Abstract
The extracellular Ca2+-sensing receptor (CaSR) is an allosteric protein that responds to changes in the extracellular concentration of Ca2+ ([Ca2+]e) and aromatic amino acids with the production of different patterns of oscillations in intracellular Ca2+ concentration ([Ca2+]i). An increase in [Ca2+]e stimulates sinusoidal oscillations in [Ca2+]i whereas aromatic amino acid-induced CaR activation in the presence of a threshold [Ca2+]e promotes transient oscillations in [Ca2+]i. Here, we examined spontaneous and ligand-evoked [Ca2+]i oscillations in single HEK-293 cells transfected with the wild type CaSR or with a mutant CaSR in which Ser170 was converted to Thr (CaSRS170T). Our analysis demonstrates that cells expressing CaSRS170T display [Ca2+]i oscillations in the presence of low concentrations of extracellular Ca2+ and respond to L-Phe with robust transient [Ca2+]i oscillations. Our results indicate that the S170T mutation induces a marked increase in CaSR sensitivity to [Ca2+]e and imply that the allosteric regulation of the CaSR by aromatic amino acids is not only mediated by an heterotropic positive effect on Ca2+ binding cooperativity but, as biased agonists, aromatic amino acids stabilize a CaSR conformation that couples to a different signaling pathway leading to transient [Ca2+]i oscillations.
Keywords: Ca2+ oscillations, amino acid signaling, CaSR mutant S170T, allosteric regulation
1. Introduction
The extracellular Ca2+-sensing receptor (CaSR), a member of the C family of heptahelical G protein-coupled receptors (GPCRs), is an allosteric protein that plays a major physiological role in correcting small changes in extracellular concentration of Ca2+ ([Ca2+]e) by inhibiting parathyroid hormone secretion and renal Ca2+ absorption [1]. Interestingly, subsequent studies demonstrated that the CaSR recognizes other ligands and is expressed in many other tissues and organs, including the gastrointestinal tract, brain, pituitary, thyroid, skin, breast, pancreas, lung, bone, and heart [2], suggesting that this receptor plays additional, yet less well defined, physiological roles in the regulation of normal and abnormal cell function [3,4,5,6]. Indeed, recent evidence indicates that the CaSR is implicated in the negative control of colon cell proliferation [7] and cancer [8], nutrient sensing [4], epithelial transport [9], inflammation [10], bone turnover [11] and stem cell differentiation [6]. Thus, the signaling mechanisms triggered via CaSR activation are attracting intense attention.
A number of studies of CaSR activation in individual living cells have shown that intracellular Ca2+ concentration ([Ca2+]i) oscillates upon stimulation of CaSR by an elevation in [Ca2+]e within a physiological range [12,13,14,15,16,17,18]. Oscillatory changes in [Ca2+]i in response to receptor stimulation is a fundamental mechanism of cell signaling implicated in the regulation of Ca2+- and calmodulin-dependent protein kinase II [19], conventional protein kinase C (PKC) isoforms [15,20], mitochondrial function [21,22], and nuclear transcriptional activity leading to differential gene expression [23,24]. We proposed that [Ca2+]i oscillations induced by activation of the CaSR in response to an increase in extracellular Ca2+ results from negative feedback involving PKC-mediated phosphorylation of the CaSR at the inhibitory residue Thr888 [14,25]. In addition to its role as sensor of [Ca2+]e, the CaSR is also stimulated by aromatic amino acids [26] which, like [Ca2+]e, induce striking and lasting CaSR-mediated [Ca2+]i oscillations [13,15,16]. However, the patterns of [Ca2+]i oscillations induced by these agonists are different. Aromatic amino acid stimulation of the CaSR in the presence of a threshold [Ca2+]e induces repetitive, low frequency [Ca2+]i spikes that return to the base-line level, a pattern known as transient oscillations [13,15,16]. The amplitude, frequency, and duration of [Ca2+]i oscillations are increasingly recognized to encode important information for a variety of biological processes, including metabolism and gene expression.
The most striking structural feature of the family C GPCR is its large extracellular domain, which consists of ~600 amino acid residues arranged in two predicted lobes referred as the Venus Flytrap Domain (VFTD), the site of numerous disease-causing mutations in this receptor [27,28]. The VFTD contains multiple binding sites for extracellular Ca2+ [29] and the allosteric binding site for amino acids. The residues Ser-147, Ser-170, Asp-190, Tyr-218 and Glu-297 of the VFTD comprise the first Ca2+ binding site which is considered the most important and operational under physiological conditions [27,30]. A number of studies identified Ser-170 as playing a critical role in Ca2+ binding and signal generation since mutation of Ser-170 to Ala completely abolished the response of the mutant CaSR to even very high [Ca2+]e [31] without affecting its membrane expression [30,31]. In contrast, a conservative mutation of Ser-170 to Thr (S170T) appeared to have very little effect on CaSR responsiveness to an increase in [Ca2+]e but impaired L-phenylalanine (L-Phe) sensing [29,31] though the ability of this CaSR mutant to mediate transient oscillations in single cells was not examined. The detailed study of the impact of mutations in the N-terminal VFTD is of importance to advance understanding of the molecular mechanisms of CaSR regulation.
