Abstract
Gastric cancer is the second most frequent cause of cancer death worldwide, although much geographical variation in incidence exists. Prevention and personalised treatment are regarded as the best options to reduce gastric cancer mortality rates. Prevention strategies should be based on specific risk profiles, including Helicobacter pylori genotype, host gene polymorphisms, presence of precursor lesions, and environmental factors. Although adequate surgery remains the cornerstone of gastric cancer treatment, this single modality treatment seems to have reached its maximum achievable effect for local control and survival. Minimally invasive techniques can be used for treatment of early gastric cancers. Achievement of locoregional control for advanced disease remains very difficult. Extended resections that are standard practice in some Asian countries have not been shown to be as effective in other developed countries. We present an update of the incidence, causes, pathology, and treatment of gastric cancer, consisting of surgery, new strategies with neoadjuvant and adjuvant chemotherapy or radiotherapy, or both, novel treatment strategies using gene signatures, and the effect of caseload on patient outcomes.
Introduction
Gastric cancer is a very common disease worldwide and the second most frequent cause of cancer death, affecting about one million people per year.1 The ratio of men to women is about 2:1. Large differences in incidence exist between continents. The highest incidence—up to 69 cases per 100 000 people per year—is in men in northeast Asia (Japan, Korea, and China).2 Intermediate incidences occur in Europe and South America; North America, Africa, south Asia, and Oceania (including Australia and New Zealand) are low-incidence regions, with rates of 4–10 cases per 100 000 people.
Explanations for these differences in incidence have been sought. High intake of various traditional salt-preserved foods and salt, and low consumption of fresh fruit and vegetables are associated with a raised risk of gastric cancer.3,4 Further in support of this idea is the finding that gastric cancer incidence in migrants from low-incidence countries increases from a low rate in first-generation migrants to the high incidence of their host country in the second generation.5 Additionally, Helicobacter pylori is a major risk factor for development of gastric cancer.6 However, not all populations with high rates of H pylori infection, such as Africa and south Asia, have a raised incidence of gastric cancer. Differences in H pylori cagA and vacA genotypes might explain these geographical variations.2 Smoking is another important environmental risk factor for gastric cancer.7
Primary prevention strategies to reduce gastric cancer include improvement of sanitation, high intake of fresh fruits and vegetables, safe food-preservation methods, and avoidance of smoking. Although frequency of distal gastric cancer has declined, incidence of proximal gastric cancer has risen. Unlike distal gastric cancer, development of proximal gastric cancer is mainly related to gastro-oesophageal reflux and obesity.8
Countries with high incidences of gastric cancer have screening programmes for groups at high risk, but clinical evidence is insufficient to recommend endoscopic screening worldwide.1 Of 880 000 people diagnosed with gastric cancer in 2000, about 650 000 (74%) died of the disease. In Japan, survival is good (52%), in part attributable to early detection in screening programmes, whereas survival in the USA, Europe, and China generally is only 20–25%.9 Survival in patients with resectable gastric cancer is better than for those with unresectable disease, but even in the resectable group more than half of patients in developed countries (excluding Japan) die.
Improved imaging techniques enable patients to be staged more adequately than previously. Minimally invasive techniques such as endoscopic resections, sentinel node, and laparoscopy have been developed and can be used for early stages of disease. For advanced gastric cancer, achievement of locoregional control remains a substantial difficulty. In the Gunderson re-operative series,10 54% of patients had locoregional recurrence only. To improve results, the extension of surgery has been studied widely. Use of neoadjuvant and adjuvant treatment to further improve results continues to be investigated. A biological approach might lead to further individualised treatment options.
Aetiology
Hereditary diffuse gastric cancer accounts for about 1–3% of gastric cancer cases. In roughly 30% of familial gastric cancers, a germline mutation in one allele of the E-cadherin gene (CDH1) is identified.11 Inactivation of the second allele happens either by mutation or hypermethylation.12 Additional genomic changes eventually lead to early onset of diffuse gastric cancer. Estimated life-time risk of gastric cancer in carriers of a CDH1 mutation is 67% in men and 83% in women. In families with at least two people with diffuse gastric cancer, of whom one is diagnosed before age 50 years, mutational analysis is recommended.13 Histopathological examination of prophylactic gastrectomy specimens has identified macroscopically invisible, small foci of signet-ring-cell formation and invasion. Although the clinical significance of such foci is not clear, prophylactic gastrectomy should be considered in mutation carriers. Other hereditary syndromes that raise gastric cancer risk include Lynch syndrome (mutation in one of the mismatch repair genes),14 and Peutz-Jeghers syndrome (STK11 mutation).15
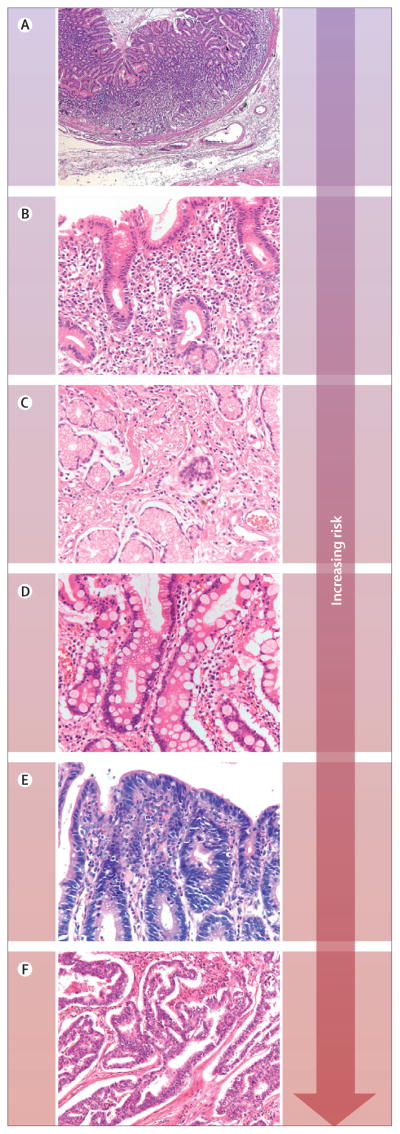
Sporadic gastric cancer of the intestinal type develops through a sequence of precursor lesions (figure 1)16 induced by H pylori infection.17–19 A meta-analysis of 12 studies, 6 including 1228 cancer cases and 3406 controls, showed that people positive for H pylori have at least a six-fold greater risk of developing gastric adenocarcinoma than do those without infection. In a subset of people, long-term H pylori infection led to atrophic gastritis and intestinal metaplasia, with increased relative risk (RR) for development of gastric cancer, ranging from 1·7 in moderate atrophy and 4·9 in severe atrophy, to 6·4 in intestinal metaplasia.20
Figure 1.
Correa sequence precursor gastric lesions
Sequence shows increasing risk for development of intestinal-type gastric carcinoma. (A) Normal mucosa. (B) Chronic gastritis. (C) Mucosal atrophy. (D) Intestinal metaplasia. (E) Dysplasia. (F) Intestinal-type carcinoma.
Host factors—such as polymorphisms in cytokine genes (eg, interleukin 1β, interferon receptor 1, and toll-like receptor 4),21–23 and predominant T-helper1 inflammatory response24—and bacterial factors (eg, presence of vacuolating toxin and the cag pathogenicity island25,26) are associated with increased intensity of inflammation and progression risk. Additional oxidative stress from bacterial overgrowth, nutritional factors (eg, high salt and low vitamin intake), and smoking is thought to cause DNA damage, thus further heightening cancer risk.7,16,27
Histopathology and molecular pathology
The intestinal-type gastric carcinoma has well defined ductal structures or cords, surrounded by a desmoplastic stroma reaction containing different amounts of a mixed inflammatory infiltration. Tumour cells are large, and nuclei are polymorphic and anisochromatic, and have a coarse chromatin pattern. Mitotic figures are easily detected. Intestinal-type carcinomas are usually well to moderately well differentiated. By contrast, diffuse-type adenocarcinomas have solitary or small groups of tumour cells without formation of glandular structures. Sometimes clear cytoplasmic vacuoles can be seen. These mucus-containing cells push the nucleus to the cell periphery (signet-ring-cell carcinoma). Generally, extensive newly formed stroma is present, making identification of separate tumour cells difficult in standard haematoxylin and eosin sections. Additional keratin staining reveals the true extent of the tumour.
Gastric carcinoma is the result of accumulated genomic damage, affecting cellular functions essential for cancer development (eg, self-sufficiency in growth signals, escaping antigrowth signals, apoptosis resistance, sustained replicative potential, angiogenesis induction, and invasive or metastatic potential).28 These genomic changes might arise through two distinct genomic instability pathways—microsatellite instability and chromosomal instability.29 Additionally, a cag-pathogenicity-island-methylator phenotype (CIMP) has been implicated as a separate mechanism causing DNA damage.30–32 Although knowledge of these pathways and the oncogenes and tumour-suppressor genes implicated in carcinogenesis are regarded as a means to reveal new therapeutic targets or predictive markers of therapy response, no such biomarkers are yet available.
About 15% of gastric carcinomas are associated with a defective mismatch repair system.33,34 During cell replication, this system recognises basepair mismatches, which occur by addition or deletion of a base. A complex of mismatch repair proteins (eg, MLH1, MSH2, MSH6, and PMS2) excise the mismatched lesion and resynthesise the DNA before the cell cycle is completed. In sporadic gastric cancer, silencing of MLH1 proteins through promoter hypermethylation is the most frequent cause of microsatellite instability,35 leading to an amplified mutation rate at the nucleotide stage. Accumulation of mutations leads to activation of oncogenes or inactivation of tumour suppressor genes, or both, by which cells can gain growth advantage and invasive capability. Microsatellite instability has been associated with clinicopathological characteristics, such as intestinal-type carcinoma, antral location, less frequent lymph-node metastases, and extended survival.36,37
The role of microsatellite instability in tumour response to fluorouracil is uncertain, and is most extensively studied in colorectal cancer.38–40 Only one study41 addressed the relation between response to chemotherapy and microsatellite instability in gastric cancer; however, patient numbers were too low to draw conclusions. Large clinical trials are needed to establish the role of this pathway in tumour response to treatment for gastric cancer.
