Abstract
Despite its function as an inhibitor of urokinase and tissue-type plasminogen activator (PA), PA inhibitor-1 (PAI-1) has a paradoxical pro-tumorigenic role in cancer promoting angiogenesis and tumor cell survival. In this review we summarize pre-clinical evidence in support of the pro-tumorigenic function of PAI-1 that has led to the testing of small molecule PAI-1 inhibitors, initially developed as anti-thrombotic agents, in animal models of cancer. The review discusses the challenges and the opportunities that lay ahead to the development of efficacious and non-toxic PAI-1 inhibitors as anti-cancer agents.
Keywords: Plasminogen activator inhibitor-1 (PAI-1), PAI-1 inhibitor, pre-clinical
Introduction
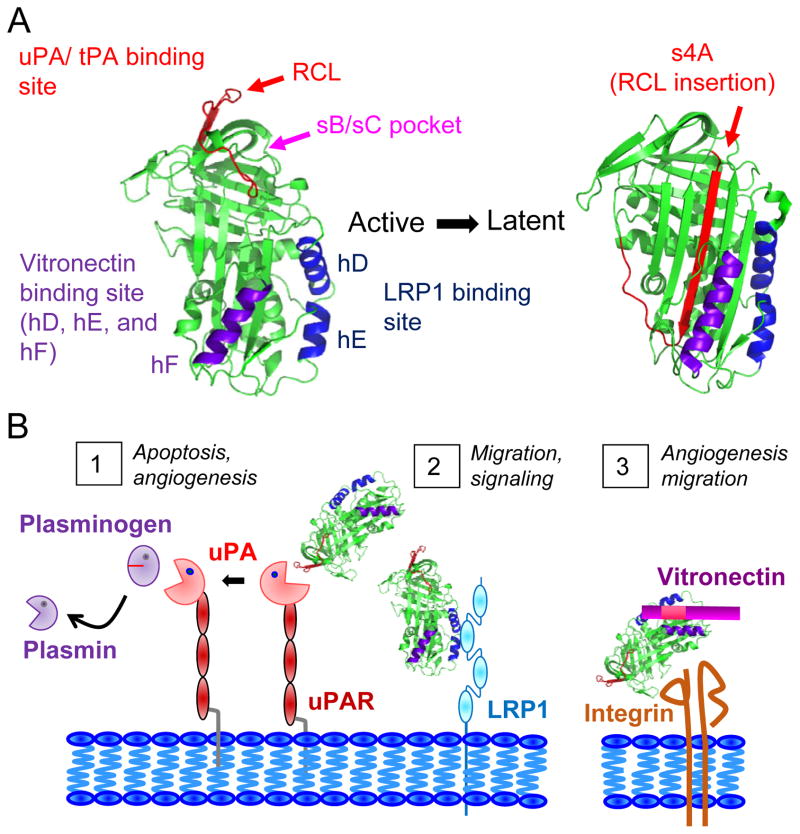
Plasminogen Activator Inhibitor-1 (PAI-1) is a serine protease inhibitor (serpin) and the main regulator of the plasminogen activation system. It acts by inhibiting tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA) (1). PAI-1 has a pleiotropic biological function which stems from its complex structure. Three specific protein-binding domains in the molecule have been well characterized (Figure 1) (1): a domain in the reactive center loop (RCL) that binds to PA, a domain in the flexible joint region that binds to vitronectin comprised of helix D (hD), helix E (hE), and helix F (hF), and a domain within hD and hE that binds to low-density lipoprotein receptor-related protein (LRP1). Upon binding to the RCL, PA cleaves PAI-1 at P1-P1′ inducing a dynamic conformational change that inserts the RCL as a β-strand (4A) into the core of the protein. The binding of PAI-1 to PA forms a catalytically inactive complex, which is internalized when combined with the receptor for uPA (uPAR) and LRP1 (2). Premature insertion of the RCL into the β-sheet is the basis for the conversion of active PAI-1 into its latent form. Binding of PAI-1 to LRP1 also promotes cell migration and signaling (3). Binding of the flexible joint region of PAI-1 to vitronectin has multiple consequences: it masks the adjacent RGD binding site for αv integrins and inhibits cell attachment to vitronectin (2) and stabilizes PAI-1 inhibiting its conversion to latency and increasing its anti-uPA ability by 200 fold (4). Adding to the complexity of the function of PAI-1 in cancer is the fact that it can have paracrine and autocrine effects as it is produced by tumor cells and non-malignant cells including endothelial cells (EC), macrophage cells or adipocytes in the tumor microenvironment.
Figure 1. Structure of PAI-1 and inhibitor targeting sites.