Here, we examined spontaneous and ligand-evoked [Ca2+]i oscillations in single HEK-293 cells transfected with the wild type CaSR or with CaSR in which Ser170 was mutated to Thr (CaSRS170T). Our analysis demonstrates that cells expressing CaSRS170T display [Ca2+]i oscillations in the presence of low concentrations of extracellular Ca2+ and respond to L-Phe with robust transient [Ca2+]i oscillations. We propose that the S170T mutation increases CaSR sensitivity to [Ca2+]e and separates the positive heterotropic effect that enhances the apparent affinity of the CaSR for extracellular Ca2+ from the stabilization of a receptor conformation that promotes transient oscillations in the presence of a threshold [Ca2+]e.
2. Materials and Methods
2.1 Materials
Fura 2-AM, Dulbecco’s Modified Eagle’s Medium (DMEM), and Hanks Balanced Salt Solution (HBSS) were obtained from Invitrogen (Carlsbad, CA).
2.2 Cell culture and transfection
Human Embryonic Kidney (HEK-293) cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) in a humidified incubator under 10% CO2 and 90% air at 37°C, as described previously [13,25,32]. For experimental purposes, cells were plated onto 18-mm diameter glass coverslips inside 35-mm plastic dishes, where they could be dually transiently transfected with a plasmid encoding the human CaSR or a mutant CaSR receptor (CaSRS170T). Site-directed mutagenesis to convert Ser170 of the CaSR into Thr was performed as previously described [33]. Identification of cells transiently transfected with pCR3.1-CaSRwt (0.5 µg/dish) or pCR3.1-CaSRS170T (0.5 µg/ dish) was achieved by co-transfection with pDsRed-Express (BD Biosciences) (0.5 µg/dish), a vector that encodes a red fluorescent protein. After 16 h, the cultures were loaded with the Ca2+ indicator Fura-2 as described below.
2.3 Measurement of [Ca2+]i
[Ca2+]i was measured in single cells loaded with the calcium indicator fura-2 as previously described [13]. Briefly, cells were incubated in saline solution containing 138 mM NaCl, 4 mM NaHCO3, 0.3 mM Na2HPO4, 5 mM KCl, 0.3 mM KH2PO4, 1.5 mM CaCl2, 0.5 mM MgCl2, 0.4 mM MgSO4, 5.6 mM D-glucose, 20 mM HEPES, pH: 7.4 which was supplemented with 5 µM fura-2 AM for 45–60 min at 37°C before [Ca2+]i imaging. The cells were then washed and placed in an experimental chamber that was perfused with saline solution at 1.5 ml/min at 37°C. The chamber in turn was placed on the stage of an inverted microscope connected to a digital imaging system. Ratios of images (340 nm excitation/ 380 nm excitation, emission filter 520 nm) were obtained at 1.5 sec intervals. A region of interest covering 15 µm × 15 µm was defined over each cell, and the average ratio intensity over the region was converted to [Ca2+]i using an standard curve constructed with a series of calibrated buffered calcium solutions (Calcium Calibration Buffer Kit #2, Invitrogen Corp).
2.4 Western blots and indirect immunofluorescence
HEK-293 cells were transfected with vectors encoding wild type human CaSR or CaSRS170T at 0.5 µg/ dish and analyzed by Western blot 16 h post-transfection using a murine monoclonal antibody against the CaSR (Affinity BioReagents). The images were captured using a luminescent image analyzer LAS-4000 mini (Fujifilm Life Sciences). For indirect immunofluorescence, the transfected cells were fixed 16 h post-transfection in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 7 min at 24°C to only reveal surface expression of the receptor. The fixed cells were first incubated during 2 h at 24°C in blocking buffer (PBS-1% gelatin) and then 4 h with an anti-CaSR murine monoclonal antibody (Affinity BioReagents) diluted in blocking buffer. The cells were then washed with PBS at 25°C and incubated during 2 h at 24°C with Alexa 488-conjugated rabbit-anti mouse (Invitrogen) diluted in blocking buffer. After extensive washes at 24°C with PBS, the samples mounted with a gelvatol-glycerol solution containing 2.5% 1,4-diazobicyclo-[2.2.2]octane. The samples were examined with a epifluorescence microscope (Zeiss Axioskop) as previously described [33]. The selected cells displayed in the appropriate figures were representative of 80% of the population of positive cells.