The other sporadic carcinomas—roughly 85%—show chromosomal instability, resulting in numerical (gains, losses, and amplifications) or structural (eg, trans locations) changes of large parts of, or even whole, chromosomes, with an aneuploid DNA pattern. By contrast with microsatellite instability, the mechanism underlying chromosomal instability is largely unknown. Mitotic chromosomal missegregation and errors in the mitotic spindle checkpoint have been implicated. Mechanisms and genes involved in these processes have been reviewed by Aguilera and Gomez-Gonzalez.42
In gastric cancer, the most frequently reported numerical aberrations by comparative genomic hybridisation are gains of chromosomes 3q, 7q, 8q, 13q, 17q, and 20q, and losses on chromosomes 4q, 5q, 6p, 9p, 17p, and 18q. Consistent high-level amplifications are located on chromosomes 7q, 8p, 8q, 17q, 19q, and 20q.43–48 Specific chromosomal changes have been associated with clinicopathological variables—eg, tumour type, tumour progression, and lymph-node metastasis.49,50 A few studies have shown an association between high-level chromosomal instability with a good response to cisplatin-based chemotherapy and poor survival.41–53 However, despite the development of high-resolution array comparative genomic hybridisation,51,52 the exact genes responsible for oncogenesis are still unknown.
CIMP might be a third pattern of genomic instability. Hypermethylation of gene promoters leads to gene silencing,54 and therefore increased methylation could be an attractive approach for investigation of carcinogenesis. However, much overlap of microsatellite instability and CIMP has been noted in gastric cancer, suggesting microsatellite instability is a confounding factor.55,56 Irrespective of CIMP being a separate pathway in gastric carcinogenesis, presence of hypermethylation of important genes could be clinically relevant, because methylation can be reverted by DNA methyltransferase inhibitors, thus reactivating genes.57 We need to establish the role of these agents.
Prevention and early detection
H pylori eradication and surveillance of precursor lesions for early detection have long been thought the best approaches to reduce gastric cancer mortality. However, follow-up studies investigating the effect of H pylori eradication have shown contradictory results for reversibility of precursor lesions and reduction of gastric cancer rate. Although eradication has a prophylactic effect on gastric cancer in experimental studies, the effect in people remains controversial. A meta-analysis58 of four randomised intervention studies, with gastric cancer incidence as a secondary outcome, showed a non-significant overall odds ratio (OR) of 0·67 (95% CI 0·42–1·07). These inconsistencies might be explained by sampling error, time of follow-up, and different baseline characteristics.59 Importantly, other factors such as dietary intake, geographical origin of patients, topographical location of the lesions, and gene polymorphisms might have affected study results. Large clinical studies incorporating all these factors into the study design are needed to identify which combinations of factors predict clinical outcome and cancer risk. Additionally, improved knowledge of molecular changes in precursor lesions might enable further discrimination between patients at high and low risk. These studies could establish which patients will benefit from H pylori eradication, and in whom surveillance of precursors should be done. Thus, evidence-based, personalised screening programmes can be designed for high-risk subgroups in a cost-effective way. Presently, insufficient clinical evidence is available to recommend endoscopic screening worldwide.1
Diagnosis and imaging
No typical signs suggestive of gastric cancer exist. In advanced disease, pain in the epigastric region, anaemia, aversion to meat, weight loss, obstruction, bleeding, and perforation might arise. Diagnosis should be made with a gastroscopic biopsy sample and histology specified by WHO criteria. Initial staging consists of clinical examination, including Virchow’s lymph nodes and digital rectal examination, blood counts, and liver and renal function tests. The currently known tumour markers are of little use in gastric cancer.60
The two major systems used to stage gastric cancer are the Japanese Classification of Gastric Cancer (JCGC), presently the 13th edition,61 and the International Union Against Cancer’s (UIAC) tumour-node-metastasis (TNM) system, which is in its sixth edition.62 These staging systems are continually evolving because of periodic validation studies.63 Diagnosis of T-stage disease by endoscopic ultrasound seems to be the most effective way to differentiate stage T1 and T2 from stage T3 and T4 (Q [overall accuracy]=0·93; 95% CI 0·91–0·95). Endoscopic ultrasound is less effective for diagnosis of nodal involvement than for tumour stage.64 In 278 patients with early gastric cancer, multidetector CT was shown to be useful for identification of extent of nodal involvement (overall accuracy 86%; 95% CI 0·82–0·90).65 Small lymph nodes, however, do not rule out lymph-node metastases.66 Multislice CT is regarded as more accurate than is single-slice CT.
PET is unique in its ability to visualise areas of enhanced metabolic activity within tissues. Most tumours larger than T1 can be identified, but differentiation between tumour stages is not possible. PET is not shown to have a high sensitivity for diagnosis of nodal involvement.67 Identification of metastases was analysed in one retrospective and three prospective studies.68–71 For liver and lung metastases, a CT scan was most useful. Endoscopic ultrasound has proved sensitive for detection of low volumes of ascites not apparent on CT, which is predictive for incurable disease.72 When peritoneal metastases are suspected, a laparoscopy is most sensitive.69 For assessment of response to preoperative treatment, PET seems promising.73
Surgical treatment
Early gastric cancer is defined as a tumour of the stomach confined to the mucosa or submucosa, irrespective of lymph-node metastases. For some of these tumours, risk of lymph-node metastasis is thought to be very low. For patients with a well to moderately well differentiated tumour of less than 2 cm in size with no submucosal invasion or lymph-angio invasion, local excision by endoscopic mucosal resection has been the preferred treatment in Japan for the past 15 years.74
A systematic review75 of the effectiveness and safety of endoscopic mucosal resection identified no randomised trials comparing endoscopic with surgical treatment. Results of cohort studies of endoscopically treated patients have shown disease-specific survival at 5 years and 10 years of more than 95%. Incidence of local recurrence is only 6%, and the chance of complications compares favourably with surgery (0·6% perforations and 14% bleeding).76 Additionally, prophylactic eradication of H pylori after endoscopic mucosal resection significantly reduced development of metachronous tumours (OR=0·353; 95% CI 0·161–0·775; p=0·009).77
Endoscopic submucosal dissection is a new technique that can remove even large tumours in one piece.78 In a comparison with endoscopic mucosal resection,79 resections removing tumours in one piece were more frequent in the endoscopic submucosal dissection group (92·7% vs 56%) and the 3-year recurrence-free rate was higher (97·6% vs 92·5%), at the expense of a higher rate of perforations (3·6% vs 1·2%), which were endoscopically managed in most cases. Gotoda and co-workers80 analysed lymph-node metastases of 5265 patients who had a gastrectomy with radical lymph-node dissection, and identified expanded criteria for endoscopic treatment of early gastric cancer, all based on tumour characteristics with a very low risk of lymph-node metastases. They showed that patients with tumours that were well differentiated intramucosally or submucosally of less than 3 cm were at very low risk of lymph-node metastases, and that those with poorly differentiated intramucosal tumours of less than 2 cm were also at very low risk.
Present indications for endoscopic submucosal dissection according to the Japanese guidelines are for well differentiated intramucosal (T1a) tumours only. In other developed countries, diagnosis of gastric cancer is made only when invasive disease is obvious from biopsy samples, and therefore pathological T1 or even T2a lesions of differentiated histology are often overlooked or managed as high-grade dysplasia. To avoid this mismanagement and ensure that minimally invasive treatment or early detection is available, endoscopic submucosal dissection should be done for lesions diagnosed as high-grade dysplasia.
For patients with no lymph-node metastases, a sentinel node procedure might avoid the risk of morbidity and mortality resulting from overtreatment by radical lymph-node dissection. Risk of lymph-node metastases rises with increased tumour stage. For early gastric cancer, the risk of lymph-node metastases is between 2% and 5% for patients with mucosal cancer, and 11–20% for those with submucosal cancer.81 Results of experimental studies82–85 show that the sentinel node technique seems to be most reliable for pathological T1 tumours with a diameter of less than 40 mm. For tumours 40 mm or more, the sentinel node technique is not recommended. Two validating studies of this technique in about 500 patients are underway in Japan.
Laparoscopic surgery has, since 1991, been adopted for treatment of gastric cancer—especially in Japan and Korea. The present status of laparoscopic surgery for gastric cancer was described in two recent reviews.86,87 Most early series and comparative studies have used laparoscopic resection for early and distal gastric cancer. However, as surgeons gain further experience, more extensive procedures are becoming more common than they were previously. Randomised controlled trials of laparoscopic gastrectomy compared with open gastrectomy were undertaken with small numbers of patients, with most operated on for early distal gastric cancer, but drawing meaningful conclusions from these studies was difficult.86
In Japan, early stage gastric cancer (T1N0 or T2N0) is regarded as the only indication for laparoscopic gastrectomy. As yet, evidence based on long-term outcomes to support laparoscopic gastrectomy for cancer is scarce. To establish laparoscopic surgery as standard treatment, multicentre randomised controlled trials87 comparing short-term and long-term outcomes of laparoscopic surgery versus open surgery are needed.
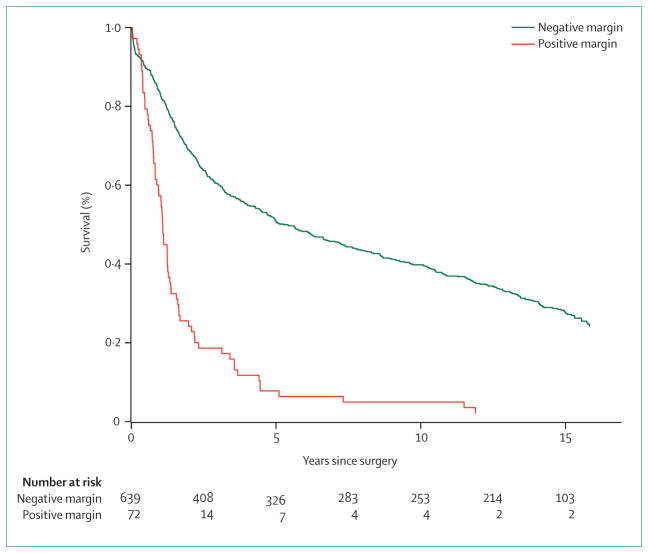
Only two randomised trials88,89 have investigated whether subtotal gastrectomy is sufficient for distal gastric cancer. Both trials identified no difference in mortality or survival. Positive resection margins, however, lead to very poor survival.90,91 In the Dutch gastric cancer trial,91 72 patients (10%) had a positive resection margin. 3-year survival was 18% compared with 63% when the resection margin was negative (figure 2). Microscopically involved margins greatly affected survival of patients with five or fewer lymph-node metastases in a comparative study of 619 patients.90 Intra-operative re-excision of microscopic disease identified from frozen section analysis resulted in a significant improvement in overall survival in patients with five or fewer positive nodes (p=0·03), but not in those with more than five positive nodes.