A) Structure of PAI-1 and targeted binding sites. The reactive center loop (RCL) is shown in red, which inserts itself to become s4A in the latent form of PAI-1. The sB/sC pocket is indicated by the arrow (pink). The vitronectin (hD, hE, and hF) and LRP1 (hD and hE) binding regions are shown in purple and blue, respectively. Molecular modeling was carried out using Pymol software version 1.2r1 and the PDB files 1B3K for active PAI-1 (57) and 1DVN for latent PAI-1 (58). B) Roles of PAI-1 in tumorigenesis: 1) the inhibitory role of PAI-1 on uPA action to prevent the cleavage of plasminogen into plasmin and subsequent substrates on the cell surface leading to angiogenesis or intracellular apoptotic signaling, 2) the ability of PAI-1 to bind to LRP1 directly leading to intracellular signaling and cell migration, and 3) the ability of PAI-1 to bind to vitronectin in the extracellular matrix to promote endothelial cell migration and angiogenesis.
On the basis of the pro-tumorigenic role of uPA in angiogenesis, tumor invasion, and metastasis, it was anticipated that PAI-1 would have an anti-tumorigenic function. Surprisingly, several studies revealed a paradoxical association between elevated levels of PAI-1 in blood and tissue samples of cancer patients and an unfavorable clinical outcome and poor response to therapy (1,5). The observation that the production of PAI-1 by EC, fibroblasts, adipocytes, smooth muscle cells, and macrophage cells in the tumor microenvironment was stimulated by pro-tumorigenic factors such as transforming growth factor (TGF)-β interleukin (IL)-6, and tumor necrosis factor (TNF)-α added further evidence supporting a pro-tumorigenic role (6,7). In this article, we review the mechanisms and evidence in mouse models supporting a pro-tumorigenic function for PAI-1. We then present recent pharmacological approaches and pre-clinical studies aimed at targeting PAI-1 in cancer and discuss the lessons learned from these studies.
PAI-1 is pro-angiogenic
A first clue explaining the paradoxical association of PAI-1 with more aggressive forms of cancer came from the observation by several laboratories, including our own, that PAI-1 has a pro-angiogenic activity through its anti-protease and vitronectin-binding functions. The activity of PAI-1 on angiogenesis is, however, dose-dependent with a promoting activity at physiological concentrations (8,9) and an inhibitory activity at pharmacological concentrations (10). This is explained by the fact that at physiological concentrations, PAI-1 inhibition of uPA limits the cleavage by pericellular plasmin of membrane associated Fas-L at R144-K145 and the release of a soluble Fas-L fragment that induces Fas-mediated apoptosis in EC (11). PAI-1 also stimulates angiogenesis through its vitronectin-binding function promoting the detachment of EC from vitronectin and their migration toward fibronectin rich tissues (9).
PAI-1 inhibits spontaneous apoptosis in cancer cells
PAI-1 protects tumor cells from apoptosis through multiple mechanisms. Similar to EC, it inhibits Fas-mediated apoptosis in several human cancer cells including brain metastasis through its control over pericellular plasmin-activity (12,13). PAI-1 also affects intrinsic apoptosis, as the absence of extracellular PAI-1 in tumor cells results in higher levels of activated caspase 9 (14). Intracellular PAI-1 also promotes cell survival through its ability to inhibit caspase 3 protecting tumor cells from chemotherapy-induced apoptosis (15).
What have we learned from mouse tumor models?
Observations in PAI-1 deficient mice have provided additional and important clues on the function of PAI-1 in tumorigenesis and angiogenesis. The vast majority of experiments using PAI-1 deficient murine tumor cells syngeneically implanted into WT or PAI-1 deficient mice consistently demonstrated the contributory role of host and tumor-derived PAI-1 to tumor growth, angiogenesis and metastasis (Table 1) (10,16–21). Consistently, maximum anti-tumor effect was shown upon PAI-1 suppression in both host and tumor cells pointing to a combined role for extracellular PAI-1. Similar observations were made when PAI-1 deficient human tumor cells were xenotransplanted in immunodeficient PAI-1 null mice. These studies typically demonstrated that suppression of PAI-1 expression in human tumor cells and in mouse host cells resulted in poor tumor take and slower tumor growth (11,12,22,23). However in some situations, such as in skin carcinoma, the effects observed depended on the tumor grade with minimal effects observed in high grade tumors (23).
Table 1. Outcome of the genetic ablation of PAI-1 in the host and/or implanted tumor cells in murine models of cancer.