4. Results
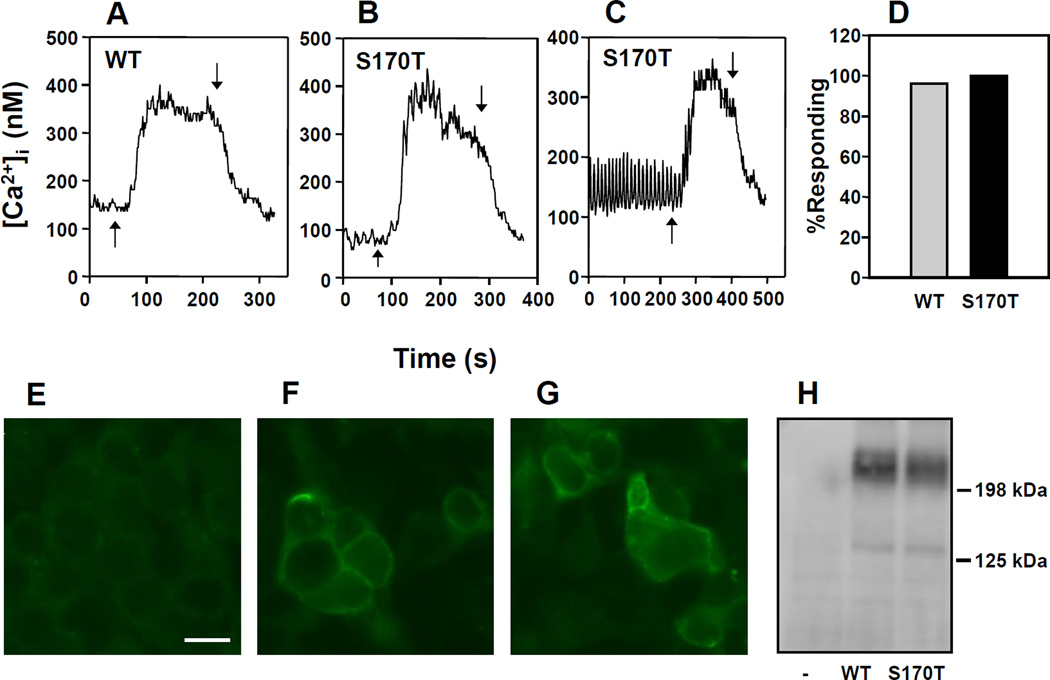
In order to examine the impact of mutations in the 1st Ca2+ binding site of the VFTD, we analyzed the pattern of [Ca2+]i response in individual HEK-293 cells transiently co-transfected with a plasmid encoding the human wild type CaSR or with a CaSR containing a highly conservative substitution in Ser-170 to Thr (CaSRS170T) and a plasmid encoding a red fluorescent protein (pDsRed-Express) to facilitate the identification of the transfected cells. After 16 h, the cells were loaded with the fluorescent Ca2+ indicator fura 2-AM and incubated in the presence of medium containing 1.5 mM [Ca2+]e. Single cell imaging of fura-2 loaded HEK-293 cells expressing the CaSR or CaSRS170T revealed that a rise in the [Ca2+]e from 1.5 mM to 5 mM produced a rapid elevation in [Ca2+]i followed by a sustained plateau in cells transfected with either the wild type CaSR (Fig. 1 A) or mutant CaSRS170T (Fig. 1 B, C). The maximal response of CaSRS170T to the increase in [Ca2+]e was similar to that of the wild type receptor (Fig. 1 A, B). Most transfected cells with CaSR or CaSRS170T responded to the increase in [Ca2+]e (Fig. 1 D). Accordingly, equivalent levels of CaSR and CaSRS170T were expressed on the cell membrane of HEK-293 cells, as judged by indirect immunofluorescence (Fig. 1 F, G) and total cellular expression of CaSR and CaSRS170T was also equal, as shown by immunoblotting (Fig. 1 H).
Figure 1.
Changes in [Ca2+]i in HEK-293 cells transfected with CaSR (WT) A, or CaSRS170T (S170T) B,C, in response to an increase in [Ca2+]e from 1.5 mM to 5 mM. The addition of Ca2+ is indicated by upward arrows whereas the return of [Ca2+]e to 1.5 mM is indicated by the downward arrows. D, Bar chart: Percentage of HEK-293 cells transfected with CaSR (WT) or CaSRS170T which responded to an increase in [Ca2+]e from 1.5 mM to 5 mM. E, F, G. Fixed cells were labeled with an anti-CaSR murine monoclonal antibody to detect surface expressed receptor. E. Control, no transfection. F. Transfected with CaSR(WT). G. Transfected with CaRS170T. Scale Bar 30 µM. H. Western Blot analysis of control, no transfection (−), CaSR (WT), and CaSRS170T (S170T) cell lysates.
Although the results in Fig. 1 indicated that the responses of wild type and mutant CaSR to an increase in the [Ca2+]e were virtually identical, analysis of [Ca2+]i prior to simulation revealed an important difference between CaSR and CaSRS170T in the dynamics of basal [Ca2+]i. In agreement with our previous results, most cells transfected with wild type CaSR and incubated in the presence of medium containing 1.5 mM [Ca2+]e exhibited a stable [Ca2+]i with only a small proportion of cells (~10%) exhibiting spontaneous oscillatory activity.