Figure 2.
Survival in patients with positive or negative resection lines
Data adapted from Songun and co-workers.91
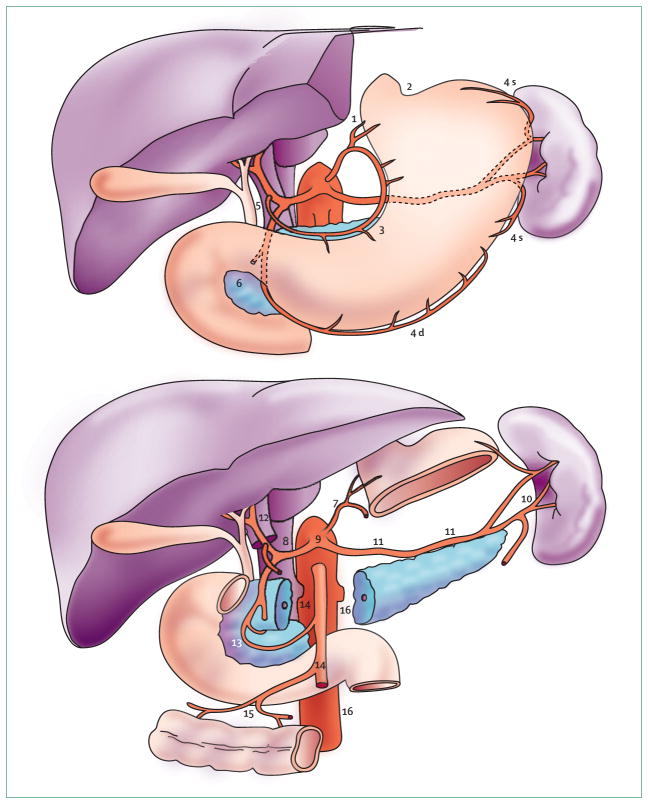
Lymph-node dissections are defined by the JCGC.61 These guidelines are also recommended by the American Joint Committee on Cancer, and by the IUAC. In these guidelines, 16 different lymph-node compartments (stations) surrounding the stomach are identified (figure 3). In general, perigastric lymph-node stations along the lesser (stations 1, 3, and 5) and greater (stations 2, 4, and 6) curvature are grouped N1, whereas nodes along the left gastric (station 7), common hepatic (station 8), coeliac (station 9), and splenic (stations 10 and 11) arteries are grouped N2. D1 dissection entails removal of the affected part of the stomach (distal or total), N1 lymph nodes, and the greater and lesser omentum. With a D2 dissection, N2 lymph nodes are also removed.
Figure 3.
Lymph-node stations surrounding stomach
1=right cardial nodes. 2=left cardial nodes. 3=nodes along lesser curvature. 4 s and 4 d=nodes along greater curvature. 5=suprapyloric nodes. 6=infrapyloric nodes. 7=nodes along left gastric artery. 8=nodes along common hepatic artery. 9=nodes around celiac axis. 10=nodes at splenic hilus. 11=nodes along splenic artery. 12=nodes in hepatoduodenal ligament. 13=nodes at posterior aspect of pancreas head. 14=nodes at root of mesenterium. 15=nodes in mesocolon of transverse colon. 16=para-aortic nodes.
For many years, clinicians have debated whether an extended lymph-node dissection (D2) for gastric cancer is beneficial. Theoretically, removal of a wide range of lymph nodes improves the chances for cure. Such resection, however, could be irrelevant when no lymph-nodes are affected, or when the cancer has developed into systemic disease, or the dissection increases morbidity and mortality substantially. So far, five randomised studies92–96 comparing D1 and D2 dissections have been completed (table 1). A Cochrane review97 showed a significantly increased mortality after D2 dissection (risk ratio 2·23, 95% CI 1·45–3·45), without a benefit in survival; hazard ratio (HR) 0·95 (95% CI 0·83–1·09).
Table 1.
Randomised trials for extension of lymphadenectomy
| Type | Number of patients | Morbidity | Mortality | 5-year survival | |
|---|---|---|---|---|---|
| 1982–8593 | D1; D2 | 22; 21 | 22%; 43% | 0; 0 | 69%; 67% |
| 1987–9195 | D1; D2 | 25; 30 | 0; 58% | 0; 3·3% | 45%; 35% |
| 1987–9492 | D1; D2 | 200; 200 | 28%; 46% | 6·5%; 13% | 35%; 33% |
| 1989–9394 | D1; D2 | 380; 331 | 25%; 43% | 4%; 10% | 45%; 47% |
| 1993–9996 | D1; D3 | 110; 111 | 7%; 17% | 0; 0 | 53·6%; 59·5% |
| 1995–200198 | D2; D4 | 263; 260 | 20·9%; 28·1% | 0·8%; 0·8% | 69%; 70% |
D1=limited lymph-node dissection. D2=extended lymph-node dissection.
A single-centre randomised trial96 comparing D1 and D3 dissections was the first to identify a difference (p=0·041) between overall survival in D1 dissections (53·6%; 95% CI 44·2–63·0) and D3 dissections (59·5%; 95% CI 50·3–68·7). No postoperative deaths occurred and morbidity was 12%. Only 13% of patients in this study had pancreatico-splenectomy compared with 23% in the Dutch gastric cancer trial.94 Analysis of the group that did not undergo a pancreatico-splenectomy in the Dutch trial showed a significant survival advantage for those who had a D2 lymph-node dissection (11-year survival 33% for D1 and 47% for D2, p=0·018; data unpublished). Thus, a D2 dissection might be beneficial if postoperative mortality can be avoided. More extended dissections than D2 with para-aortic lymph-node dissections did not seem to have any survival benefit in a large randomised Japanese trial.98
Splenectomy and pancreatectomy are important risk factors for morbidity and hospital mortality after D2 dissection. In randomised trials99,100 in Chile and Korea, researchers reported no survival benefit from splenectomy in patients with total gastrectomy, whereas morbidity was raised. One Japanese trial is underway,101 and two previous Japanese studies showed no improvement in survival when pancreatosplenectomy was combined with total gastrectomy, whereas morbidity was increased.102,103 The only comparative study of pancreatectomy was done by Wang and co-workers,104 who reported a rise in morbidity in the pancreatectomy group but no survival advantage.
On the basis of available data, we recommend that the pancreas and spleen should only be removed when there is direct tumour growth into these organs.
Caseload
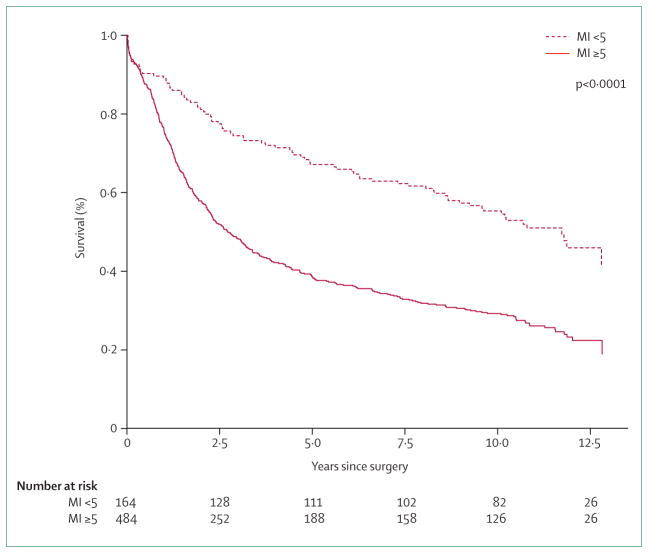
The Maruyama index of unresected disease is based on a study of 3843 patients.105 From each patient, the involvement of all separate lymph-node regions (figure 3) was registered. Based on seven input variables (age, sex, Borrmann type, tumour size, tumour location, tumour position, and histology) the likelihood for nodal involvement for each regional lymph-node station can be calculated. The Maruyama index can be calculated with the Maruyama computer program.105 This index is defined as the sum of regional nodal disease percentages for regional stations (1–12) not removed by the surgeon. In the Dutch gastric cancer trial,94 this index was calculated for 648 patients. A Maruyama index of less than five was associated with a significantly enhanced survival and a reduced relapse risk compared with patients who scored five or more (figure 4).106 Furthermore, in the Intergroup 0116 trial107 this index proved—on both univariate analysis (p=0·005) and multivariate analysis (p=0·036)—to be a significant predictor of survival.
Figure 4.
Overall survival based on Maruyama Index (MI) analysis
Overall survival for 648 patients with MI<5 and MI>5 status. Patients from Dutch D1–D2 trial cases (p<0·0001).106
In the Dutch trial94 (n=711), autopsy results were available for 441 deaths on study. Distant-only recurrence did not differ between Maruyama index categories, but isolated regional recurrence and regional plus distant recurrence occurred less frequently in the less than five index group than in the five or more group (p<0·001) (table 2).108 Thus, low Maruyama-index surgery seems to enhance regional control and survival. Furthermore, this index and the number of removed lymph nodes are good indicators of the quality of surgery. Both indicators could be used to identify patients with a high risk of recurrence and those for whom adjuvant treatment might be beneficial.
Table 2.
Analysis by autopsy of disease progression by Maruyama index
| MI <5 | MI ≥5 | |
|---|---|---|
| Died, no recurrence | 44 (59) | 110 (30) |
| Regional recurrence | 6 (8) | 78 (21) |
| Regional+distant | 14 (19) | 130 (36) |
| Distant only | 11 (15) | 48 (13) |
| Total | 75 (100) | 366 (100) |
Data are number of patients (%). p<0·001. Distant=distance recurrence. MI=Maruyama index. Data adapted from Hundahl and co-workers.108
Several studies have focused on the effect of hospital and surgeon caseload on patient outcomes, but no randomised trials have yet been done. A systematic review109 of 135 studies showed that high caseload is associated with improved outcomes across a wide range of procedures and conditions. However, only three of the studies were related to gastric cancer. Analyses110–114 of national cancer registry databases from the USA, Sweden, and Taiwan showed a clear benefit of high hospital caseload for postoperative mortality and survival, whereas studies from Scotland and the Netherlands did not report this relation.
