight infants received ABR testing at 3 months, and 45 infants were tested at 10 months. Comparison of the ABR variables were made between infants with and those without evidence of prenatal iron deficiency (maternal 3rd trimester haemoglobin <110 g/L, cord blood ferritin <75 μg/L); or anaemia at 10 months (haemoglobin <110 g/L).
Results
Latencies for wave V and wave III–V and I–V intervals were prolonged at 3 months in infants of anaemic mothers (effect sizes 1.02–1.19 SD). At 10 months, infants with low cord blood serum ferritin (indicating low iron stores at birth) showed longer wave I latency and possibly wave V latency also, besides demonstrating a smaller wave V amplitude (effect sizes 0.58–0.62 SD). Infants with low ferritin at birth and anemia at 10 months had longer wave III–V latency than other groups.
Conclusion
In full-term healthy infants, prenatal iron deficiency appears to have adverse effects on the developing central nervous system and auditory system as assessed by ABRs at 3 and/or 10 months.
Keywords: Auditory brainstem response, Central nervous system, Iron deficiency, Myelination, Prenatal
Introduction
The developing brain during late fetal and early postnatal life may be particularly vulnerable to nutritional insults due to the rapid trajectory of several processes, such as neuronal growth, differentiation, synapse formation, and myelination.1 Iron is an essential nutrient and both iron excess and deficiency have adverse health effects.2 Iron deficiency has peak prevalence in late pregnancy and infancy, when the brain is undergoing rapid development. Iron is involved in neuronal oxidative metabolism, myelin synthesis, and neurotransmitter metabolism.3,4 Animal studies show that iron intake and accumulation during critical temporal and spatial phases of brain maturation affects brain development.3,5 Histochemical and behavioural studies demonstrate long-lasting abnormalities in early iron deficiency.3,6,7 In human infants, iron-deficiency anaemia is associated with short- and long-term sensory, motor, cognitive, social-emotional, and neuroregulatory alterations despite iron therapy.3,4
Some of the most direct evidence of iron deficiency effects on human brain development comes from studies of auditory brainstem responses (ABRs).8–12 ABRs, which provide a noninvasive means to delineate the rapid development and maturation of the central nervous system (CNS), represent the progressive activation of different levels of the auditory pathway from the cochlear nerve (wave I and II) to the cochlear nuclear complex (wave III) and the lateral lemniscus (wave V). During late fetal and early postnatal life, there is a rapid maturation of the ABR that parallels an important period of brainstem myelination, neuronal development, and axonal growth.13 With increasing gestational age, ABR maturation is characterised by shorter absolute and interpeak latencies.14 The decrease in interpeak latencies reflects increased nerve conduction velocity. Both absolute and interpeak latencies are influenced by degree of myelination, axonal growth, and synaptic function.15,16
Findings of several studies of ABRs and iron-deficiency anaemia have been considered consistent with impaired myelination, especially such results as longer I–V interval and higher central-to-peripheral ratio (wave III–V interval divided by wave I–III interval).8,9,11 However, iron-deficiency anaemia was identified at 6 months of age or later, and prenatal iron status was unknown. To our knowledge, only two previous studies addressed the issue of prenatal iron deficiency and auditory system development.17,18 and both involved risk groups. ABRs were compared in infants with or without low cord-blood ferritin who were preterm17 or ≽35 weeks gestational age with intrauterine growth restriction or both to mothers with gestational diabetes or hypertension. Those with low cord blood ferritin, indicating low iron stores at birth, had prolonged latencies for waves I, III, and V, and decreased frequency of mature ABR waveforms,17 or prolonged interpeak latencies.18 These results point to effects of prenatal iron deficiency, but follow-up data were not presented and it is thus unknown whether effects are transient or long-lasting.
This pilot study was conducted to examine the effects of prenatal iron deficiency on maturation of the auditory pathway in the first year of life in healthy full-term infants. In keeping with iron’s role in myelination, we predicted that prenatal iron deficiency would be associated with prolonged absolute and interpeak latencies later in infancy. Due to the lack of previous research, we did not predict what specific latencies would be affected.
Methods
Subjects
The study was conducted in China, where generalised undernutrition is no longer a major problem, but iron deficiency in pregnant women and infants remains common (e.g., the prevalence of iron-deficiency anaemia and iron deficiency overall was 21% and 65%, respectively, among 7- to 12-month-old infants in a 2004 survey).19 Subjects were enrolled in Zhejiang province, a rapidly industrialising area on the southeast coast. The province has excellent maternal and child health care services and a mainly middle-class ethnic Han population.