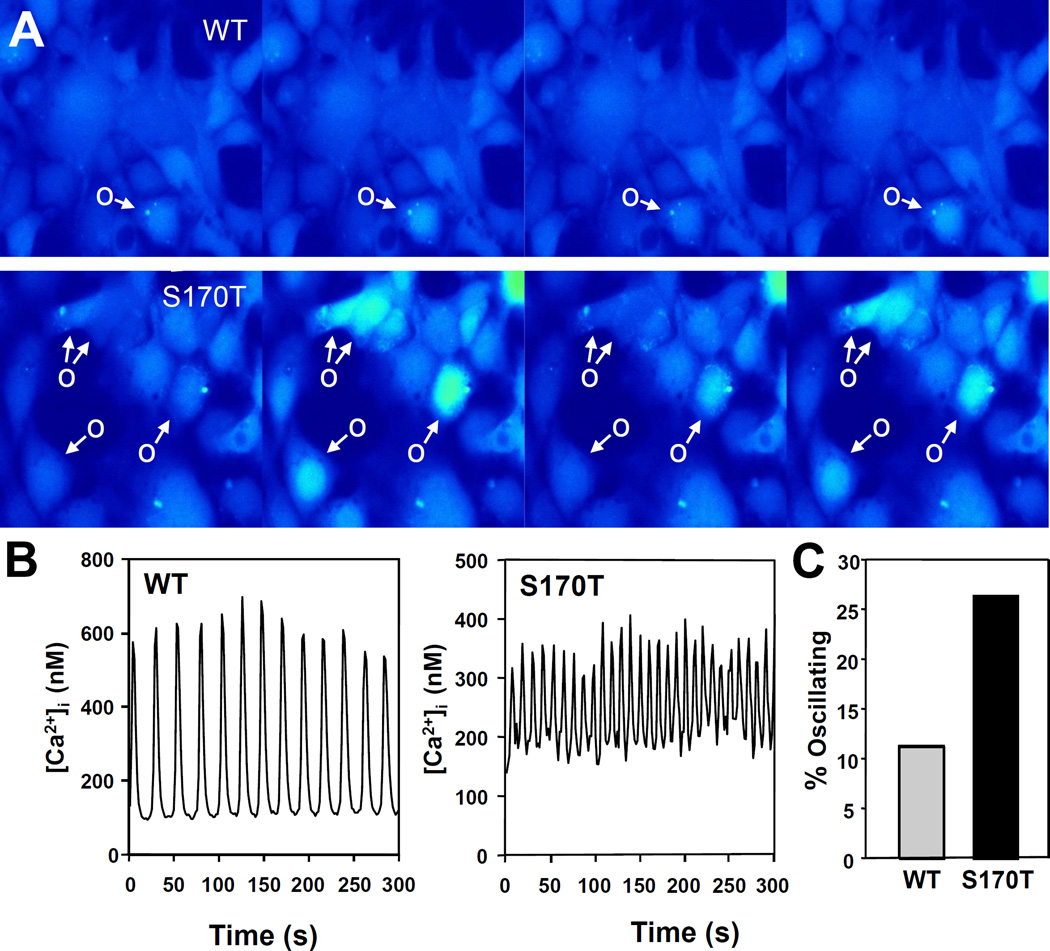
In striking contrast, a substantial proportion of cells transfected with CaSRS170T display spontaneous oscillatory fluctuations in [Ca2+]i. The difference in spontaneous [Ca2+]i oscillations is illustrated in Fig. 2 A, showing a pseudo-colored images of clusters of cells transfected with wild type CaSR or CaSRS170T incubated for various times in medium containing 1.5 mM [Ca2+]e. Cells labeled O show a spontaneous increase in [Ca2+]i which declines, and then increases again (oscillatory response) while other cells exhibited a stable [Ca2+]i. Examples of tracings corresponding to HEK-293 cells transfected with CaSRS170T displaying spontaneous [Ca2+]i oscillations are illustrated in Fig. 2 B. The examples also illustrate that the oscillatory frequency is markedly higher in cells expressing CaSRS170T. The difference in basal [Ca2+]i oscillations between CaSR and CaSRS170T was substantiated by analyzing 714 individual cells. We found a marked increase in the proportion of cells that display spontaneous [Ca2+]i oscillations when transfected with CaSRS170T (Fig. 2 C, bars). These results demonstrate, for the first time, that a sub-population of HEK-293 cells expressing CaSRS170T (~27%) displays spontaneous [Ca2+]i oscillations while the majority of cells transfected with wild type CaSR exhibited a stable [Ca2+]i. In agreement with our previous results [13], we did not detect any [Ca2+]i oscillations in non-transfected HEK-293 cells or in cells transfected with vector.
Figure 2.
Spontaneous [Ca2+]i oscillations of HEK-293 cells transfected with wild type CaSR (WT) or CaSRS170T (S170T) and incubated in medium containing 1.5 mM [Ca2+]e. A. Pseudo-colored images of clusters of cells showing changes in [Ca2+]i with time. Shown is one cycle of rest to peak response. Spontaneously oscillating cells (O) are marked with an arrow. B. Typical traces of [Ca2+]i as a function of time (s) for a single HEK-293 cell transfected with the wild type CaSR or a single HEK-293 cells transfected with CaSRS170T. C. Percentage of spontaneously oscillating HEK-293 cells from a total of 544 transfected with the wild type CaSR and from 170 cells transfected with CaSRS170T.