Effect of caseload and the extent of resection on treatment outcomes in gastric cancer varies widely.115 Effect of hospital caseload was more important to patient outcomes than was surgeon caseload, although best results are seen in hospitals in which many patients are treated by surgeons with much experience. A study by Bachmann and co-workers116 supports management of gastric resections in specialised hospitals. Operative mortality rate fell by 41% (OR 0·59, 95% CI 0·32–1·07) for each addition of ten patients to doctors’ yearly surgical caseloads, and risk of death fell by 7% (HR 0·93, 95% CI 0·89–0·98; p=0·009) for every ten additional patients to a hospitals’ yearly caseload.116
Birkmeyer and co-workers117 assessed the effect of surgeon skill in large operations and concluded that, for many procedures, observed associations between hospital caseload and operative mortality are largely mediated by surgeon caseload. They suggested that patients can often improve their chances of survival substantially, even at hospitals with high caseloads, by selecting surgeons who frequently do the operation.
Neoadjuvant and adjuvant treatment
Radiotherapy
The optimum effect of surgery alone on local control and survival seems to have been reached—at least in developed countries. Therefore, preoperative and postoperative strategies with chemotherapy or radiotherapy, or both, have been and are presently being assessed. Radiotherapy is used as palliative treatment for uncontrolled gastric bleeding and unresectable tumours. In these cases, radiotherapy did not improve survival, but locoregional control rates of 70% were reported.118 Importantly, because of the high incidence of locoregional failures after surgical treatment, radiotherapy has been regarded as an attractive modality for curative treatment of gastric cancer.119,120 Radiotherapycanbegivenintra-operatively(intra-operative radio ther apy), or preoperatively, or postoperatively (with or with out concurrent chemotherapy) with external beam radiotherapy.
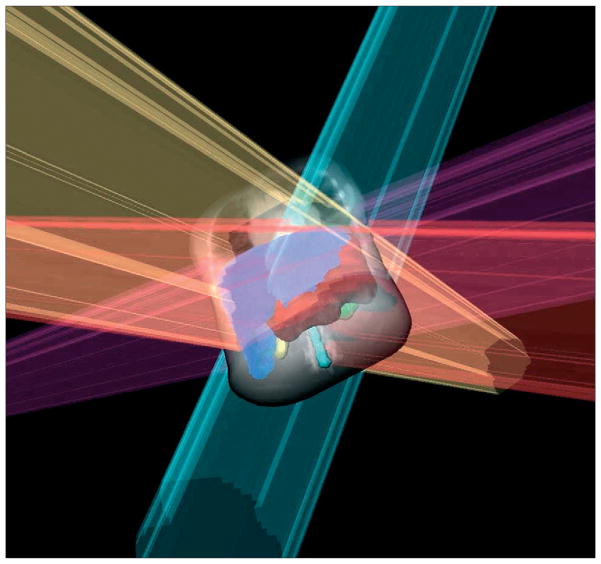
In a small prospective randomised trial,121 patients with non-metastatic disease at surgery were randomly assigned to either 20 Gy intra-operative radiotherapy to the gastric bed, or 50 Gy postoperative external beam radiotherapy in 25 fractions. Median survival was equal, but locoregional control was significantly better with intra-operative than with external beam radiotherapy (92% vs 44%, p<0·001), without a difference in toxicity. However, results of further studies122 showed that lower locoregional recurrence rates in intra-operative radiotherapy did not translate to improved survival, but morbidity was raised. Logistical difficulties, concerns of late toxicity, and emergence of other conformal external-beam techniques (eg, 3D and intensity modulated radiotherapy) are probably the reasons that intra-operative radiotherapy is not presently used widely (figure 5).
Figure 5.
Typical intensity modulated radiotherapy (IMRT) beam setup
IMRT beam setup for postoperative gastric cancer treatment. Blue=liver. Red=clinical target volume. Yellow=right kidney. Green=left kidney.
Adjuvant radiotherapy in gastric cancer has been assessed in several studies. In the British Stomach Cancer group study, 123 436 stage II and stage III patients were randomly assigned to either surgery only, or surgery then 45–50 Gy radiotherapy, or surgery plus eight courses of fluorouracil, adriamycin, and mitomycin chemotherapy. 5-year survival was identical in all three arms. The European Organisation for Research and Treatment of Cancer (EORTC) 124 randomly assigned 115 patients after surgery to four groups; 55·5 Gy radiotherapy only, radiotherapy with short-term concurrent fluorouracil chemotherapy, radiotherapy with long-term (1–18 months postoperatively) fluorouracil, and combined short-term and long-term chemotherapy. After correction for prognostic factors—such as tumour stage, age, and type of surgery—survival did not differ.
Theoretically, preoperative radiotherapy could be a good strategy because: radiotherapy will not be delayed by postoperative recovery; treatment target area is easy to demarcate because the tumour and stomach are still in the normal position, with good vascularisation and oxygenation of tumour tissue without major anatomical deviations; and tumour downsizing could facilitate surgery. A disadvantage is that pathological staging is unavailable. However, because most patients in countries without screening programmes present with advanced disease, overtreatment will happen in few patients.
In a Russian trial,125 152 patients were randomly assigned to surgery alone or 20 Gy (5 fractions) of radiotherapy in the week before surgery. Surgery plus radiotherapy did not lead to a significant improvement in 5-year overall survival. No increase in postoperative complications was reported, but radiation doses were rather low. In China in a prospective trial,126 370 patients received either surgery or surgery with preoperative 40 Gy (20 fractions in 4 weeks) radiotherapy. 5-year overall survival was 19·8% and 30·1%, respectively. Only gastric cardia cases were included, which might explain these favourable results.
Furthermore, a meta-analysis127 comparing surgery with surgery preceded by radiotherapy showed significant improvement in 3-year (p=0·0001) and 5-year (p=0·002) survival without a rise in postoperative mortality and mobidity. Although these studies show the advantages of preoperative radiotherapy and surgery, further studies with this subject are unlikely because research efforts are directed towards perioperative chemotherapy and postoperative chemoradiotherapy.
Chemoradiotherapy
Several randomised and retrospective studies128–131 from the 1980s showed a potential beneficial effect of radiotherapy in combination with fluorouracil-based chemotherapy on local control and survival. On the basis of these studies, between 1991 and 1998, investigators for the SWOG-Intergroup 0116 trial132 randomly assigned 556 patients to surgery only and surgery plus postoperative chemoradiotherapy. Adjuvant treatment consisted of 45 Gy radiotherapy at 1·8 Gy per day, given 5 days per week for 5 weeks, with modified doses of fluorouracil and leucovorin on the first 4 days and last 3 days of radiotherapy. Two 5-day cycles of fluorouracil and leucovorin were given after and one cycle was given before chemoradiotherapy. Although clinically significant acute toxic effects—mainly haematological and gastrointestinal—were recorded after chemoradiotherapy, median overall survival was 27 months in the surgery group and 36 months in the surgery plus chemoradiotherapy group (p=0·005). Furthermore, relapse-free survival was extended from 19 months (surgery only) to 30 months with chemoradiotherapy.
Present consensus guidelines in the USA recommend postoperative chemoradiotherapy as a treatment option, which according to the Surveillance, Epidemiology and End Results (SEER) database might improve survival.133,134 However, this study has been criticised, mainly for suboptimum surgery. 54% of patients underwent a limited dissection (D0), instead of the advised D2 lymph-node dissection, which could have undermined survival.107 However, a Korean observational study135 in 990 patients showed that chemoradiotherapy after a D2 resection improved survival. Because this study was not randomised, conclusions drawn from it should be cautious.
Results of a randomised study from this Korean group—in which patients were given either capecitabine and cisplatin chemotherapy, or capecitabine and cisplatin chemo radiotherapy after D2 resection—are awaited with great interest (ARTIST trial; Clinicaltrials.gov NCT 00323830). In a meta-analysis,127 postoperative chemo-radiotherapy was reported to improve survival significantly.
Late toxicity data of combined treatment are scarce. Progressive renal toxicity after chemoradiotherapy for gastric cancer with commonly used 2D or 3D radiation techniques have been reported.136 Results of radiotherapy dose-planning studies137,138 showed that modern, intensity modulated radiotherapy techniques are able to spare kidneys and other crucial organs (figure 6). The SWOG-Intergroup study132 began in the early 1990s when concurrent chemoradiotherapy was not widely accepted. Nowadays, studies139 that combine radiotherapy with cytostatic drugs, such as epirubicin and paclitaxel, show that these regimens are feasible, but effects on survival are unknown. Results of phase I and phase II studies show that radiotherapy can be intensively combined with chemotherapy.140,141
Figure 6.
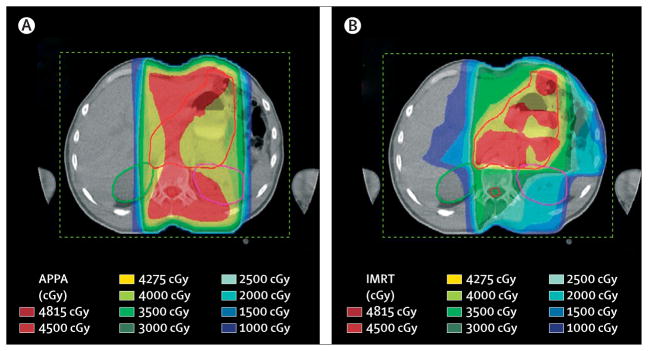
Radiotherapy and IMRT planning for gastric cancer
IMRT=intensity modulated radiotherapy. Red line=clinical target volume. Green line=right kidney. Purple line=left kidney. Improved sparing of kidneys and optimum coverage of clinical target volume is possible with present radiotherapy techniques. (A) Result of a two-dimensional anterior-posterior posterior-anterior (APPA) radiotherapy plan. (B) Result of an IMRT plan.
Preoperative chemoradiotherapy improves surgical outcomes in oesophageal and rectal cancer, and thus might be a good approach in gastric cancer. The MD Anderson Cancer Center142 reported outcomes for 33 patients who completed a preoperative regimen of fluorouracil, leucovorin, and cisplatin, with 45 Gy radiotherapy in 25 fractions. A negative-margin resection was achieved in 23 patients, with pathological complete response and partial response rates of 36% and 29%, respectively. In another study143 from the same centre, 41 patients with operable gastric cancer were given radiotherapy combined with fluorouracil, paclitaxel, and cisplatin. Negative-margin resection, pathological complete response, and partial response rates were 78%, 20%, and 15%, respectively. This schedule was tested in a multicentre (RTOG 9904) phase II trial,144 which resulted in a negative-margin resection rate of 77% and a pathological complete response rate of 26%. Of note, 18 of 43 patients had a major radiotherapy protocol violation, drawing attention to the need for strict but clear protocols.