ABR data were collected as part of a pilot study on the developmental effects of prenatal iron deficiency. Subjects for the pilot study were identified in conjunction with a survey of maternal and newborn iron status, which was conducted in Fuyang, Huzhou, and Yongkang counties between December 2005 and April 2007.20 To increase the likelihood that the developmental study had relevant representation of prenatal iron deficiency, laboratory personnel prepared lists of identification numbers with “low” or “normal” iron stores based on cord blood serum ferritin concentrations (see below). Approximately equal numbers of infants were selected randomly from each list and invited for developmental testing. A total of 31 infants with “low” cord ferritin and 38 infants with “normal” cord ferritin participated in developmental testing (Table 1). Other study personnel were unaware of maternal or infant iron status.
Table 1.
Characteristics of iron status
| Variable | N | Mean±SD | Range | N (%) | |
|---|---|---|---|---|---|
| Maternal iron status | |||||
| Hb (g/L) | 66 | 116.47±10.58 | 89.0~141.0 | <110 g/L 25.8% (17/66) |
|
| Ferritin (μg/L) | 68 | 17.42±21.29 | 2.60~135.0 | <20 μg/L 85.3% (58/68) |
<16 μg/L 72.1% (49/68) |
| Cord blood | |||||
| Hb (g/L) | 64 | 154.89±15.09 | 126.0–197.0 | <130 g/L 1.6% (1/64) |
|
| Ferritin (μg/L) | 69 | 130.82±80.06 | 27.7–279.0 | <75 μg/L 44.9% (31/69) |
|
| 10-month infant Hb (g/L) |
39 | 115.74±10.63 | 97.0–148.0 | <110 g/L 28.2% (11/39) |
Enrollment criteria included uncomplicated pregnancy, singleton term birth, birth weight 2.5–4.0 kg, no major congenital anomalies, no major birth or neonatal complications, no emergency delivery, no jaundice requiring phototherapy or lasting longer than 14 days, no hospitalisation for other than an uncomplicated problem (e.g., 2 days for diarrhoea), and no chronic illness. Another inclusion criterion was parental willingness to bring the infant to the Department of Child Health Care, Children’s Hospital Zhejiang University, in Hangzhou for routine paediatric visits at 3 and/or 9–10 months, study-specific developmental tests including ABR at 3 and/or 9–10 months, and a venous blood sample to test for anaemia at 9–10 months. If parents agreed verbally but the infant could not be scheduled in time for the 3-month visit, they were invited to participate in the assessment at 9–10 months.
The study protocol was approved by the ethics committees of Children’s Hospital Zhejiang University and the University of Michigan. All aspects of the study were explained to parents of qualifying infants, and signed informed consent was obtained when the infant came for developmental assessment for the first time.
Iron Status
Maternal and cord blood profiles included haemoglobin (Hb) (Sysmex SE-9000 Auto Hematology Analyzer, Japan), serum ferritin (SF) (IMMULITE chemiluminescent immunoassay system, Diagnostic Products Corporation, USA), C-reactive protein (CRP)(QuikRead 101, Orion Diagnostica, Finland), and whole blood lead (graphite furnace atomic absorption spectrometry, AA700, Perkin Elmer Company, USA).
Maternal anaemia in the 3rd trimester was defined as Hb <110 g/L, and low iron stores was defined as serum ferritin <16 μg/L.21 In the newborn, low Hb was defined as cord blood Hb <130 g/L. This cutoff is about 2 SD below the mean for term births using modern machine. The mean Hb was 151–166 g/L and one SD was about 11–16 g/L according to our survey20 and Macphail et al’s study22,23 Laboratory personal used a cutoff for “low” iron stores of cord blood serum ferritin <75 μg/L in making the lists of potential participants. A ferritin concentration of 75 μg/L represented the lower 10th percentile in the survey. Cord blood serum ferritin <75 μg/L is also the cutoff used in Tamura et al’s study relating cord blood serum ferritin to long-term developmental effects and Amin et al’s recent studies of newborn ABR and in utero iron status.17,18,24 “Normal” cord blood iron stores was defined as ferritin ≥75 μg/L. Cord blood samples suggestive of inflammation (CRP >5 mg/L or SF >370 μg/L)25,26 or high lead exposure (≽100 μg/L) were not considered for the developmental study. Because iron deficiency is common in later infancy, we wanted to take into account current iron status in assessing the effects of prenatal iron deficiency on ABR at 10 months. A full panel of iron status measures was not available due to funding constraints, but some infants had serum ferritin determinations, and almost all (86.7%, 39/45) had a venous Hb determination, together with a complete blood count. Rather than exclude infants with good quality ABR data who were missing ferritin, we used Hb as the primary haematology measure at 10 months and the Mentzer index to assess the likelihood of iron deficiency anaemia in anaemic infants.27 Anaemia at 10 months was defined as venous Hb <110 g/L.21 The Mentzer index is calculated as mean corpuscular volume divided by red blood cell count. A Mentzer index >13 has been shown to have a high positive predictive value (98.2%) in differentiating iron deficiency anaemia from β-thalassaemia trait.27
ABR Testing
All ABR measures were obtained and processed without knowledge of maternal or infant iron status. The infants were tested in the supine position during an afternoon nap, generally while in quiet sleep. No sedation was used. ABR recording was carried out in a quiet, dimly lit, and electrically shielded room using an EMG/EP Systems (Medtronic, USA). ABRs were recorded using silver-silver chloride disk electrodes placed according to the 10–20 International System as follows: the active electrode was placed on the vertex (Cz) and the reference one on the earlobe (A1 or A2) ipsilateral to the stimulation; an electrode placed on the forehead was used as a ground. Interelectrode impedance was kept at <5 kiloohms. ABRs were elicited by stimulating the test ear with a series of square wave rarefaction clicks (0.14 ms) through headphones at 105 dB Sound Pressure Level (SPL) (equivalent to 70–75 dB Hearing Level, HL). The contralateral ear was masked by white noise at 60 dB SPL. The bioelectrical activity recorded at the vertex was amplified, filtered (100–3000 Hz), and averaged by the evoked potential system. The recording window was 10 ms from click onset. Each trial consisted of 2000 artifact-free individual responses. The data acquisition program automatically rejected any traces with high-amplitude artifacts due to electrical interference or subject motion. Two consecutive averages were obtained for each ear to determine response replicability.