GEMM: genetically engineered mouse model. +/+ indicates transgenic overexpressing PAI-1 mice, −/− indicates PAI-1 deficient mice. n/e indicates that the experimental parameter was not evaluated.
| Model | PAI-1 Host Mouse | Implanted Cells | Tumor Growth | Angiogenesis | Metastasis | Reference |
|---|---|---|---|---|---|---|
| Syngeneic Implantation | +/+ | B16 melanoma | No difference | n/e | No difference | 21 |
| −/− | B16 melanoma | No difference | n/e | No difference | 21 | |
| −/− | Malignant keratinocytes | Decreased | Decreased | n/e | 17 | |
| −/− | T241 fibrosarcoma | Decreased | Decreased | Decreased | 16 | |
| +/+ | Malignant keratinocytes | Decreased | Decreased | n/e | 10 | |
| −/− | Malignant keratinocytes | Decreased | Decreased | n/e | 10 | |
| −/− | Spontaneously transformed primary lung fibroblasts | Decreased | n/e | n/e | 18 | |
| −/− | Spontaneously transformed primary fibrosarcoma | Decreased | n/e | n/e | 19 | |
| −/− | Spontaneously transformed primary fibrosarcoma | Decreased | n/e | n/e | 20 | |
| Xenograft | −/− | Human neuroblastoma | Decreased | Decreased | n/e | 11 |
| +/+ | Human M21 melanoma | Increased | Increased | n/e | 22 | |
| −/− | Human M21 melanoma | Decreased | Decreased | n/e | 22 | |
| −/− | Human HaCaTII-4 and HaCaT A5-RT3 | Decreased | Decreased | n/e | 23 | |
| −/− | Human PAI-1 KD HT1080 fibrosarcoma, colon cancer HCT116, breast cancer MDA-MB-231, lung cancer A549 | Decreased | Decreased | n/e | 12 | |
| GEMM | MMTV-PymT/PAI-1−/− | No difference | No difference | No difference | 24 | |
| Apc/Apc1638N/PAI-1−/− | No difference | n/e | n/e | 25 | ||
| TRP-1/SV40 Tag/PAI-1−/− | No difference | No difference | Decreased | 26 | ||
| K14-HPV16/PAI-1−/− | No difference | No difference | n/e | 27 |
In contrast to the above data, experiments using genetically engineered mice (GEM) prone to develop cancer crossed with PAI-1 null mice failed to demonstrate any effect of PAI-1 suppression on tumor initiation, growth, or metastasis. For example, when FVB-PymT mice prone to develop mammary tumors were crossed with PAI-1 null mice, there was no effect on the development of mammary tumors, metastasis, or survival (24). Similarly, genetic ablation of PAI-1 had no effect on tumor development in Apc/Apc1638N mice prone to develop colon cancer (25), in TRP-1SV40 Tag/PAI-1−/− mice prone to develop ocular tumors (26), and in K14-HPV16/PAI-1−/− mice prone to develop skin cancer (27). An explanation for this difference of effect between transplanted and GEM mice is the possible presence of compensatory serpins including PAI-2, protein C inhibitor, protease nexin-1, or maspin (28–31), whose overexpression in transformed cells may have compensated for a lack of PAI-1 (27–31).
Pharmacological inhibition of PAI-1
Over the last two decades, several laboratories and pharmaceutical companies have developed a variety of small molecule PAI-1 inhibitors using high throughput screening (32). The vast majority of these inhibitors are molecules interfering with the molecular interactions of PAI-1 to inhibit tPA and uPA by binding to the reactive center loop and by inducing a conformational change that promotes an irreversible conversion of PAI-1 into its latent form. To our knowledge, no inhibitors specifically interacting with the vitronectin-binding domain of PAI-1 have been reported yet. The main focus of investigations on the activity of these inhibitors in biological processes has been in cardiovascular diseases (thrombus repermeabilization), lung fibrosis, Alzheimers disease and to a lesser degree in cancer. A first family of PAI-1 inhibitors developed consisted of diketopiperazine inhibitors that interfere with uPA binding to the protease binding domain of the RCL. These inhibitors (XR334, XR1853, XR5082, and XR5118) block tPA inhibition by PAI-1 in vitro (33,34). However, their in vivo efficacy is limited by their low solubility, poor oral availability, or high IC50 values (in the 5 μM to > 1000 μM range) that are unachievable in the blood upon administration in animals. Other menthol-based, benzothiophene, and butadiene small molecules with lower IC50 values (644 nM to 44 μM) in vitro were developed (35–37), however despite their good anti-PAI-1 activity in vitro, they were either not tested or not further pursued in animal experiments for reasons not reported.
One indole oxoacetic acid PAI-1 inhibitor, PAI-039 (tiplaxtinin) (38), was extensively and successfully tested for its anti-thrombotic activity in pre-clinical rat and canine models of acute arterial thrombosis (38,39). Other inhibitors derived from the structure of this inhibitor (PAI-749 and PAZ-417) then underwent human phase I clinical trials in healthy volunteers and in patients with Alzheimer disease (40–42). To date, the results of these studies have not been reported.