Studies145,146 from Switzerland and Poland showed good results with preoperative chemoradiotherapy. Thus, conceptually, preoperative chemoradiotherapy unifies the proven benefit of chemoradiotherapy with the advantages of a neoadjuvant approach, and, therefore, should be further explored in clinical phase III trials. Additionally, chemoradiotherapy provides durable responses and symptom control in patients with locally advanced disease not amenable for surgery, or for patients refusing surgery.147,148
Chemotherapy
Preoperative or neoadjuvant chemotherapy could potentially downstage advanced gastric cancer and improve resectability and survival. Pilot phase II studies seemed to have promising results.149,150 A randomised study by the Dutch Gastric Cancer group,151 however, was unable to show a benefit from neoadjuvant chemotherapy with fluorouracil, adriamycin, and methotrexate chemotherapy.
Many studies have been done with chemotherapy in the postoperative setting. These studies have been included in several meta-analyses,152–155 reporting no survival benefit or at the most a modest benefit for adjuvant chemotherapy. However, most chemotherapy regimens used in adjuvant studies seemed to have low response rates and are now regarded as outdated. In a Japanese phase III study,156 530 patients were randomly assigned to surgery only, and 529 to surgery with 1 year of an adjuvant oral fluoro-pyrimidine, called S-1. Patients with stage II or stage III disease underwent gastrectomy with D2 lymph-node dissection. After a median follow-up of 2·9 years, overall survival was 80·1% in the S-1 group versus 70·1% in the surgery only group (p=0·002); relapse-free survival was 72·2% and 59·6% (p<0·001) respectively. Therefore, at least for Japanese patients, this treatment seems a reasonable option after a D2 dissection.
In the UK, the Medical Research Council (MRC)157 randomly assigned 503 patients with resectable gastric carcinoma to either surgery only, or to surgery with three preoperative and three postoperative courses of epirubicin, cisplatin, and fluorouracil. After a median follow-up of 4 years, perioperative chemotherapy improved 5-year overall survival (36 vs 23%) and progression-free survival, despite only 42% of patients in the chemotherapy group completing treatment. About 40% of patients had a D2 dissection. Results of a French phase III trial158 confirmed improvement of disease-free survival and 5-year overall survival with preoperative fluorouracil and cisplatin chemotherapy (38% vs 24%).
Up to 50% of curatively resected gastric cancer patients develop peritoneal carcinomatosis. Adjuvant intra-peritoneal chemotherapy could prevent such recurrence. In a randomised trial,159 248 patients were given either adjuvant postoperative intraperitoneal chemotherapy (mitomycin and fluorouracil) or surgery alone. The intraperitoneal group had higher morbidity and mortality than did the surgery alone group, but no improvement in surviv al was recorded. A meta-analysis160 of ten of 13 published randomised controlled trials reported a significant improvement in survival with hyperthermic intraoperative intraperitoneal chemotherapy (HIIC) alone (p=0·002) or HIIC combined (p=0·0002) with early postoperative intraperitoneal chemotherapy (EPIC). However, HIIC is not standard of care for gastric cancer because of a high risk of intra-abdominal abscess and neutropenia,160 and it is not proven to be better than is systemic chemotherapy.
In advanced-stage gastric cancer, randomised studies161 show that chemotherapy has a beneficial effect on survival and quality of life. A meta-analysis162 reported a three-drug regimen with fluorouracil, cisplatin, and an anthracycline offers the best chance for extended survival.
The REAL-2 study,163 comparing capecitabine with fluorouracil, and oxaliplatin with cisplatin, in 1002 advanced gastric cancer patients in a two-by-two design, showed that capecitabine and oxaliplatin are at least as effective as cisplatin and fluorouracil, respectively, with a favourable toxicity profile and ease of administration. New taxane and irinotecan-based regimens show promise, but their place in treatment strategies is yet to be established.
Targeted therapy
Chemotherapy is useful in advanced gastric cancer,157 but overall survival does not exceed 1 year in phase III studies. Good biomarkers of chemotherapy response might improve quality of life of non-responders, reduce time until surgery in non-responders, and reduce costs. Additionally, optimum treatment can be achieved for patients. Several tumour markers are thought to be predictive of therapy response in gastric cancer (eg, microsatellite instability, chromosomal instability, and overexpression of thymidylate synthase, thymidine phosphorylase, GADD45A, and ERCC).38,41,53,164–167 Likewise, gene polymorphisms, in specific genes, have been associated with clinical outcome and response to treatment.168–170
Additionally, specific antibodies against molecular targets are being investigated in clinical trials, such as ERBB2, epidermal growth-factor receptor and vascular endothelial growth factor.171 In a review172 of phase II studies integrating a targeted drug into chemotherapeutic regimens, objective response rates were 11–65% and time to progression was 2·5–16·0 months in patients with advanced gastric cancer. The role of these targeted agents needs to be established in randomised phase III trials.
Optimum locoregional treatment for gastric cancer will be achieved with a combination of radical surgery and individualised neoadjuvant or adjuvant treatment, with modern conformal radiotherapy and optimum cytostatic drugs or biological agents.173 We agree with Cunningham and Chua174 that, except for early gastric cancer, surgery alone is no longer acceptable as standard treatment for resectable gastric cancer. Only randomised trials can confirm the value of new strategies. The Dutch Colorectal Cooperative Group is currently accruing patients to the CRITICS study (Clinicaltrials.gov NCT 00407186) a phase III trial in which patients are randomly assigned after neoadjuvant chemotherapy (epirubicin, cisplatin, and capecitabine) and standardised (D1 or higher) surgery to either postoperative chemotherapy (epirubicin, cisplatin, and capecitabine) and 3D, or intensity modulated radio therapy based chemo radiotherapy. The MRC has started accruing patients to the MRC-ST03 phase II and III study, in which patients are given either perioperative epirubicin, cisplatin, and capecitabine with or without bevacizumab, a humanised monoclonal antibody against vascular endothelial growth factor.
The Cancer and Leukaemia Group B (CALBG 80101) has almost completed accruing patients to a phase III trial,175 in which patients are randomly assigned to either postoperative fluorouracil and leucovorin before and after fluorouracil-based chemoradiation, or postoperative epirubicin, cisplatin, and fluorouracil before and after fluorouracil-based chemoradiation. Preliminary results show a better toxicity profile with epirubicin, cisplatin, and fluorouracil than with fluorouracil and leucovorin. In Europe, a collaboration has been founded (the European Union Network of Excellence for Gastric Cancer) to improve clinical and translational studies for gastric cancer.176
Conclusion
Reduction of gastric cancer mortality can be achieved by implementation of prevention programmes and personalised treatment. Effective prevention strategies should be based on specific risk profiles, including H pylori genotype, host gene polymorphisms, and environmental factors. Treatment and the extent of resection is still decided on the basis of the disease stage identified with conventional techniques. For improvement of locoregional control, new strategies with neoadjuvant and adjuvant chemotherapy, and radiotherapy, or both, have been investigated, with some clinical trials underway. Novel treatment strategies using gene signatures for therapy response and specific targets to further individualise treatment are promising, but are not yet clinically validated. Treatment of gastric cancer patients should be centralised in high-caseload hospitals to further improve outcomes and help with trial and research participation. Finally, although guidelines for treatment of gastric cancer differ throughout the world, an algorithm for clinicians is available on the United States National Comprehensive Cancer Network website.
Search strategy and selection criteria.
We searched the Cochrane Library, Medline, and Embase for publications from January 1, 2000, to August 31, 2008. We used the search terms “gastric cancer” or “stomach cancer” in combination with the terms “review”, “randomised”, and “clinical trial”. We largely selected publications in the past 5 years, but did not exclude commonly referenced older publications. We also searched the reference lists of articles identified by this search strategy and selected those we judged relevant. Review articles and book chapters are cited to provide readers with more details and references than are given in this Seminar.
Footnotes
Conflicts of interest
We declare that we have no conflicts of interest.