ABR Processing
ABR amplitudes and latencies were analysed off-line. With the aid of cursors, the individual peaks of each response were identified, marked, and measured. The following variables were used in statistical analyses: absolute latency and amplitude for wave I, III, and V, and interpeak latencies I–III, III–V, and I–V. In keeping with usual practice, the latency and amplitude values obtained for the right and left ears were combined, resulting in a mean value for each measure for each infant.
Statistical Analysis
Analysis of covariance was used to identify differences in ABR measures between infants with or without maternal anaemia or low ferritin or low cord ferritin, controlling for background factors. Pearson correlation analyses were performed to assess the association between ABR variables (i.e., wave I, III, V latencies and amplitudes and the corresponding interpeak latencies) and background factors, including sex, birth weight, gestational age, and age, weight-for-age z-score, and length-for-age z-score at testing, breastfeeding vs. formula. A given background factor was included as a covariate if it correlated with two or more ABR variables with two-tailed alpha level <0.10.
To consider concurrent iron status at 10 months, we performed analysis of covariance for 10-month ABR variables with two between-subjects factors and their interaction: poor iron status at birth (cord blood serum ferritin <75 μg/L) and anaemia at 10 months (venous Hb <110 g/L). We viewed this analysis as exploratory and hypothesis-generating, since the cell size for some combinations of cord blood and 10-month haematology was as small as 5.
Results
Sample
Among 115 infants invited to join the developmental study, 84 (73.0%) agreed to developmental testing. There were no significant background differences between infants who did or did not participate. ABR data were obtained for 74 of the infants, but 5 did not have satisfactory recordings at either 3 or 10 months due to infant state or equipment malfunction.
Thus a total of 69 infants had ABR assessments; 24 with records at both ages, 24 were assessed at 3 months only, and 21 at 10 months only. The net results were 48 infants had data at 3 months and 45 at 10 months respectively. Table 2 shows the sample background characteristics. About 88% of the infants were breastfed, 70% exclusively for 6 months.
Table 2.
Characteristics for all samples with ABRs (N=69)*
| N | 69 |
|---|---|
| Sex (% male, N) | 58%, 40 |
| Birth weight (kg) | 3.41 (0.37) |
| Gestational age (wk) | 39.3 (0.9) |
| 3 months (N=48) | |
| Age (d) | 86.0 (9.5) |
| Weight (kg) | 6.78 (0.72) |
| Weight-for-age z-score | 1.44 (0.89) |
| Length (cm) | 60.1 (2.4) |
| Length-for-age z-score | 0.07 (0.90) |
| Weight-for-length z-score | 1.63 (0.86) |
| 10 months (N=45) | |
| Age (d) | 301.3 (13.3) |
| Weight (kg) | 9.34 (1.07) |
| Weight-for-age z-score | 0.08 (1.01) |
| Length (cm) | 72.4 (2.3) |
| Length-for-age z-score | −0.12 (0.72) |
| Weight-for-length z-score | 0.30 (0.91) |
Values are mean (SD) except for sex. Z-scores were calculated according to Centers for Disease Control and Prevention growth curves. Infants were also growing normally by World Health Organization growth curves (data not shown).
Iron Status
Regarding prenatal iron status (Table 1), Hb was missing for 3 mothers and ferritin for 1. Seventeen infants (25.8%, 17/66) had mothers who were anaemic in the 3rd trimester (Hb <110 g/L). Maternal anaemia was generally mild (mean Hb=103.8±5.5 g/L). Iron stores were compromised in virtually all mothers; 72.1% (49/68) had ferritin concentrations <16 μg/L, and all but 10 values were below 20 μg/L. Only 1 infant had cord blood Hb <130 g/L, making it impossible to analyse the effects of neonatal anaemia. Thirty-one infants (44.9%, 31/69) had cord blood serum ferritin <75 μg/L. Maternal anaemia and low cord blood serum ferritin identified different infants; only 8 infants had prenatal iron deficiency according to both indicators.