As an alternative approach, using computer simulation models mimicking the RCL insertion within the strands of β-sheet A, conformation disrupting agents were designed. Such compounds include a family of dimeric 2-acylamino-3-thiophenecarboxylic acid derivatives (TM5001 and TM5007) developed with the anticipation that by binding to the s4A position of the A β-sheet, they would induce the conversion of PAI-1 into its latent and inactive form (43). These inhibitors were found active against vascular thrombosis in a rat model and against lung fibrosis in murine models. Their pharmacokinetic properties, however, were sub-optimal due to their relatively high lipophilicity [calculated octanol/water partition coefficient (ClogP) value of 5.79, above the ideal 2–3 value]. A second generation molecule (TM5275) with better oral pharmacokinetic properties and lower ClogP of 3.37 was then developed (44). TM5275 showed activity in rat and mouse models of acute arterial thrombosis and lung fibrosis. Importantly, this inhibitor had no systemic toxicity as it did not prolong bleeding time in non-human primates (45).
A limiting element in the activity of these inhibitors so far, has been their lack of activity against the stable form of PAI-1 bound to vitronectin (46). Thus, the recent focus has been on the development of inhibitors active against vitronectin-bound PAI-1. These efforts, however, have been limited by a lack of crystal structure information on vitronectin-bound PAI-1 (47). Polyphenolic inhibitors of PAI-1 (CDE-066 and 096) synthesized on the basis of the structure of small molecules identified by a high throughput screen were found to bind to the sB/sC pocket of PAI-1 with an IC50 of 32 μM and 25 nM, respectively (Figure 1) (48,49). CDE-096 induces allosteric conformational changes affecting the flexibility of PAI-1 and preventing it from binding to PA and decreasing vitronectin binding (49). CDE-096 was still able to interact with vitronectin-bound PAI-1, although the magnitude of polarization was decreased 7.3-fold compared to binding free PAI-1. The in vivo pharmacokinetic profile of these inhibitors is unknown.
Pharmacological inhibition of PAI-1 in cancer therapy
Pharmacologic inhibition of PAI-1 in cardiovascular diseases has been the primary goal with the objective to prevent inhibition of intravascular fibrinolysis and subsequently promote thrombus repermeabilization in an acute setting. In contrast, pre-clinical evidence supporting the therapeutic efficacy of small PAI-1 inhibitors in cancer has been limited (Table 2). In cancer, the objective is to prevent inhibition of peri-cellular activation of plasminogen and interfere with PAI-1-vitronectin interactions. Whereas in cardiovascular and thrombotic disease the desired inhibition is short-term; in cancer (or other chronic conditions) it is long-term requiring a pharmacological profile suitable for chronic administration (32).
Table 2. Outcome of pharmacologic inhibition of PAI-1 in pre-clinical murine models of cancer.
n/e indicates that the experimental parameter was not evaluated.
| Compound | Inhibitor Class | IC50 (uM) | Half-life (hours) | Cancer Model | Efficacy in cancer models | |||
|---|---|---|---|---|---|---|---|---|
| in vitro | Tumor growth | Angiogenesis | Metastasis | |||||
| PAI-039 | Indole oxoacetic acid | 2.7 | 2.95 – 3.73 | Bladder and cervical cancer | Yes | Yes | Yes | n/e |
| TM5275 | N-acylanthranilic acid | 6.9 | 2.5 | Ovarian cancer | Yes | n/e | n/e | n/e |
| SK-116, SK-216 | not stated | 35, 44 | n/e | ApcMin/+ GEMM | Yes | Yes | n/e | n/e |
| SK-216 | not stated | 44 | n/e | Lung cancer and melanoma | Yes | Yes | Yes | Yes |
Insofar, four small molecule PAI-1 inhibitors have been tested in pre-clinical models of cancer. Although limited, these studies have shown anti-tumor activity. SK-116 and SK-216 inhibitors administered orally for 9 weeks to Min mice that spontaneously developed intestinal polyps caused an almost 2-fold reduction in the number of small intestinal polyps (50). When administered to PAI-1 producing Lewis lung carcinoma and PAI-1-non-producing B16 melanoma tumor-bearing mice, SK-216 caused a 2-fold reduction in subcutaneous primary tumor size and inhibited angiogenesis and metastases (51). The oral administration of PAI-039 to mice xenotransplanted with human T24 bladder and HeLa cervical cancer cells resulted in a 2-fold reduction of tumor volume after 14 days associated with a decrease in tumor cell proliferation and vascularization and an increase in apoptosis (52). The TM5275 inhibitor was recently shown to increase apoptosis in vitro in ovarian cancer cell lines (53). The in vivo activity of these inhibitors in cancer models have not been reported with the exception of TM5275 and TM5441 in a most recent study (54).