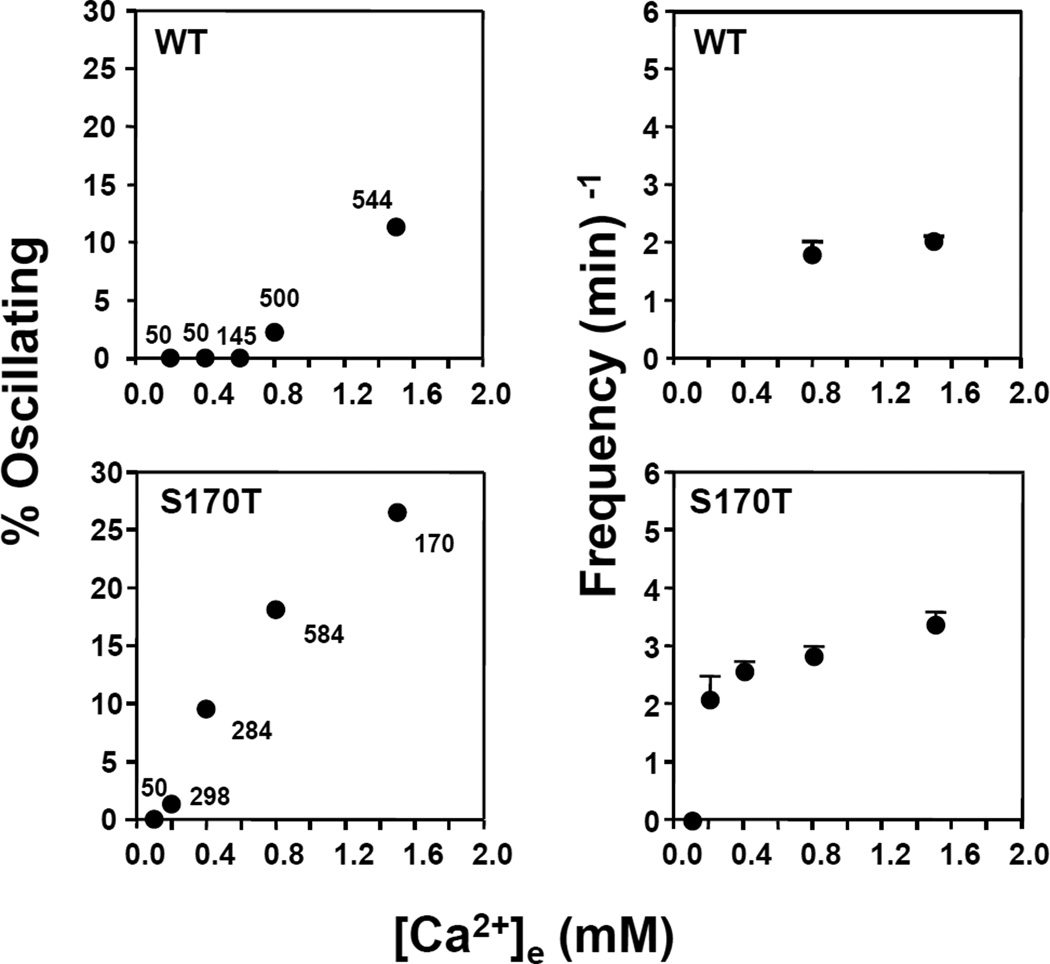
The results shown in Fig. 2 prompted us to hypothesize that HEK-293 cells transfected with CaSRS170T have increased sensitivity to extracellular Ca2+. To examine this possibility, we monitored basal [Ca2+]i oscillations in HEK-293 cells transfected with wild type CaSR or CaSRS170T incubated in medium containing different [Ca2+]e. Cells incubated in medium containing 0.8 mM Ca2+ show striking differences, as shown in Fig. 3. We found that ~20% of HEK-293 cells expressing CaSRS170T exhibit spontaneous [Ca2+]i oscillations with a frequency of 3 spikes/min. In sharp contrast, only ~3% of cells transfected with wild type CaSR exhibited oscillatory activity of lower frequency (2 spikes/min) whereas 97% of transfected cells showed stable [Ca2+]i. A further decrease in [Ca2+]e to 0.4 mM completely eliminated any spontaneous [Ca2+]i oscillations in HEK 293 cells transfected with wild type CaSR while 10% of HEK-293 cells expressing CaSRS170T exhibited spontaneous [Ca2+]i oscillations with a frequency of 2.7 spikes/min (Fig. 3). A small subpopulation of HEK 293 cells expressing CaSRS170T show spontaneous [Ca2+]i oscillations at a [Ca2+]e as low as 0.2 mM. The threshold [Ca2+]e required for spontaneous [Ca2+]i oscillations in individual HEK-293 cells transfected with either CaSR or CaSRS170T was 0.6mM and 0.2 mM, respectively, as extrapolated from the data in Fig. 3. These results support the hypothesis that a highly conserved mutation of the CaSR at Ser170 increases the sensitivity of the CaSR for extracellular Ca2+.
Figure 3.
Percentage of spontaneously oscillating HEK-293 cells transfected with wild type CaSR (WT) or CaSRS170T (S170T), (left panels) and their oscillating frequencies (right panels) as a function of varying [Ca2+]e. The data is a summary derived from a total of 1,289 HEK-293 cells transfected with wild type CaSR and 1,386 HEK-293 cells transfected with CaSRS170T. Numbers show number of cells measured at each [Ca2+]e.