At 10 months, 6 infants did not have blood work, resulting in 39 infants for analyses relating anaemia at 10 months to ABR variables. Eleven infants (28.2%, 11/39) had Hb <110 g/L; anaemia was again generally mild (mean Hb = 104.0±3.8 g/L). All 11 anaemic infants had a Mentzer index >13 (mean = 17.7±2.1), indicating that anaemia was likely due to iron deficiency.
Background Characteristics and Outcomes
There were no statistically significant differences in background characteristics between infants with or without evidence of prenatal iron deficiency, whether assessed by maternal anaemia or low cord blood serum ferritin. In terms of background factors that correlated with 2 or more ABR variables, birth weight, sex, and length-for-age z-score did so at 3 months. At 10 months, current length-for-age z-score was the only background variable to do so. These background variables were included as covariates in all analyses of ABR outcomes at the appropriate ages.
Maternal Iron Status and ABR Outcomes
At 3 months, infants whose mothers were anaemic in the 3rd trimester had significantly longer latencies for wave V and wave III–V and I–V intervals, compared to infants of non-anaemic mothers (Table 3). Effect sizes were large (>1.0 SD unit). There was no relation between maternal anaemia and ABR measures at 10 months. Low maternal ferritin did not relate to infant ABR variables at 3 or 10 months. Iron depletion in almost all mothers likely restricted our ability to detect relations with ABR outcomes.
Table 3.
Maternal 3rd-trimester anaemia (Hb <110 g/L) and infant ABR at 3 months (N=47)a
| Infants of anaemic mothers | Infants of non-anaemic mothers | F(1,42) | p | Effect sizeb | Significant background variables | |
|---|---|---|---|---|---|---|
| N | 8 | 39 | ||||
| Absolute latencies (ms) | ||||||
| Wave I | 1.63 (0.18) | 1.67 (0.14) | 0.14 | 0.71 | 0.32 | Birth weight |
| Wave III | 4.46 (0.17) | 4.43 (0.17) | 0.02 | 0.88 | 0.17 | Birth weight |
| Wave V | 6.72 (0.22) | 6.53 (0.19) | 4.62 | 0.04 | 1.02 | Sex |
| Interpeak latencies (ms) | ||||||
| Wave I–III interval | 2.84 (0.12) | 2.76 (0.17) | 0.26 | 0.61 | 0.47 | Length-for-age z-score |
| Wave III–V interval | 2.26 (0.19) | 2.10 (0.15) | 5.55 | 0.02 | 1.06 | |
| Wave I–V interval | 5.09 (0.16) | 4.86 (0.21) | 5.39 | 0.03 | 1.19 | Sex |
| Amplitude (μV) | ||||||
| Wave I | 0.14 (0.04) | 0.14 (0.06) | 0.09 | 0.77 | 0.02 | |
| Wave III | 0.19 (0.06) | 0.21 (0.05) | 0.79 | 0.78 | 0.33 | Sex |
| Wave V | 0.17 (0.08) | 0.21 (0.06) | 1.12 | 0.30 | 0.62 |
Values are mean (SD). One infant with ABR data at 3-month was not included due to missing maternal Hb. Statistical significance was determined by analysis of covariance with birth weight, sex, or length-for-age z-score at 3-month as covariates as indicated. Significant differences are shown in bold.
Effect size is the difference between group means divided by the overall SD. Moderate to large effect sizes are shown in bold, regardless of statistical significance, since the number of infants of anaemic mothers was small and effect size is relatively independent of sample size.
Infant Iron Status and ABR Outcomes
Low cord blood serum ferritin (<75 μg/L) did not relate to 3-month ABR variables. However, at 10 months, infants with low ferritin at birth showed longer latency for wave I, a suggestive trend for longer latency of wave V, and smaller amplitude for wave V, compared to infants with normal cord blood serum ferritin (Table 4). Effect sizes were considerable (0.6 SD unit). There were no statistically significant ABR differences related to anaemia at 10 months. In exploratory analyses that considered the combined impact of low ferritin at birth and anaemia at 10 months, there was a statistically significant interaction between iron status at birth and anaemia at 10 months for the wave III–V interval, F(1,34)=6.27, p=0.017). Post-hoc tests (Bonferroni) showed that the latency of the wave III–V interval was longer for infants with low cord ferritin and anaemia at 10 months, compared to the other groups (i.e., infants with low ferritin at birth and no anaemia at 10 months, anaemia at 10 months and normal cord ferritin, or normal ferritin at birth and no anaemia at 10 months) (Figure 1).