Contributors
All authors participated in writing the report and have approved the final version.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47:1077–83. doi: 10.2169/internalmedicine.47.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 4.Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and stomach cancer risk: a quantitative systematic review. Gastric Cancer. 2008;11:23–32. doi: 10.1007/s10120-007-0447-2. [DOI] [PubMed] [Google Scholar]
- 5.Hanley AJ, Choi BC, Holowaty EJ. Cancer mortality among Chinese migrants: a review. Int J Epidemiol. 1995;24:255–65. doi: 10.1093/ije/24.2.255. [DOI] [PubMed] [Google Scholar]
- 6.Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–53. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladeiras-Lopes R, Pereira AK, Nogueira A, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 8.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;29:533–43. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 10.Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 1982;8:1–11. doi: 10.1016/0360-3016(82)90377-7. [DOI] [PubMed] [Google Scholar]
- 11.Guilford PJ, Hopkins JB, Grady WM, et al. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat. 1999;14:249–55. doi: 10.1002/(SICI)1098-1004(1999)14:3<249::AID-HUMU8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Barber M, Murrell A, Ito Y, et al. Mechanisms and sequelae of E-cadherin silencing in hereditary diffuse gastric cancer. J Pathol. 2008;216:295–306. doi: 10.1002/path.2426. [DOI] [PubMed] [Google Scholar]
- 13.Brooks-Wilson AR, Kaurah P, Suriano G, et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004;41:508–17. doi: 10.1136/jmg.2004.018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gylling A, Abdel-Rahman WM, Juhola M, et al. Is gastric cancer part of the tumour spectrum of hereditary non-polyposis colorectal cancer? A molecular genetic study. Gut. 2007;56:926–33. doi: 10.1136/gut.2006.114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinmura K, Goto M, Tao H, et al. A novel STK11 germline mutation in two siblings with Peutz-Jeghers syndrome complicated by primary gastric cancer. Clin Genet. 2005;67:81–86. doi: 10.1111/j.1399-0004.2005.00380.x. [DOI] [PubMed] [Google Scholar]
- 16.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—first American Cancer Society Award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 17.Forman D, Newell DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 302:1302–05. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–36. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 19.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 20.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–89. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 21.Canedo P, Corso G, Pereira F, et al. The interferon gamma receptor 1 (IFNGR1) -56C/T gene polymorphism is associated with increased risk of early gastric carcinoma. Gut. 2008;57:1504–08. doi: 10.1136/gut.2007.143578. [DOI] [PubMed] [Google Scholar]
- 22.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 23.Hold GL, Rabkin CS, Chow WH, et al. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007;132:905–12. doi: 10.1053/j.gastro.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Fox JG, Beck P, Dangler CA, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–42. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 25.Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–87. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palli D, Masala G, Del GG, et al. CagA+ Helicobacter pylori infection and gastric cancer risk in the EPIC-EURGAST study. Int J Cancer. 2007;120:859–67. doi: 10.1002/ijc.22435. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Russell RM. Nutrition and gastric cancer risk: an update. Nutr Rev. 2008;66:237–49. doi: 10.1111/j.1753-4887.2008.00029.x. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 29.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–49. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 30.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–96. [PubMed] [Google Scholar]
- 31.Ottini L, Falchetti M, Lupi R, et al. Patterns of genomic instability in gastric cancer: clinical implications and perspectives. Ann Oncol. 2006;17 (suppl 7):97–102. doi: 10.1093/annonc/mdl960. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Suzuki I, Leodolter A, et al. Global DNA demethylation in gastrointestinal cancer is age dependent and precedes genomic damage. Cancer Cell. 2006;9:199–207. doi: 10.1016/j.ccr.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Bacani J, Zwingerman R, di Nicola N, et al. Tumor microsatellite instability in early onset gastric cancer. J Mol Diagn. 2005;7:465–77. doi: 10.1016/S1525-1578(10)60577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ottini L, Falchetti M, Saieva C, et al. MRE11 expression is impaired in gastric cancer with microsatellite instability. Carcinogenesis. 2004;25:2337–43. doi: 10.1093/carcin/bgh257. [DOI] [PubMed] [Google Scholar]
- 35.Fleisher AS, Esteller M, Wang S, et al. Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res. 1999;59:1090–95. [PubMed] [Google Scholar]
- 36.Beghelli S, de Manzoni G, Barbi S, et al. Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery. 2006;139:347–56. doi: 10.1016/j.surg.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Wu MS, Lee CW, Shun CT, et al. Distinct clinicopathologic and genetic profiles in sporadic gastric cancer with different mutator phenotypes. Genes Chromosomes Cancer. 2000;27:403–11. [PubMed] [Google Scholar]
- 38.Meyers M, Wagner MW, Hwang HS, Kinsella TJ, Boothman DA. Role of the hMLH1 DNA mismatch repair protein in fluoropyrimidine-mediated cell death and cell cycle responses. Cancer Res. 2001;61:5193–201. [PubMed] [Google Scholar]
- 39.Elsaleh H, Iacopetta B. Microsatellite instability is a predictive marker for survival benefit from adjuvant chemotherapy in a population-based series of stage III colorectal carcinoma. Clin Colorectal Cancer. 2001;1:104–09. doi: 10.3816/CCC.2001.n.010. [DOI] [PubMed] [Google Scholar]
- 40.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–57. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grundei T, Vogelsang H, Ott K, et al. Loss of heterozygosity and microsatellite instability as predictive markers for neoadjuvant treatment in gastric carcinoma. Clin Cancer Res. 2000;6:4782–88. [PubMed] [Google Scholar]
- 42.Aguilera A, Gomez-Gonzalez B. Genome instability: a mechanistic view of its causes and consequences. Nat Rev Genet. 2008;9:204–17. doi: 10.1038/nrg2268. [DOI] [PubMed] [Google Scholar]
- 43.Buffart TE, Carvalho B, Hopmans E, et al. Gastric cancers in young and elderly patients show different genomic profiles. J Pathol. 2007;211:45–51. doi: 10.1002/path.2085. [DOI] [PubMed] [Google Scholar]
- 44.Kokkola A, Monni O, Puolakkainen P, et al. Presence of high-level DNA copy number gains in gastric carcinoma and severely dysplastic adenomas but not in moderately dysplastic adenomas. Cancer Genet Cytogenet. 1998;107:32–36. doi: 10.1016/s0165-4608(98)00092-2. [DOI] [PubMed] [Google Scholar]
- 45.Koo SH, Kwon KC, Shin SY, et al. Genetic alterations of gastric cancer: comparative genomic hybridization and fluorescence In situ hybridization studies. Cancer Genet Cytogenet. 2000;117:97–103. doi: 10.1016/s0165-4608(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 46.Sakakura C, Mori T, Sakabe T, et al. Gains, losses, and amplifications of genomic materials in primary gastric cancers analyzed by comparative genomic hybridization. Genes Chromosomes Cancer. 1999;24:299–305. doi: 10.1002/(sici)1098-2264(199904)24:4<299::aid-gcc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 47.van Grieken NC, Weiss MM, Meijer GA, et al. Helicobacter pylori-related and -non-related gastric cancers do not differ with respect to chromosomal aberrations. J Pathol. 2000;192:301–06. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH697>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 48.Weiss MM, Kuipers EJ, Hermsen MA, et al. Barrett’s adenocarcinomas resemble adenocarcinomas of the gastric cardia in terms of chromosomal copy number changes, but relate to squamous cell carcinomas of the distal oesophagus with respect to the presence of high-level amplifications. J Pathol. 2003;199:157–65. doi: 10.1002/path.1260. [DOI] [PubMed] [Google Scholar]
- 49.Kimura Y, Noguchi T, Kawahara K, Kashima K, Daa T, Yokoyama S. Genetic alterations in 102 primary gastric cancers by comparative genomic hybridization: gain of 20q and loss of 18q are associated with tumor progression. Mod Pathol. 2004;17:1328–37. doi: 10.1038/modpathol.3800180. [DOI] [PubMed] [Google Scholar]
- 50.Weiss MM, Kuipers EJ, Postma C, et al. Genomic profiling of gastric cancer predicts lymph node status and survival. Oncogene. 2003;22:1872–79. doi: 10.1038/sj.onc.1206350. [DOI] [PubMed] [Google Scholar]
- 51.Snijders AM, Nowak N, Segraves R, et al. Assembly of microarrays for genome-wide measurement of DNA copy number. Nat Genet. 2001;29:263–64. doi: 10.1038/ng754. [DOI] [PubMed] [Google Scholar]
- 52.Ylstra B, van den IJssel I, Carvalho B, Brakenhoff RH, Meijer GA. BAC to the future! or oligonucleotides: a perspective for micro array comparative genomic hybridization (array CGH) Nucleic Acids Res. 2006;34:445–50. doi: 10.1093/nar/gkj456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ott K, Vogelsang H, Mueller J, et al. Chromosomal instability rather than p53 mutation is associated with response to neoadjuvant cisplatin-based chemotherapy in gastric carcinoma. Clin Cancer Res. 2003;9:2307–15. [PubMed] [Google Scholar]
- 54.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 55.An C, Choi IS, Yao JC, et al. Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin Cancer Res. 2005;11:656–63. [PubMed] [Google Scholar]
- 56.Carvalho B, Pinto M, Cirnes L, et al. Concurrent hypermethylation of gene promoters is associated with an MSI-H phenotype and diploidy in gastric carcinomas. Eur J Cancer. 2003;39:1222–27. doi: 10.1016/s0959-8049(03)00177-1. [DOI] [PubMed] [Google Scholar]
- 57.Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer. 2008;123:8–13. doi: 10.1002/ijc.23607. [DOI] [PubMed] [Google Scholar]
- 58.Fuccio L, Zagari RM, Minardi ME, Bazzoli F. Systematic review: Helicobacter pylori eradication for the prevention of gastric cancer. Aliment Pharmacol Ther. 2007;25:133–41. doi: 10.1111/j.1365-2036.2006.03183.x. [DOI] [PubMed] [Google Scholar]
- 59.de Vries AC, Kuipers EJ. Review article: Helicobacter pylori eradication for the prevention of gastric cancer. Aliment Pharmacol Ther. 2007;26 (suppl 2):25–35. doi: 10.1111/j.1365-2036.2007.03475.x. [DOI] [PubMed] [Google Scholar]
- 60.Lai IR, Lee WJ, Huang MT, Lin HH. Comparison of serum CA72-4, CEA, TPA, CA19-9 and CA125 levels in gastric cancer patients and correlation with recurrence. Hepatogastroenterology. 2002;49:1157–60. [PubMed] [Google Scholar]
- 61.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma–2nd English Edn. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 62.UICC. TNM classification of malignant tumors. 6. New York: John Wiley; 2002. [Google Scholar]
- 63.Yamashita K, Sakuramoto S, Kikuchi S, Katada N, Kobayashi N, Watanabe M. Validation of staging systems for gastric cancer. Gastric Cancer. 2008;11:111–18. doi: 10.1007/s10120-008-0466-7. [DOI] [PubMed] [Google Scholar]