Challenges and opportunities
Despite the fact that much is known on the structure of PAI-1, its complex biological function, and its pro-tumorigenic role in cancer progression, its potential as a target for therapeutic intervention in cancer has only been recently considered and explored. These studies provide important insight on the need to develop better inhibitors for cancer. The need for chronic administration and thus a pharmacological profile with a prolonged plasma half-life of orally available inhibitors is an important consideration. Although much work has been done on the development of orally available compounds, the half-life of these inhibitors in vivo is 2–3 hours and therefore would be impractical in long term cancer therapy. The fact that most inhibitors developed until now are active in vitro at concentrations in the μM range represents another limitation. Such concentrations are typically difficult to reach in blood and more importantly in tumor tissues for an extensive period of time. The lack of activity of most inhibitors against the stable vitronectin-bound form of PAI-1 is a third limitation, since this is the predominant form of PAI-1 in vitronectin-rich tumor tissues (55). A fourth limitation is the potential systemic effect that the chronic administration of PAI-1 inhibitors may have on hemostasis. By suppressing the control that PAI-1 exerts on plasmin-mediated fibrinolysis, small molecule PAI-1 inhibitors may impair hemostasis and cause excessive spontaneous bleeding (56). Although studies in non-human primates have shown an absence of side effects on systemic hemostasis following the chronic administration of some PAI-1 inhibitors (44,45), this aspect deserves more extensive investigation.
In summary, targeting PAI-1 in cancer therapy remains an attractive but also challenging approach that will require the design and development of inhibitors that address some of the current limitations outlined above. As new inhibitors with better pharmacological profiles are developed, they should continue to be tested in pre-clinical models of cancer.
Acknowledgments
We would like to thank Dr. Marta Kubala for reading through our manuscript and showing us how to generate the crystal structure to highlight the inhibitor binding sites.
Grant Support
This work was supported by the US Department of Health and Human Services/National Institutes of Health with a grant to Y. A. DeClerck (grant 5R01 CA 129377).
Footnotes
Conflict of Interest: All authors report no conflicts of interest.
References
- 1.McMahon B, Kwaan HC. The plasminogen activator system and cancer. Pathophysiology of haemostasis and thrombosis. 2008;36(3–4):184–94. doi: 10.1159/000175156. [DOI] [PubMed] [Google Scholar]
- 2.Schroeck F, Arroyo de Prada N, Sperl S, Schmitt M, Viktor M. Interaction of plasminogen activator inhibitor type-1 (PAI-1) with vitronectin (Vn): mapping the binding sites on PAI-1 and Vn. Biological chemistry. 2002;383(7–8):1143–9. doi: 10.1515/BC.2002.125. [DOI] [PubMed] [Google Scholar]
- 3.Kwaan HC, Mazar AP, McMahon BJ. The apparent uPA/PAI-1 paradox in cancer: more than meets the eye. Seminars in thrombosis and hemostasis. 2013;39(4):382–91. doi: 10.1055/s-0033-1338127. [DOI] [PubMed] [Google Scholar]
- 4.Wind T, Hansen M, Jensen JK, Andreasen PA. The molecular basis for anti-proteolytic and non-proteolytic functions of plasminogen activator inhibitor type-1: roles of the reactive centre loop, the shutter region, the flexible joint region and the small serpin fragment. Biological chemistry. 2002;383(1):21–36. doi: 10.1515/BC.2002.003. [DOI] [PubMed] [Google Scholar]
- 5.Foekens JA, Peters HA, Look MP, Portengen H, Schmitt M, Kramer MD, et al. The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients. Cancer research. 2000;60(3):636–43. [PubMed] [Google Scholar]
- 6.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17(11):3091–100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown NJ. Therapeutic potential of plasminogen activator inhibitor-1 inhibitors. Therapeutic advances in cardiovascular disease. 2010;4(5):315–24. doi: 10.1177/1753944710379126. [DOI] [PubMed] [Google Scholar]
- 8.Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, et al. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. The Journal of cell biology. 2001;152(4):777–84. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isogai C, Laug WE, Shimada H, Declerck PJ, Stins MF, Durden DL, et al. Plasminogen activator inhibitor-1 promotes angiogenesis by stimulating endothelial cell migration toward fibronectin. Cancer research. 2001;61(14):5587–94. [PubMed] [Google Scholar]