Table 4.
Low cord blood ferritin (<75 μg/L) and infant ABR at 10 months (N=45)a
| Low cord blood iron status | Normal cord blood iron status | F(1,42) | p | Effect sizeb | Significant background variables | |
|---|---|---|---|---|---|---|
| N | 19 | 26 | ||||
| Absolute latencies (ms) | ||||||
| Wave I | 1.65 (0.17) | 1.57 (0.12) | 5.50 | 0.02 | 0.58 | Length-for-age z-score |
| Wave III | 4.12 (0.26) | 4.06 (0.21) | 0.94 | 0.34 | 0.25 | |
| Wave V | 6.11 (0.23) | 6.00 (0.17) | 3.69 | 0.06 | 0.62 | |
| Interpeak latencies (ms) | ||||||
| Wave I–III interval | 2.47 (0.20) | 2.49 (0.17) | 0.20 | 0.66 | 0.12 | |
| Wave III–V interval | 2.00 (0.17) | 1.94 (0.15) | 0.95 | 0.33 | 0.39 | |
| Wave I–V interval | 4.47 (0.18) | 4.43 (0.16) | 0.24 | 0.62 | 0.25 | Length-for-age z-score |
| Amplitude (μV) | ||||||
| Wave I | 0.15 (0.06) | 0.18 (0.06) | 4.64 | 0.04 | 0.61 | |
| Wave III | 0.22 (0.08) | 0.25 (0.08) | 2.35 | 0.13 | 0.47 | |
| Wave V | 0.27 (0.08) | 0.31 (0.07) | 2.16 | 0.15 | 0.52 |
Values are mean (SD). Statistical significance was determined by analysis of covariance controlling for length-for-age z-score at 10 months as indicated. Significant differences are shown in bold.
Moderate to large effect sizes are shown in bold.
Figure 1.
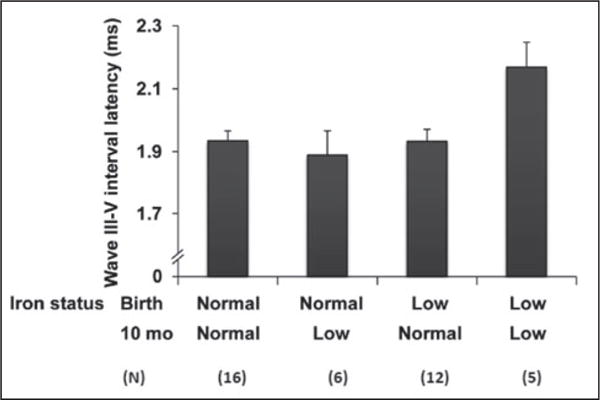
The effects of low ferritin at birth and anaemia at 10 months on wave III–V interval latency. Latency was significantly longer in the group with low iron status at both ages, compared to each other group (p values <0.05).
The absence of a full iron status panel and missing ferritin data at 10 months prevented us from determining iron status in all infants at that age. However, 10-month ferritin values were available for all 5 infants with low ferritin at birth and anaemia at 10 months. The mean ferritin was 11.9 μg/L (highest value 19.1 μg/L), indicating that their iron status was compromised both at birth and 10 months.
Discussion
In this pilot study, ABR latencies for wave V and wave III–V and I–V intervals were prolonged at 3 months for infants whose mothers were anaemic in the last trimester. At 10 months, infants with low cord blood ferritin showed longer latencies for wave I and wave V (suggestive trend) and smaller amplitude for wave V. In addition, wave III–V interval latency was longer for infants with low ferritin at birth and anaemia at 10 months, compared to the other combinations. These findings provide evidence that prenatal iron deficiency may adversely affects the functional status of the auditory system in full-term healthy infants, with effects observed throughout much of the 1st year of life.
It is generally accepted that interpeak latency changes relate to increases in conduction velocity during axonal myelination,13 whereas amplitude changes are probably the result of improvements in synchronisation at the axonal or synaptic levels.28 Given that most of our findings relate to differences in latency, we postulate that impaired myelination is the most likely explanation. Studies in animal models provide evidence that the peak uptake of brain iron coincides with the peak period of myelination, which develops rapidly during the late gestational and early postnatal periods.29,30 Oligodendrocytes, which are responsible for making myelin, are particularly sensitive to iron deficiency, resulting in altered composition and amount of myelin in white matter.31 As myelination is initiated, iron, ferritin and transferrin (Tf) mRNA are found within oligogendrocytes.32,33 In rats, iron deficiency during gestational and early postnatal periods is associated with less total myelin protein as well as decreased cholesterol, phospholipids, and galactolipids.31 We previously reported that perinatal iron deficiency in rats delayed myelination of subcortical white matter and the fimbria of the hippocampus and also altered behavioural outcomes.5 Many such changes appear to be persistent and do not reverse with iron treatment.3 This evidence supports the interpretation that our ABR results are primarily due to impaired myelination, especially when considering the temporal and spatial sequence of auditory system myelination during development. However, iron deficiency also disrupts neurotransmitter function and energy metabolism,3 which could contribute to the lower 10-month wave V amplitude observed among infants with low cord blood ferritin. In addition, a study in growing rats showed that iron deficiency adversely affected cochlear structure, such as loss and altered structure of stereocilia.34 If cochlear effects also occur in the human infant, they might help explain the longer wave I latency at 10 months among infants born with low iron stores.