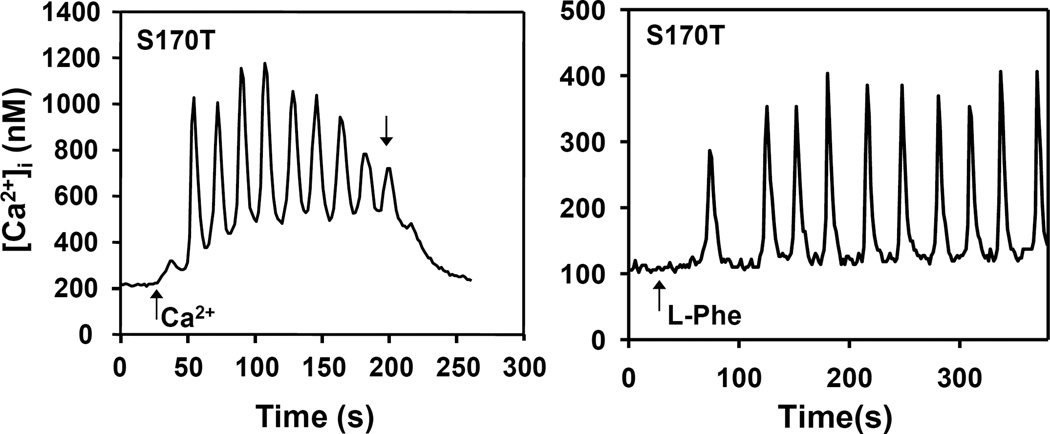
In addition to its role as sensor of [Ca2+]e, the CaSR is also stimulated by aromatic amino acids [26] that induce striking and lasting transient [Ca2+]i oscillations characterized by low frequency [Ca2+]i spikes that return to the base-line level [13]. These oscillations are mediated by a phospholipase C-independent pathway that involves Rho, filamin and TRPC1 [13,15,16,18]. In contrast, CaSR activation in response to modest increase in [Ca2+]e (e.g. from 1.5 to 3 mM) produces high frequency sinusoidal oscillations upon a raised plateau level of [Ca2+]i [13,15,16]. Consequently, we next determined whether CaSRS170T mediates different oscillatory patterns, i.e. sinusoidal and transient oscillations in response to different agonists in cells initially incubated in low [Ca2+]e. To examine this possibility, HEK-293 cells transfected with CaSRS170T were incubated in medium containing 0.8 mM [Ca2+]e to diminish spontaneous [Ca2+]i oscillations and then stimulated by an increase of [Ca2+]e to 3 mM or by addition of 5 mM L-Phe. As shown in Fig. 4, HEK-293 cells expressing CaSRS170T displayed sinusoidal oscillations in response to an increase in [Ca2+]e to 3 mM and striking transient oscillations in response to 5 mM L-Phe in the presence of 0.8 mM [Ca2+]e (Fig. 4). The responses were comparable to those showed by HEK-293 cells expressing wild type CaSR but incubated initially in medium containing 1.5 mM [Ca2+]e and thus substantiating that the Ser170 to Thr mutation decreases the [Ca2+]e required for detecting transient oscillations in response to aromatic amino acids.
Figure 4.
Oscillatory responses in [Ca2+]i in HEK-293 expressing CaSRS170T (S170T) cells when challenged with increases in [Ca2+]e or L-phenylalanine (L-Phe). The cells were initially incubated in medium containing [Ca2+]e at 0.8 mM. After recording basal [Ca2+]i the cells were challenged by either increasing the [Ca2+]e to 3mM or 5 mM L-Phe (upward arrows). Downward arrow marks return to medium containing [Ca2+]e at 1.5 mM.
5. Discussion
Oscillatory changes in [Ca2+]i in response to receptor stimulation is a fundamental mechanism of cell signaling implicated in the regulation of signal transduction [15,19,20], metabolism [21,22], and differential gene expression [23,24]. Previous studies identified Ser-170 in the N-terminal region of the VFTD as a critical residue in Ca2+ binding and signal generation since mutation of Ser-170 to Ala completely abolished the response to even very high concentrations of extracellular Ca2+ [31] without affecting its membrane expression [30,31]. In the current study we analyzed [Ca2+]i in single HEK-293 cells expressing wild type CaSR or a receptor with a highly conservative mutation of Ser-170 to Thr. Our results demonstrated that cells expressing CaSRS170T exhibit spontaneous oscillatory activity when incubated in medium containing 1.5 mM [Ca2+]e. Analysis of spontaneous [Ca2+]i oscillations as a function of [Ca2+]e revealed that HEK-293 cells expressing CaSRS170T display [Ca2+]i oscillations of higher frequency at markedly lower [Ca2+]e as compared with HEK-293 cells expressing wild type CaSR. Furthermore, HEK-293 cells expressing CaSRS170T display sinusoidal [Ca2+]i oscillations in response to an increase in [Ca2+]e or transient oscillations when challenged by L-Phe, provided that cells are initially incubated in medium containing a low [Ca2+]e. We conclude that a small increase in the size of the side chain of Ser170 (Ser-to-Thr substitution in the VTFD) is sufficient to induce a marked increase in sensitivity to [Ca2+]e of the CaSR.