- 10.Bajou K, Maillard C, Jost M, Lijnen RH, Gils A, Declerck P, et al. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene. 2004;23(41):6986–90. doi: 10.1038/sj.onc.1207859. [DOI] [PubMed] [Google Scholar]
- 11.Bajou K, Peng H, Laug WE, Maillard C, Noel A, Foidart JM, et al. Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer cell. 2008;14(4):324–34. doi: 10.1016/j.ccr.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang H, Placencio VR, DeClerck YA. Protumorigenic activity of plasminogen activator inhibitor-1 through an antiapoptotic function. J Natl Cancer Inst. 2012;104(19):1470–84. doi: 10.1093/jnci/djs377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156(5):1002–16. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balsara RD, Castellino FJ, Ploplis VA. A novel function of plasminogen activator inhibitor-1 in modulation of the AKT pathway in wild-type and plasminogen activator inhibitor-1-deficient endothelial cells. The Journal of biological chemistry. 2006;281(32):22527–36. doi: 10.1074/jbc.M512819200. [DOI] [PubMed] [Google Scholar]
- 15.Schneider DJ, Chen Y, Sobel BE. The effect of plasminogen activator inhibitor type 1 on apoptosis. Thrombosis and haemostasis. 2008;100(6):1037–40. [PubMed] [Google Scholar]
- 16.Gutierrez LS, Schulman A, Brito-Robinson T, Noria F, Ploplis VA, Castellino FJ. Tumor development is retarded in mice lacking the gene for urokinase-type plasminogen activator or its inhibitor, plasminogen activator inhibitor-1. Cancer research. 2000;60(20):5839–47. [PubMed] [Google Scholar]
- 17.Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nature medicine. 1998;4(8):923–8. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 18.Lademann U, Romer MU, Jensen PB, Hofland KF, Larsen L, Christensen IJ, et al. Malignant transformation of wild-type but not plasminogen activator inhibitor-1 gene-deficient fibroblasts decreases cellular sensitivity to chemotherapy-mediated apoptosis. European journal of cancer. 2005;41(7):1095–100. doi: 10.1016/j.ejca.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Romer MU, Kirkebjerg Due A, Knud Larsen J, Hofland KF, Christensen IJ, Buhl-Jensen P, et al. Indication of a role of plasminogen activator inhibitor type I in protecting murine fibrosarcoma cells against apoptosis. Thrombosis and haemostasis. 2005;94(4):859–66. [PubMed] [Google Scholar]
- 20.Romer MU, Larsen L, Offenberg H, Brunner N, Lademann UA. Plasminogen activator inhibitor 1 protects fibrosarcoma cells from etoposide-induced apoptosis through activation of the PI3K/Akt cell survival pathway. Neoplasia. 2008;10(10):1083–91. doi: 10.1593/neo.08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eitzman DT, Krauss JC, Shen T, Cui J, Ginsburg Lack of plasminogen activator inhibitor-1 effect in a transgenic mouse model of metastatic melanoma. Blood. 1996;87(11):4718–22. [PubMed] [Google Scholar]
- 22.McMahon GA, Petitclerc E, Stefansson S, Smith E, Wong MK, Westrick RJ, et al. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. The Journal of biological chemistry. 2001;276(36):33964–8. doi: 10.1074/jbc.M105980200. [DOI] [PubMed] [Google Scholar]
- 23.Maillard C, Jost M, Romer MU, Brunner N, Houard X, Lejeune A, et al. Host plasminogen activator inhibitor-1 promotes human skin carcinoma progression in a stage-dependent manner. Neoplasia. 2005;7(1):57–66. doi: 10.1593/neo.04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almholt K, Nielsen BS, Frandsen TL, Brunner N, Dano K, Johnsen M. Metastasis of transgenic breast cancer in plasminogen activator inhibitor-1 gene-deficient mice. Oncogene. 2003;22(28):4389–97. doi: 10.1038/sj.onc.1206601. [DOI] [PubMed] [Google Scholar]
- 25.Fen Li C, Kandel C, Baliko F, Nadesan P, Brunner N, Alman BA. Plasminogen activator inhibitor-1 (PAI-1) modifies the formation of aggressive fibromatosis (desmoid tumor) Oncogene. 2005;24(9):1615–24. doi: 10.1038/sj.onc.1208193. [DOI] [PubMed] [Google Scholar]
- 26.Maillard CM, Bouquet C, Petitjean MM, Mestdagt M, Frau E, Jost M, et al. Reduction of brain metastases in plasminogen activator inhibitor-1-deficient mice with transgenic ocular tumors. Carcinogenesis. 2008;29(11):2236–42. doi: 10.1093/carcin/bgn204. [DOI] [PubMed] [Google Scholar]