- 64.Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25:2107–16. doi: 10.1200/JCO.2006.09.5224. [DOI] [PubMed] [Google Scholar]
- 65.Shinohara T, Ohyama S, Yamaguchi T, et al. Clinical value of multidetector row computed tomography in detecting lymph node metastasis of early gastric cancer. Eur J Surg Oncol. 2005;31:743–48. doi: 10.1016/j.ejso.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Noda N, Sasako M, Yamaguchi N, Nakanishi Y. Ignoring small lymph nodes can be a major cause of staging error in gastric cancer. Br J Surg. 1998;85:831–34. doi: 10.1046/j.1365-2168.1998.00691.x. [DOI] [PubMed] [Google Scholar]
- 67.Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247–53. doi: 10.1007/s00268-003-7191-5. [DOI] [PubMed] [Google Scholar]
- 68.Blackshaw GR, Barry JD, Edwards P, Allison MC, Thomas GV, Lewis WG. Laparoscopy significantly improves the perceived preoperative stage of gastric cancer. Gastric Cancer. 2003;6:225–29. doi: 10.1007/s10120-003-0257-0. [DOI] [PubMed] [Google Scholar]
- 69.de Graaf GW, Ayantunde AA, Parsons SL, Duffy JP, Welch NT. The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol. 2007;33:988–92. doi: 10.1016/j.ejso.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 70.Kayaalp C, Arda K, Orug T, Ozcay N. Value of computed tomography in addition to ultrasound for preoperative staging of gastric cancer. Eur J Surg Oncol. 2002;28:540–43. doi: 10.1053/ejso.2002.1296. [DOI] [PubMed] [Google Scholar]
- 71.Kim HJ, Kim AY, Oh ST, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. 2005;236:879–85. doi: 10.1148/radiol.2363041101. [DOI] [PubMed] [Google Scholar]
- 72.Sultan J, Robinson S, Hayes N, Griffin SM, Richardson DL, Preston SR. Endoscopic ultrasonography-detected low-volume ascites as a predictor of inoperability for oesophagogastric cancer. Br J Surg. 2008;95:1127–30. doi: 10.1002/bjs.6299. [DOI] [PubMed] [Google Scholar]
- 73.Ott K, Herrmann K, Lordick F, et al. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res. 2008;14:2012–18. doi: 10.1158/1078-0432.CCR-07-0934. [DOI] [PubMed] [Google Scholar]
- 74.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 75.Wang YP, Bennett C, Pan T. Endoscopic mucosal resection for early gastric cancer. Cochrane Database Syst Rev. 2006;1:CD004276. doi: 10.1002/14651858.CD004276.pub2. [DOI] [PubMed] [Google Scholar]
- 76.Kim JJ, Lee JH, Jung HY, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007;66:693–700. doi: 10.1016/j.gie.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 77.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–97. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 78.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–42. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 79.Oda I, Saito D, Tada M, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262–70. doi: 10.1007/s10120-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 80.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–25. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 81.Yasuda K, Shiraishi N, Suematsu T, Yamaguchi K, Adachi Y, Kitano S. Rate of detection of lymph node metastasis is correlated with the depth of submucosal invasion in early stage gastric carcinoma. Cancer. 1999;85:2119–23. doi: 10.1002/(sici)1097-0142(19990515)85:10<2119::aid-cncr4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 82.Orsenigo E, Tomajer V, di Palo S, et al. Sentinel node mapping during laparoscopic distal gastrectomy for gastric cancer. Surg Endosc. 2008;22:118–21. doi: 10.1007/s00464-007-9385-7. [DOI] [PubMed] [Google Scholar]
- 83.Park DJ, Lee HJ, Lee HS, et al. Sentinel node biopsy for cT1 and cT2a gastric cancer. Eur J Surg Oncol. 2006;32:48–54. doi: 10.1016/j.ejso.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Saikawa Y, Otani Y, Kitagawa Y, et al. Interim results of sentinel node biopsy during laparoscopic gastrectomy: possible role in function-preserving surgery for early cancer. World J Surg. 2006;30:1962–68. doi: 10.1007/s00268-006-0142-1. [DOI] [PubMed] [Google Scholar]
- 85.Tajima Y, Yamazaki K, Masuda Y, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg. 2009;249:58–62. doi: 10.1097/SLA.0b013e3181927267. [DOI] [PubMed] [Google Scholar]
- 86.Shehzad K, Mohiuddin K, Nizami S, et al. Current status of minimal access surgery for gastric cancer. Surg Oncol. 2007;16:85–98. doi: 10.1016/j.suronc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 87.Shiraishi N, Yasuda K, Kitano S. Laparoscopic gastrectomy with lymph node dissection for gastric cancer. Gastric Cancer. 2006;9:167–76. doi: 10.1007/s10120-006-0380-9. [DOI] [PubMed] [Google Scholar]
- 88.Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170–78. doi: 10.1097/00000658-199908000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gouzi JL, Huguier M, Fagniez PL, et al. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg. 1989;209:162–66. doi: 10.1097/00000658-198902000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim SH, Karpeh MS, Klimstra DS, Leung D, Brennan MF. Effect of microscopic resection line disease on gastric cancer survival. J Gastrointest Surg. 1999;3:24–33. doi: 10.1016/s1091-255x(99)80004-3. [DOI] [PubMed] [Google Scholar]
- 91.Songun I, Bonenkamp JJ, Hermans J, van Krieken JH, van de Velde CJ. Prognostic value of resection-line involvement in patients undergoing curative resections for gastric cancer. Eur J Cancer. 1996;32:433–37. doi: 10.1016/0959-8049(95)00591-9. [DOI] [PubMed] [Google Scholar]
- 92.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–30. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg. 1988;75:110–12. doi: 10.1002/bjs.1800750206. [DOI] [PubMed] [Google Scholar]
- 94.Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–77. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 95.Robertson CS, Chung SC, Woods SD, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg. 1994;220:176–82. doi: 10.1097/00000658-199408000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu C-W, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309–15. doi: 10.1016/S1470-2045(06)70623-4. [DOI] [PubMed] [Google Scholar]
- 97.McCulloch P, Nita ME, Kazi H, Gama-Rodrigues J. Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2004;4:CD001964. doi: 10.1002/14651858.CD001964.pub2. [DOI] [PubMed] [Google Scholar]
- 98.Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–62. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 99.Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery. 2002;131:401–07. doi: 10.1067/msy.2002.121891. [DOI] [PubMed] [Google Scholar]
- 100.Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006;93:559–63. doi: 10.1002/bjs.5353. [DOI] [PubMed] [Google Scholar]
- 101.Sano T, Yamamoto S, Sasako M. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol. 2002;32:363–64. doi: 10.1093/jjco/hyf085. [DOI] [PubMed] [Google Scholar]
- 102.Kitamura K, Nishida S, Ichikawa D, et al. No survival benefit from combined pancreaticosplenectomy and total gastrectomy for gastric cancer. Br J Surg. 1999;86:119–22. doi: 10.1046/j.1365-2168.1999.00967.x. [DOI] [PubMed] [Google Scholar]
- 103.Kodera Y, Yamamura Y, Shimizu Y, et al. Lack of benefit of combined pancreaticosplenectomy in D2 resection for proximal-third gastric carcinoma. World J Surg. 1997;21:622–27. doi: 10.1007/s002689900283. [DOI] [PubMed] [Google Scholar]
- 104.Wang JY, Huang TJ, Chen FM, Huang CJ, Huang YS, Hsieh JS. A comparative study of pancreatectomy and pancreas-preserving gastrectomy in advanced gastric carcinomas. Hepatogastroenterology. 2004;51:1229–32. [PubMed] [Google Scholar]
- 105.Kampschoer GH, Maruyama K, van de Velde CJ, Sasako M, Kinoshita T, Okabayashi K. Computer analysis in making preoperative decisions: a rational approach to lymph node dissection in gastric cancer patients. Br J Surg. 1989;76:905–08. doi: 10.1002/bjs.1800760910. [DOI] [PubMed] [Google Scholar]
- 106.Peeters KC, Hundahl SA, Kranenbarg EK, Hartgrink H, van de Velde CJ. Low Maruyama index surgery for gastric cancer: blinded reanalysis of the Dutch D1-D2 trial. World J Surg. 2005;29:1576–84. doi: 10.1007/s00268-005-7907-9. [DOI] [PubMed] [Google Scholar]
- 107.Hundahl SA, Macdonald JS, Benedetti J, Fitzsimmons T. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Ann Surg Oncol. 2002;9:278–86. doi: 10.1007/BF02573066. [DOI] [PubMed] [Google Scholar]
- 108.Hundahl SA, Peeters KC, Kranenbarg EK, Hartgrink H, van de Velde CJ. Improved regional control and survival with low Maruyama Index surgery in gastric cancer: autopsy findings from the Dutch D1-D2 Trial. Gastric Cancer. 2007;10:84–86. doi: 10.1007/s10120-007-0426-7. [DOI] [PubMed] [Google Scholar]
- 109.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–20. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 110.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–83. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Damhuis RA, Meurs CJ, Dijkhuis CM, Stassen LP, Wiggers T. Hospital volume and post-operative mortality after resection for gastric cancer. Eur J Surg Oncol. 2002;28:401–05. doi: 10.1053/ejso.2001.1246. [DOI] [PubMed] [Google Scholar]
- 112.Thompson AM, Rapson T, Gilbert FJ, Park KG. Hospital volume does not influence long-term survival of patients undergoing surgery for oesophageal or gastric cancer. Br J Surg. 2007;94:578–84. doi: 10.1002/bjs.5729. [DOI] [PubMed] [Google Scholar]
- 113.Wenner J, Zilling T, Bladstrom A, Alvegard TA. The influence of surgical volume on hospital mortality and 5-year survival for carcinoma of the oesophagus and gastric cardia. Anticancer Res. 2005;25:419–24. [PubMed] [Google Scholar]
- 114.Xirasagar S, Lien YC, Lin HC, Lee HC, Liu TC, Tsai J. Procedure volume of gastric cancer resections versus 5-year survival. Eur J Surg Oncol. 2008;34:23–29. doi: 10.1016/j.ejso.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 115.Meyer HJ. The influence of case load and the extent of resection on the quality of treatment outcome in gastric cancer. Eur J Surg Oncol. 2005;31:595–604. doi: 10.1016/j.ejso.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 116.Bachmann MO, Alderson D, Edwards D, et al. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg. 2002;89:914–22. doi: 10.1046/j.1365-2168.2002.02135.x. [DOI] [PubMed] [Google Scholar]
- 117.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 118.Henning GT, Schild SE, Stafford SL, et al. Results of irradiation or chemoirradiation for primary unresectable, locally recurrent, or grossly incomplete resection of gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2000;46:109–18. doi: 10.1016/s0360-3016(99)00379-x. [DOI] [PubMed] [Google Scholar]
- 119.Jansen EP, Boot H, Verheij M, van de Velde CJ. Optimal locoregional treatment in gastric cancer. J Clin Oncol. 2005;23:4509–17. doi: 10.1200/JCO.2005.21.196. [DOI] [PubMed] [Google Scholar]
- 120.Smalley SR, Gunderson L, Tepper J, et al. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002;52:283–93. doi: 10.1016/s0360-3016(01)02646-3. [DOI] [PubMed] [Google Scholar]
- 121.Sindelar WF, Kinsella TJ, Tepper JE, et al. Randomized trial of intraoperative radiotherapy in carcinoma of the stomach. Am J Surg. 1993;165:178–86. doi: 10.1016/s0002-9610(05)80423-4. [DOI] [PubMed] [Google Scholar]
- 122.Drognitz O, Henne K, Weissenberger C, et al. Long-term results after intraoperative radiation therapy for gastric cancer. Int J Radiat Oncol Biol Phys. 2008;70:715–21. doi: 10.1016/j.ijrobp.2007.07.2331. [DOI] [PubMed] [Google Scholar]