Previous studies concluded that mutation of Ser-170 to Thr impairs amino acid sensing [30,31], as judged by experiments in which the EC50 value for [Ca2+]e remains unchanged in the presence of L-Phe. In contrast, we demonstrated that L-Phe induced transient oscillations in cells expressing CaSRS170T incubated in medium containing a low [Ca2+]e. We propose that the S170T mutation separates two different allosteric effects of aromatic amino acids on the CaSR. Specifically, (1) the stabilization of a conformation that promotes transient oscillations in the presence of a threshold level of [Ca2+]e via a multi-protein complex that includes Rho, filamin-A, and TRPC1 from (2) the positive heterotropic effect that causes an increase in the affinity of the CaSR for extracellular Ca2+. We obtained similar results using a different CaSR mutant in which Glu127 was replaced by Ala, an activating mutation. As with CaSRS170T, cells expressing the CaSRE127A mutation displayed [Ca2+]i transient oscillations in response to L-Phe. We conclude that the allosteric regulation of the CaSR by aromatic amino acids is not only mediated by an heterotropic positive effect on Ca2+ binding cooperativity but, as a biased agonist, aromatic amino acids stabilize a CaSR conformation that couples to a different signaling pathway leading to transient [Ca2+]i oscillations.
Supplementary Material
HIGHLIGHTS.
[Ca2+]i oscillations were analyzed in HEK-293 cells expressing CaSR and CaSRS170T
CaSRS170T mediated basal [Ca2+]i oscillations even in medium with low [Ca2+]e
CaSRS170T produced transient [Ca2+]i oscillations in response to L-phenylalanine.
CaSRS170T separates enhanced sensitivity to [Ca2+]e from transient oscillations
Acknowledgements
The work was supported by the Veterans Affair Merit grant (I01BX001473) and by National Institutes of Health Grants R0-1DK100405, P30-DK41301 and P01CA163200 (all to ER). Cell Imaging Services were provided by the Imaging and Stem Cell Biology Core, CURE: Digestive Diseases Research Center supported by P30DK41301. E.R. holds the Ronald S. Hirshberg Chair of Pancreatic Cancer Research. O.R. is the recipient of PICT 2012-0875 and PICT 2013-0891, FONCyT-MINCyT, Argentina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 2.Alfadda TI, Saleh AMA, Houillier P, Geibel JP. Calcium-sensing receptor 20 years later. Am J Physiol Cell Physiol. 2014;307:C221–C231. doi: 10.1152/ajpcell.00139.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 4.Geibel JP, Hebert SC. The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract. Annu Rev Physiol. 2009;71:205–217. doi: 10.1146/annurev.physiol.010908.163128. [DOI] [PubMed] [Google Scholar]
- 5.Saidak Z, Mentaverri R, Brown EM. The Role of the Calcium-Sensing Receptor in the Development and Progression of Cancer. Endocr Rev. 2009;30:178–195. doi: 10.1210/er.2008-0041. [DOI] [PubMed] [Google Scholar]
- 6.Riccardi D, Kemp PJ. The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol. 2012;74:271–297. doi: 10.1146/annurev-physiol-020911-153318. [DOI] [PubMed] [Google Scholar]
- 7.Rey O, Chang W, Bikle D, Rozengurt N, Young SH, Rozengurt E. Negative Cross-talk between Calcium-sensing Receptor and beta-Catenin Signaling Systems in Colonic Epithelium. J Biol Chem. 2012;287:1158–1167. doi: 10.1074/jbc.M111.274589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal A, Prinz-Wohlgenannt M, Tennakoon S, Höbaus J, Boudot C, Mentaverri R, Brown EM, Baumgartner-Parzer S, Kállay E. The calcium-sensing receptor: A promising target for prevention of colorectal cancer. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2015;1853:2158–2167. doi: 10.1016/j.bbamcr.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang L, Peng M, Liu L, Chang W, Binder HJ, Cheng SX. Calcium-sensing receptor stimulates Cl−- and SCFA-dependent but inhibits cAMP-dependent HCO3− secretion in colon. Am J Physiol Gastrointest Liver Physiol. 2015;308:G874–G883. doi: 10.1152/ajpgi.00341.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goltzman D, Hendy GN. The calcium-sensing receptor in bone[mdash]mechanistic and therapeutic insights. Nat Rev Endocrinol. 2015;11:298–307. doi: 10.1038/nrendo.2015.30. [DOI] [PubMed] [Google Scholar]
- 12.Breitwieser GE, Gama L. Calcium-sensing receptor activation induces intracellular calcium oscillations. Am J Physiol Cell Physiol. 2001;280:C1412–C1421. doi: 10.1152/ajpcell.2001.280.6.C1412. [DOI] [PubMed] [Google Scholar]
- 13.Young SH, Rozengurt E. Amino acids and Ca2+ stimulate different patterns of Ca2+ oscillations through the Ca2+-sensing receptor. Amer J Physiol-Cell Physiol. 2002;282:C1414–C1422. doi: 10.1152/ajpcell.00432.2001. [DOI] [PubMed] [Google Scholar]