Our study design differs from the few previous studies of ABR in early iron deficiency.8–12 Two recent studies involving at-risk neonates did not report effects beyond the neonatal period.17,18 In all other studies, iron-deficiency anaemia was identified postnatally at 6 months of age or later. The age range was often wide (from 6–7 months up to 36–60 months), and there was no information on iron status at birth. Nonetheless, the majority of studies show prolonged absolute and/or interpeak latencies in the iron-deficient anaemic group.8,9,11 Long-term follow-up data come from a study in Chile. At 6 months, infants with iron-deficiency anaemia showed a tendency toward longer latency for the wave I–V interval than non-anaemic infants. The magnitude of the differences in wave V latency and wave III–V and I–V intervals increased over the next year, despite iron therapy.9 The increasing magnitude of differences suggests that effects on myelinating systems may take some time to be fully apparent. At 4 years, absolute latencies for all ABR waves and interpeak latencies (except I–III interval) were still >1.0 SD longer in former iron-deficient anaemic children.35 Thus, the effects appear to be long-lasting.
An unanswered question is whether previous results are due to postnatal iron-deficiency anaemia or combined pre-and postnatal iron deficiency. Since iron deficiency is common in pregnant women and infants worldwide, many infants, especially in developing countries, are likely to be exposed to iron deficiency pre- and postnatally. For instance, it is likely that many of the Chilean infants had pre- and postnatal iron deficiency, since iron-deficiency anaemia was detected at the young age of 6 months. Our results and those of the recent studies of premature and infants with other risk factors,18 indicate that prenatal iron status is an important factor in ABR development.
Our exploratory analysis of iron status at birth and anaemia at 10 months points to the combined impact of pre- and postnatal iron deficiency. The latency of the interpeak wave III–V interval was prolonged only among infants with both low ferritin at birth and anaemia at 10 months, all of whom had poor iron status, as indicated by low-to-marginal ferritin and high Mentzer index. The interpeak wave III–V interval reflects the speed of transmission in the more central part of the auditory pathway and might be particularly sensitive to early iron deficiency, given iron’s role in myelination and the centripetal nature of myelination of the auditory pathway.36 This exploratory analysis leads us to hypothesise that prenatal iron deficiency is a necessary but not sufficient condition for alterations in interpeak latencies in later infancy. It is possible that there may be recovery if prenatal iron deficiency is not followed by postnatal iron-deficiency anaemia and resilience if postnatal iron-deficiency anaemia is not preceded by poor prenatal iron status. However, the sample size for this analysis was very small.
Our study is limited by small sample size for infants with longitudinal ABR data both at 3 and 10 months and the lack of more iron status measures at 10 months. Although our sample size for the cross-sectional analyses at 3 and 10 months is comparable to other ABR and iron deficiency studies, the missing data at both 3 and 10 months is a major drawback to longitudinal analysis. Therefore, our observations on combined effects of pre- and/or postnatal iron deficiency are best considered as an impetus for further investigation. There is also need for replication of several key findings. For instance, our findings that maternal anaemia predicted longer ABR latencies in early infancy (3-month) and low cord blood ferritin predicted longer latencies later on (10-month) have no counterpart in other studies. We could speculate that maternal anaemia and low ferritin at birth capture prenatal iron deficiency at somewhat different time periods and that ABR changes might be detectable at different ages, given that the effect of any compromise to myelination probably takes some time to be observable.
Despite these limitations, the study adds to the little available research on prenatal iron deficiency. There is increasing evidence that prenatal iron deficiency impacts more than the developing auditory system. One previous study found that neuromotor reflexes were more immature in preterm infants with iron-deficiency anaemia.37 In another study, social-emotional or temperament-like behaviours related to iron status at birth.38 Tamura et al found that low cord ferritin concentrations predicted poorer neurodevelopment at 5 years of age.24 A longitudinal study of infants of diabetic mothers observed that those with low cord blood ferritin had impaired recognition memory processing throughout early childhood.39 These studies, combined with the ABR results from our study and two other studies,17,18 suggest that prenatal iron deficiency affects several developing brain and behavioural systems in the human infant.