- 27.Masset A, Maillard C, Sounni NE, Jacobs N, Bruyere F, Delvenne P, et al. Unimpeded skin carcinogenesis in K14-HPV16 transgenic mice deficient for plasminogen activator inhibitor. International journal of cancer Journal international du cancer. 2011;128(2):283–93. doi: 10.1002/ijc.25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biliran H, Jr, Sheng S. Pleiotrophic inhibition of pericellular urokinase-type plasminogen activator system by endogenous tumor suppressive maspin. Cancer research. 2001;61(24):8676–82. [PubMed] [Google Scholar]
- 29.Espana F, Estelles A, Fernandez PJ, Gilabert J, Sanchez-Cuenca J, Griffin JH. Evidence for the regulation of urokinase and tissue type plasminogen activators by the serpin, protein C inhibitor, in semen and blood plasma. Thrombosis and haemostasis. 1993;70(6):989–94. [PubMed] [Google Scholar]
- 30.Montemurro P, Barbuti G, Conese M, Gabriele S, Petio M, Colucci M, et al. Retinoic acid stimulates plasminogen activator inhibitor 2 production by blood mononuclear cells and inhibits urokinase-induced extracellular proteolysis. British journal of haematology. 1999;107(2):294–9. doi: 10.1046/j.1365-2141.1999.01698.x. [DOI] [PubMed] [Google Scholar]
- 31.Scott RW, Bergman BL, Bajpai A, Hersh RT, Rodriguez H, Jones BN, et al. Protease nexin. Properties and a modified purification procedure. The Journal of biological chemistry. 1985;260(11):7029–34. [PubMed] [Google Scholar]
- 32.Fortenberry YM. Plasminogen activator inhibitor-1 inhibitors: a patent review (2006-present) Expert opinion on therapeutic patents. 2013;23(7):801–15. doi: 10.1517/13543776.2013.782393. [DOI] [PubMed] [Google Scholar]
- 33.Charlton PA, Faint RW, Bent F, Bryans J, Chicarelli-Robinson I, Mackie I, et al. Evaluation of a low molecular weight modulator of human plasminogen activator inhibitor-1 activity. Thrombosis and haemostasis. 1996;75(5):808–15. [PubMed] [Google Scholar]
- 34.Friederich PW, Levi M, Biemond BJ, Charlton P, Templeton D, van Zonneveld AJ, et al. Novel low-molecular-weight inhibitor of PAI-1 (XR5118) promotes endogenous fibrinolysis and reduces postthrombolysis thrombus growth in rabbits. Circulation. 1997;96(3):916–21. [PubMed] [Google Scholar]
- 35.Liang A, Wu F, Tran K, Jones SW, Deng G, Ye B, et al. Characterization of a small molecule PAI-1 inhibitor, ZK4044. Thrombosis research. 2005;115(4):341–50. doi: 10.1016/j.thromres.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Rupin A, Gaertner R, Mennecier P, Richard I, Benoist A, De Nanteuil G, et al. S35225 is a direct inhibitor of Plasminogen Activator Inhibitor type-1 activity in the blood. Thrombosis research. 2008;122(2):265–70. doi: 10.1016/j.thromres.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki H, Ogiku T, Sai H, Ohmizu H, Murakami J, Ohtani A. Design, synthesis, and evaluation of orally active inhibitors of plasminogen activator inhibitor-1 (PAI-1) production. Bioorganic & medicinal chemistry letters. 2008;18(24):6419–22. doi: 10.1016/j.bmcl.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 38.Elokdah H, Abou-Gharbia M, Hennan JK, McFarlane G, Mugford CP, Krishnamurthy G, et al. Tiplaxtinin, a novel, orally efficacious inhibitor of plasminogen activator inhibitor-1: design, synthesis, and preclinical characterization. Journal of medicinal chemistry. 2004;47(14):3491–4. doi: 10.1021/jm049766q. [DOI] [PubMed] [Google Scholar]
- 39.Hennan JK, Morgan GA, Swillo RE, Antrilli TM, Mugford C, Vlasuk GP, et al. Effect of tiplaxtinin (PAI-039), an orally bioavailable PAI-1 antagonist, in a rat model of thrombosis. Journal of thrombosis and haemostasis : JTH. 2008;6(9):1558–64. doi: 10.1111/j.1538-7836.2008.03063.x. [DOI] [PubMed] [Google Scholar]
- 40.Lucking AJ, Visvanathan A, Philippou H, Fraser S, Grant PJ, Connolly TM, et al. Effect of the small molecule plasminogen activator inhibitor-1 (PAI-1) inhibitor, PAI-749, in clinical models of fibrinolysis. Journal of thrombosis and haemostasis : JTH. 2010;8(6):1333–9. doi: 10.1111/j.1538-7836.2010.03872.x. [DOI] [PubMed] [Google Scholar]
- 41.Jacobsen JS, Comery TA, Martone RL, Elokdah H, Crandall DL, Oganesian A, et al. Enhanced clearance of Abeta in brain by sustaining the plasmin proteolysis cascade. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(25):8754–9. doi: 10.1073/pnas.0710823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barker R, Kehoe PG, Love S. Activators and inhibitors of the plasminogen system in Alzheimer’s disease. Journal of cellular and molecular medicine. 2012;16(4):865–76. doi: 10.1111/j.1582-4934.2011.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izuhara Y, Takahashi S, Nangaku M, Takizawa S, Ishida H, Kurokawa K, et al. Inhibition of plasminogen activator inhibitor-1: its mechanism and effectiveness on coagulation and fibrosis. Arterioscler Thromb Vasc Biol. 2008;28(4):672–7. doi: 10.1161/ATVBAHA.107.157479. [DOI] [PubMed] [Google Scholar]