- 123.Hallissey MT, Dunn JA, Ward LC, Allum WH. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet. 1994;343:1309–12. doi: 10.1016/s0140-6736(94)92464-3. [DOI] [PubMed] [Google Scholar]
- 124.Bleiberg H, Goffin JC, Dalesio O, et al. Adjuvant radiotherapy and chemotherapy in resectable gastric cancer. A randomized trial of the gastro-intestinal tract cancer cooperative group of the EORTC. Eur J Surg Oncol. 1989;15:535–43. [PubMed] [Google Scholar]
- 125.Skoropad V, Berdov B, Zagrebin V. Concentrated preoperative radiotherapy for resectable gastric cancer: 20-years follow-up of a randomized trial. J Surg Oncol. 2002;80:72–78. doi: 10.1002/jso.10102. [DOI] [PubMed] [Google Scholar]
- 126.Zhang ZX, Gu XZ, Yin WB, Huang GJ, Zhang DW, Zhang RG. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)–report on 370 patients. Int J Radiat Oncol Biol Phys. 1998;42:929–34. doi: 10.1016/s0360-3016(98)00280-6. [DOI] [PubMed] [Google Scholar]
- 127.Fiorica F, Cartei F, Enea M, et al. The impact of radiotherapy on survival in resectable gastric carcinoma: a meta-analysis of literature data. Cancer Treat Rev. 2007;33:729–40. doi: 10.1016/j.ctrv.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 128.Moertel CG, Childs DS, O’Fallon JR, Holbrook MA, Schutt AJ, Reitemeier RJ. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol. 1984;2:1249–54. doi: 10.1200/JCO.1984.2.11.1249. [DOI] [PubMed] [Google Scholar]
- 129.Gastrointestinal Tumor Study Group. A comparison of combination chemotherapy and combined modality therapy for locally advanced gastric carcinoma. Cancer. 1982;49:1771–77. doi: 10.1002/1097-0142(19820501)49:9<1771::aid-cncr2820490907>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 130.Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373–78. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed] [Google Scholar]
- 131.The Gastrointestinal Tumor Study Group. The concept of locally advanced gastric cancer. Effect of treatment on outcome. Cancer. 1990;66:2324–30. doi: 10.1002/1097-0142(19901201)66:11<2324::aid-cncr2820661112>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 132.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 133.Kozak KR, Moody JS. The survival impact of the intergroup 0116 trial on patients with gastric cancer. Int J Radiat Oncol Biol Phys. 2008;72:517–21. doi: 10.1016/j.ijrobp.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 134.Coburn NG, Guller U, Baxter NN, et al. Adjuvant therapy for resected gastric cancer—rapid, yet incomplete adoption following results of intergroup 0116 trial. Int J Radiat Oncol Biol Phys. 2008;70:1073–80. doi: 10.1016/j.ijrobp.2007.07.2378. [DOI] [PubMed] [Google Scholar]
- 135.Kim S, Lim DH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63:1279–85. doi: 10.1016/j.ijrobp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 136.Jansen EP, Saunders MP, Boot H, et al. Prospective study on late renal toxicity following postoperative chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2007;67:781–85. doi: 10.1016/j.ijrobp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 137.Ringash J, Perkins G, Brierley J, et al. IMRT for adjuvant radiation in gastric cancer: a preferred plan? Int J Radiat Oncol Biol Phys. 2005;63:732–38. doi: 10.1016/j.ijrobp.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 138.Wieland P, Dobler B, Mai S, et al. IMRT for postoperative treatment of gastric cancer: covering large target volumes in the upper abdomen: a comparison of a step-and-shoot and an arc therapy approach. Int J Radiat Oncol Biol Phys. 2004;59:1236–44. doi: 10.1016/j.ijrobp.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 139.Kollmannsberger C, Budach W, Stahl M, et al. Adjuvant chemoradiation using 5-fluorouracil/folinic acid/cisplatin with or without paclitaxel and radiation in patients with completely resected high-risk gastric cancer: two cooperative phase II studies of the AIO/ARO/ACO. Ann Oncol. 2005;16:1326–33. doi: 10.1093/annonc/mdi252. [DOI] [PubMed] [Google Scholar]
- 140.Jansen EP, Boot H, Dubbelman R, Bartelink H, Cats A, Verheij M. Postoperative chemoradiotherapy in gastric cancer—a phase I/II dose-finding study of radiotherapy with dose escalation of cisplatin and capecitabine chemotherapy. Br J Cancer. 2007;97:712–16. doi: 10.1038/sj.bjc.6603965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jansen EP, Boot H, Saunders MP, et al. A phase I-II study of postoperative capecitabine-based chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2007;69:1424–28. doi: 10.1016/j.ijrobp.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 142.Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22:2774–80. doi: 10.1200/JCO.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 143.Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237–44. doi: 10.1200/JCO.2005.01.305. [DOI] [PubMed] [Google Scholar]
- 144.Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953–58. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 145.Allal AS, Zwahlen D, Brundler MA, et al. Neoadjuvant radiochemotherapy for locally advanced gastric cancer: long-term results of a phase I trial. Int J Radiat Oncol Biol Phys. 2005;63:1286–89. doi: 10.1016/j.ijrobp.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 146.Wydmanski J, Suwinski R, Poltorak S, et al. The tolerance and efficacy of preoperative chemoradiotherapy followed by gastrectomy in operable gastric cancer, a phase II study. Radiother Oncol. 2007;82:132–36. doi: 10.1016/j.radonc.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 147.Kim MM, Mansfield PF, Das P, et al. Chemoradiation therapy for potentially resectable gastric cancer: clinical outcomes among patients who do not undergo planned surgery. Int J Radiat Oncol Biol Phys. 2008;71:167–72. doi: 10.1016/j.ijrobp.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 148.Saikawa Y, Kubota T, Kumagai K, et al. Phase II study of chemoradiotherapy with S-1 and low-dose cisplatin for inoperable advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2008;71:173–79. doi: 10.1016/j.ijrobp.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 149.Ajani JA, Ota DM, Jessup JM, et al. Resectable gastric carcinoma. An evaluation of preoperative and postoperative chemotherapy. Cancer. 1991;68:1501–06. doi: 10.1002/1097-0142(19911001)68:7<1501::aid-cncr2820680706>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 150.Ott K, Sendler A, Becker K, et al. Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric Cancer. 2003;6:159–67. doi: 10.1007/s10120-003-0245-4. [DOI] [PubMed] [Google Scholar]
- 151.Hartgrink HH, van de Velde CJ, Putter H, et al. Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol. 2004;30:643–49. doi: 10.1016/j.ejso.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 152.Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059–64. doi: 10.1016/s0959-8049(99)00076-3. [DOI] [PubMed] [Google Scholar]
- 153.Gianni L, Panzini I, Tassinari D, Mianulli AM, Desiderio F, Ravaioli A. Meta-analyses of randomized trials of adjuvant chemotherapy in gastric cancer. Ann Oncol. 2001;12:1178–80. [PubMed] [Google Scholar]
- 154.Hermans J, Bonenkamp JJ, Boon MC, et al. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441–47. doi: 10.1200/JCO.1993.11.8.1441. [DOI] [PubMed] [Google Scholar]
- 155.Mari E, Floriani I, Tinazzi A, et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell’Apparato Digerente) Ann Oncol. 2000;11:837–43. doi: 10.1023/a:1008377101672. [DOI] [PubMed] [Google Scholar]
- 156.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 157.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 158.Boige V, Pignon J, Saint-Aubert B, et al. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol; ASCO Annual Meeting Proceedings Part I; 2007. p. 4510. [Google Scholar]
- 159.Yu W, Whang I, Averbach A, Chang D, Sugarbaker PH. Morbidity and mortality of early postoperative intraperitoneal chemotherapy as adjuvant therapy for gastric cancer. Am Surg. 1998;64:1104–08. [PubMed] [Google Scholar]
- 160.Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14:2702–13. doi: 10.1245/s10434-007-9487-4. [DOI] [PubMed] [Google Scholar]
- 161.Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–68. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 162.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–09. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 163.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 164.Johnston PG, Lenz HJ, Leichman CG, et al. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995;55:1407–12. [PubMed] [Google Scholar]
- 165.Lenz HJ, Leichman CG, Danenberg KD, et al. Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumor response and overall survival. J Clin Oncol. 1996;14:176–82. doi: 10.1200/JCO.1996.14.1.176. [DOI] [PubMed] [Google Scholar]
- 166.Napieralski R, Ott K, Kremer M, et al. Combined GADD45A and thymidine phosphorylase expression levels predict response and survival of neoadjuvant-treated gastric cancer patients. Clin Cancer Res. 2005;11:3025–31. doi: 10.1158/1078-0432.CCR-04-1605. [DOI] [PubMed] [Google Scholar]
- 167.Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16:309–16. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
- 168.Keam B, Im SA, Han SW, et al. Modified FOLFOX-6 chemotherapy in advanced gastric cancer: Results of phase II study and comprehensive analysis of polymorphisms as a predictive and prognostic marker. BMC Cancer. 2008;8:148. doi: 10.1186/1471-2407-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grau JJ, Caballero M, Monzo M, et al. Dihydropyrimidine dehydrogenases and cytidine-deaminase gene polymorphisms as outcome predictors in resected gastric cancer patients treated with fluoropyrimidine adjuvant chemotherapy. J Surg Oncol. 2008;98:130–34. doi: 10.1002/jso.21096. [DOI] [PubMed] [Google Scholar]
- 170.Moutinho C, Mateus AR, Milanezi F, Carneiro F, Seruca R, Suriano G. Epidermal growth factor receptor structural alterations in gastric cancer. BMC Cancer. 2008;8:10. doi: 10.1186/1471-2407-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ohtsu A. Chemotherapy for metastatic gastric cancer: past, present, and future. J Gastroenterol. 2008;43:256–64. doi: 10.1007/s00535-008-2177-6. [DOI] [PubMed] [Google Scholar]
- 172.Rivera F, Vega-Villegas ME, Lopez-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev. 2007;33:315–24. doi: 10.1016/j.ctrv.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 173.Petrelli NJ. The debate is over; it’s time to move on. J Clin Oncol. 2004;22:2041–42. doi: 10.1200/JCO.2004.03.956. [DOI] [PubMed] [Google Scholar]
- 174.Cunningham D, Chua YJ. East meets west in the treatment of gastric cancer. N Engl J Med. 2007;357:1863–65. doi: 10.1056/NEJMe078182. [DOI] [PubMed] [Google Scholar]
- 175.Fuchs C, Tepper JE, Niedwiecki D, et al. Postoperative adjuvant chemoradiation for gastric or gastro-esophageal adenocarcinoma using epirubicin, cisplatin and infusional (CI) 5-FU (ECF) before and after CI 5-FU and radiotherapy (RT): interim toxicity results from Intergroup trial CALGB 80101. 2006 Gastrointestinal Cancer Symposium; San Francisco (CA). 2008. p. 115. [Google Scholar]
- 176.Gastric cancer in Europe. Br J Surg. 2008;95:406–08. doi: 10.1002/bjs.6197. [DOI] [PubMed] [Google Scholar]