In conclusion, we postulate that the developmental effects of iron deficiency depend on the timing, duration, and severity of iron deficiency. These parameters can be experimentally manipulated in animal models, and the accumulating evidence points to the importance of prenatal iron deficiency.3,4 Well-designed human studies, with specific and sensitive measures, can also help determine the combined and separate effects of pre- and/or postnatal iron deficiency on short- and long-term sensory, motor, cognitive, language, and social-emotional functioning. These are urgently needed, since iron deficiency is common in both periods and developmental effects of prenatal iron deficiency have received little attention in human infants.
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (#30671773), University of Michigan Global REACH, and the US National Institutes of Health (P01 HD039386). The preliminary data in this paper has been presented in a poster session at the Paediatric Academic Societies meeting in Vancouver, Canada, 2010 (Abstract #751721).
The authors thank Yaping Shi, Dongbo Zhu, Xiurong Yi, and members of Fuyang, Yongkang and Huzhou Maternal and Child Health Hospitals for their assistance with subject enrollment and cord blood collection, Liqin Chen for ferritin analyses, and the infants and families who participated in the study.
Footnotes
Conflict of Interest Statement
The authors declare that there is no actual or potential conflict of interest that could inappropriately influence this work.
Contributor Information
J Lou (樓港旰), Department of Gastroenterology, Children’s Hospital Zhejiang University School of Medicine, 57 Zhugan Xiang Road, Hangzhou, Zhejiang 310003, China.
X Mai, Center for Human Growth and Development, University of Michigan, 300 North Ingalls Street, Ann Arbor, MI 48109, United States.
B Lozoff, Center for Human Growth and Development, University of Michigan, 300 North Ingalls Street, Ann Arbor, MI 48109, United States; Department of Pediatrics and Communicable Diseases, University of Michigan, 300 North Ingalls Street, Ann Arbor, MI 48109, United States.
BT Felt, Center for Human Growth and Development, University of Michigan, 300 North Ingalls Street, Ann Arbor, MI 48109, United States; Department of Pediatrics and Communicable Diseases, University of Michigan, 300 North Ingalls Street, Ann Arbor, MI 48109, United States.
PR Kileny, Department of Otorhinolaryngology, University of Michigan, 1500 East Medical Center Drive, Ann Arbor, MI 48109, United States.
Z Zhao (牧正瘀), Department of Child Health Care, Children’s Hospital Zhejiang University School of Medicine, 57 Zhugan Xiang Road, Hangzhou, Zhejiang 310003, China.
J Shao (潋潔), Department of Child Health Care, Children’s Hospital Zhejiang University School of Medicine, 57 Zhugan Xiang Road, Hangzhou, Zhejiang 310003, China.
References
- 1.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85:614S–20S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 2.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–65. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beard JL. Recent evidence from human and animal studies regarding iron status and infant development. J Nutr. 2007;137:524S–30S. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- 5.Wu LL, Zhang L, Shao J, et al. Effect of perinatal iron deficiency on myelination and associated behaviors in rat pups. Behav Brain Res. 2008;188:263–70. doi: 10.1016/j.bbr.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Felt BT, Beard JL, Schallert T, et al. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–70. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz E, Pasquini JM, Thompson K, et al. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res. 2004;77:681–9. doi: 10.1002/jnr.20207. [DOI] [PubMed] [Google Scholar]
- 8.Li YY, Wang HM, Wang WG. The effect of iron deficiency anemia on the auditory brainstem response in infant [Chinese] Zhonghua Yi Xue Za Zhi. 1994;74:367–9. [PubMed] [Google Scholar]
- 9.Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: Delayed maturation of auditory brain stem responses. Am J Clin Nutr. 1998;68:683–90. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- 10.Kurekci AE, Sarici SU, Karaoglu A, et al. Effects of iron deficiency versus iron deficiency anemia on brainstem auditory evoked potentials in infancy. Turk J Pediatr. 2006;48:334–9. [PubMed] [Google Scholar]
- 11.Cankaya H, Oner AF, Egeli E, Caksen H, Uner A, Akcay G. Auditory brainstem response in children with iron deficiency anemia. Acta Paediatr Taiwan. 2003;44:21–4. [PubMed] [Google Scholar]
- 12.Sarici SU, Serdar MA, Dundaroz MR, et al. Brainstem auditory-evoked potentials in iron-deficiency anemia. Pediatr Neurol. 2001;24:205–8. doi: 10.1016/s0887-8994(00)00270-8. [DOI] [PubMed] [Google Scholar]
- 13.Hecox K, Burkard R. Developmental dependencies of the human brainstem auditory evoked response. Ann NY Acad Sci. 1982;388:538–56. doi: 10.1111/j.1749-6632.1982.tb50815.x. [DOI] [PubMed] [Google Scholar]