- 44.Izuhara Y, Yamaoka N, Kodama H, Dan T, Takizawa S, Hirayama N, et al. A novel inhibitor of plasminogen activator inhibitor-1 provides antithrombotic benefits devoid of bleeding effect in nonhuman primates. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30(5):904–12. doi: 10.1038/jcbfm.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaoka N, Kawano Y, Izuhara Y, Miyata T, Meguro K. Structure-activity relationships of new 2-acylamino-3-thiophenecarboxylic acid dimers as plasminogen activator inhibitor-1 inhibitors. Chemical & pharmaceutical bulletin. 2010;58(5):615–9. doi: 10.1248/cpb.58.615. [DOI] [PubMed] [Google Scholar]
- 46.Gorlatova NV, Cale JM, Elokdah H, Li D, Fan K, Warnock M, et al. Mechanism of inactivation of plasminogen activator inhibitor-1 by a small molecule inhibitor. The Journal of biological chemistry. 2007;282(12):9288–96. doi: 10.1074/jbc.M611642200. [DOI] [PubMed] [Google Scholar]
- 47.Rouch A, Vanucci-Bacque C, Bedos-Belval F, Baltas M. Small molecules inhibitors of plasminogen activator inhibitor-1 - An overview. European journal of medicinal chemistry. 2015;92:619–36. doi: 10.1016/j.ejmech.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Cale JM, Li SH, Warnock M, Su EJ, North PR, Sanders KL, et al. Characterization of a novel class of polyphenolic inhibitors of plasminogen activator inhibitor-1. The Journal of biological chemistry. 2010;285(11):7892–902. doi: 10.1074/jbc.M109.067967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li SH, Reinke AA, Sanders KL, Emal CD, Whisstock JC, Stuckey JA, et al. Mechanistic characterization and crystal structure of a small molecule inactivator bound to plasminogen activator inhibitor-1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(51):E4941–9. doi: 10.1073/pnas.1216499110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mutoh M, Niho N, Komiya M, Takahashi M, Ohtsubo R, Nakatogawa K, et al. Plasminogen activator inhibitor-1 (Pai-1) blockers suppress intestinal polyp formation in Min mice. Carcinogenesis. 2008;29(4):824–9. doi: 10.1093/carcin/bgn028. [DOI] [PubMed] [Google Scholar]
- 51.Masuda T, Hattori N, Senoo T, Akita S, Ishikawa N, Fujitaka K, et al. SK-216, an inhibitor of plasminogen activator inhibitor-1, limits tumor progression and angiogenesis. Molecular cancer therapeutics. 2013;12(11):2378–88. doi: 10.1158/1535-7163.MCT-13-0041. [DOI] [PubMed] [Google Scholar]
- 52.Gomes-Giacoia E, Miyake M, Goodison S, Rosser CJ. Targeting plasminogen activator inhibitor-1 inhibits angiogenesis and tumor growth in a human cancer xenograft model. Molecular cancer therapeutics. 2013;12(12):2697–708. doi: 10.1158/1535-7163.MCT-13-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mashiko S, Kitatani K, Toyoshima M, Ichimura A, Dan T, Usui T, et al. Inhibition of plasminogen activator inhibitor-1 is a potential therapeutic strategy in ovarian cancer. Cancer biology & therapy. 2015:0. doi: 10.1080/15384047.2014.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Placencio VR, Ichimura A, Miyata T, DeClerck YA. Small Molecule Inhibitors of Plasminogen Activator Inhibitor-1 Elicit Anti-Tumorigenic and Anti-Angiogenic Activity. PLOS ONE. 2015 doi: 10.1371/journal.pone.0133786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Declerck PJ, De Mol M, Alessi MC, Baudner S, Paques EP, Preissner KT, et al. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multimeric form of S protein (vitronectin) The Journal of biological chemistry. 1988;263(30):15454–61. [PubMed] [Google Scholar]
- 56.Fay WP, Parker AC, Condrey LR, Shapiro AD. Human plasminogen activator inhibitor-1 (PAI-1) deficiency: characterization of a large kindred with a null mutation in the PAI-1 gene. Blood. 1997;90(1):204–8. [PubMed] [Google Scholar]
- 57.Sharp AM, Stein PE, Pannu NS, Carrell RW, Berkenpas MB, Ginsburg D, et al. The active conformation of plasminogen activator inhibitor 1, a target for drugs to control fibrinolysis and cell adhesion. Structure. 1999;7(2):111–8. doi: 10.1016/S0969-2126(99)80018-5. [DOI] [PubMed] [Google Scholar]
- 58.Stout TJ, Graham H, Buckley DI, Matthews DJ. Structures of active and latent PAI-1: a possible stabilizing role for chloride ions. Biochemistry. 2000;39(29):8460–9. doi: 10.1021/bi000290w. [DOI] [PubMed] [Google Scholar]