- 14.Starr A, Amlie RN, Martin WH, Sanders S. Development of auditory function in newborn infants revealed by auditory brainstem potentials. Pediatrics. 1977;60:831–9. [PubMed] [Google Scholar]
- 15.Eggermont JJ, Salamy A. Maturational time course for the ABR in preterm and full term infants. Hear Res. 1988;33:35–47. doi: 10.1016/0378-5955(88)90019-6. [DOI] [PubMed] [Google Scholar]
- 16.Moore JK, Ponton CW, Eggermont JJ, Wu BJ, Huang JQ. Perinatal maturation of the auditory brain stem response: Changes in path length and conduction velocity. Ear Hear. 1996;17:411–8. doi: 10.1097/00003446-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr. 2010;156:377–81. doi: 10.1016/j.jpeds.2009.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin SB, Orlando M, Wang H. Latent iron deficiency in utero is associated with abnormal auditory neural myelination in >/= 35 weeks gestational age infants. J Pediatr. 2013;163:1267–71. doi: 10.1016/j.jpeds.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Zhu YP, Liao QK, Collaborative Study Group Prevalence of iron deficiency in children aged 7 months to 7 years in China [Chinese] Chin J Pediatr. 2004;42:886–91. [PubMed] [Google Scholar]
- 20.Shao J, Lou J, Rao R, et al. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142:2004–9. doi: 10.3945/jn.112.162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47:1–29. [PubMed] [Google Scholar]
- 22.MacPhail AP, Charlton RW, Bothwell TH, Torrance JD. The relationship between maternal and infant iron status. Scand J Haematol. 1980;25:141–50. doi: 10.1111/j.1600-0609.1981.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 23.Fleming RE. Cord serum ferritin levels, fetal iron status, and neurodevelopmental outcomes: correlations and confounding variables. J Pediatr. 2002;140:145–8. doi: 10.1067/mpd.2002.121931. [DOI] [PubMed] [Google Scholar]
- 24.Tamura T, Goldenberg RL, Hou J, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–70. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- 25.Beard JL, Murray-Kolb LE, Rosales FJ, Solomons NW, Angelilli ML. Interpretation of serum ferritin concentrations as indicators of total-body iron stores in survey populations: the role of biomarkers for the acute phase response. Am J Clin Nutr. 2006;84:1498–505. doi: 10.1093/ajcn/84.6.1498. [DOI] [PubMed] [Google Scholar]
- 26.Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92:73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vehapoglu A, Ozgurhan G, Demir AD, et al. Hematological indices for differential diagnosis of Beta thalassemia trait and iron deficiency anemia. Anemia. 2014;2014:576738. doi: 10.1155/2014/576738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudell AP. A fiber tract model of auditory brain-stem responses. Electroencephalogr Clin Neurophysiol. 1987;67:53–62. doi: 10.1016/0013-4694(87)90163-5. [DOI] [PubMed] [Google Scholar]
- 29.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. GLIA. 1996;17:83–93. doi: 10.1002/(SICI)1098-1136(199606)17:2<83::AID-GLIA1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Crowe A, Morgan EH. Iron and transferrin uptake by brain and cerebrospinal fluid in the rat. Brain Res. 1992;592:8–16. doi: 10.1016/0006-8993(92)91652-u. [DOI] [PubMed] [Google Scholar]
- 31.Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. GLIA. 2009;57:467–78. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 32.Bartlett WP, Li XS, Connor JR. Expression of transferrin mRNA in the CNS of normal and jimpy mice. J Neurochem. 1991;57:318–22. doi: 10.1111/j.1471-4159.1991.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 33.Cheepsunthorn P, Palmer C, Connor JR. Cellular distribution of ferritin subunits in postnatal rat brain. J Comp Neurol. 1998;400:73–86. [PubMed] [Google Scholar]
- 34.Sun AH, Xiao SZ, Zheng Z, Li BS, Li ZJ, Wang TY. A scanning electron microscopic study of cochlear changes in iron-deficient rats. Acta Otolaryngol. 1987;104:211–6. doi: 10.3109/00016488709107320. [DOI] [PubMed] [Google Scholar]
- 35.Algarin C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: Long-lasting effects on auditory and visual systems functioning. Pediatr Res. 2003;53:217–23. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- 36.Jiang ZD. Maturation of the auditory brainstem in low risk-preterm infants: A comparison with age-matched full term infants up to 6 years. Early Hum Dev. 1995;42:49–65. doi: 10.1016/0378-3782(95)01639-k. [DOI] [PubMed] [Google Scholar]
- 37.Armony-Sivan R, Eidelman AI, Lanir A, Sredni D, Yehuda S. Iron status and neurobehavioral development of premature infants. J Perinatol. 2004;24:757–62. doi: 10.1038/sj.jp.7211178. [DOI] [PubMed] [Google Scholar]
- 38.Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol. 2005;46:141–53. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- 39.Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood. Dev Neuropsychol. 2009;34:762–79. doi: 10.1080/87565640903265145. [DOI] [PMC free article] [PubMed] [Google Scholar]