- 14.Young SH, Wu SV, Rozengurt E. Ca2+-stimulated Ca2+ Oscillations Produced by the Ca2+-sensing Receptor Require Negative Feedback by Protein Kinase C. J. Biol. Chem. 2002;277:46871–46876. doi: 10.1074/jbc.M207083200. [DOI] [PubMed] [Google Scholar]
- 15.Rey O, Young SH, Yuan J, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1,4,5-trisphosphate-independent pathway that requires G12, Rho, filamin-A, and the actin cytoskeleton. J Biol Chem. 2005;280:22875–22882. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- 16.Rey O, Young SH, Papazyan R, Shapiro MS, Rozengurt E. Requirement of the TRPC1 Cation Channel in the Generation of Transient Ca2+ Oscillations by the Calcium-sensing Receptor. J. Biol. Chem. 2006;281:38730–38737. doi: 10.1074/jbc.M605956200. [DOI] [PubMed] [Google Scholar]
- 17.Davies SL, Ozawa A, McCormick WD, Dvorak MM, Ward DT. Protein Kinase C-mediated Phosphorylation of the Calcium-sensing Receptor Is Stimulated by Receptor Activation and Attenuated by Calyculin-sensitive Phosphatase Activity. J Biol Chem. 2007;282:15048–15056. doi: 10.1074/jbc.M607469200. [DOI] [PubMed] [Google Scholar]
- 18.Rey O, Young SH, Jacamo R, Moyer MP, Rozengurt E. Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J Cell Physiol. 2010;225:73–83. doi: 10.1002/jcp.22198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soderling TR, Chang B, Brickey D. Cellular Signaling through Multifunctional Ca2+/Calmodulin-dependent Protein Kinase II. J. Biol. Chem. 2001;276:3719–3722. doi: 10.1074/jbc.R000013200. [DOI] [PubMed] [Google Scholar]
- 20.Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95:307–318. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- 21.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of Cytosolic Calcium Oscillations in the Mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 22.Drago I, Pizzo P, Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J. 2011;30:4119–4125. doi: 10.1038/emboj.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 24.Hu Q, Deshpande S, Irani K, Ziegelstein RC. (Ca2+)i oscillation frequency regulates agonist-stimulated NF-kappaB transcriptional activity. J Biol Chem. 1999;274:33995–33998. doi: 10.1074/jbc.274.48.33995. [DOI] [PubMed] [Google Scholar]
- 25.Young SH, Rey O, Sinnett-Smith J, Rozengurt E. Intracellular Ca2+ oscillations generated via the Ca2+-sensing receptor are mediated by negative feedback by PKCα at Thr888. Am J Physiol Cell Physiol. 2014;306:C298–C306. doi: 10.1152/ajpcell.00194.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci USA. 2000;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannan FM, Nesbit MA, Zhang C, Cranston T, Curley AJ, Harding B, Fratter C, Rust N, Christie PT, Turner JJO, Lemos MC, Bowl MR, Bouillon R, Brain C, Bridges N, Burren C, Connell JM, Jung H, Marks E, McCredie D, Mughal Z, Rodda C, Tollefsen S, Brown EM, Yang JJ, Thakker RV. Identification of 70 calcium-sensing receptor mutations in hyper- and hypo-calcaemic patients: evidence for clustering of extracellular domain mutations at calcium-binding sites. Hum Mol Genet. 2012;21:2768–2778. doi: 10.1093/hmg/dds105. [DOI] [PubMed] [Google Scholar]
- 28.Hu J, Spiegel AM. Naturally occurring mutations of the extracellular Ca2+-sensing receptor: implications for its structure and function. Trends Endocrinol Metabol. 2003;14:282–288. doi: 10.1016/s1043-2760(03)00104-8. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Zhou Y, Castiblanco A, Yang W, Brown EM, Yang JJ. Multiple Ca2+-Binding Sites in the Extracellular Domain of the Ca2+-Sensing Receptor Corresponding to Cooperative Ca2+ Response†. Biochemistry. 2009;48:388–398. doi: 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Huang Y, Jiang Y, Mulpuri N, Wei L, Hamelberg D, Brown EM, Yang JJ. Identification of an l-Phenylalanine Binding Site Enhancing the Cooperative Responses of the Calcium-sensing Receptor to Calcium. J Biol Chem. 2014;289:5296–5309. doi: 10.1074/jbc.M113.537357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mun H-C, Culverston EL, Franks AH, Collyer CA, Clifton-Bligh RJ, Conigrave AD. A Double Mutation in the Extracellular Ca2+-sensing Receptor's Venus Flytrap Domain That Selectively Disables l-Amino Acid Sensing. J Biol Chem. 2005;280:29067–29072. doi: 10.1074/jbc.M500002200. [DOI] [PubMed] [Google Scholar]
- 32.Needham LK, Rozengurt E. Gα12 and Gα13 stimulate Rho-dependent tyrosine phosphorylation of focal adhesion kinase, paxillin and p130 Crk-associated substrate. J Biol Chem. 1998;273:14626–14632. doi: 10.1074/jbc.273.23.14626. [DOI] [PubMed] [Google Scholar]
- 33.Rey O, Young SH, Cantrell D, Rozengurt E. Rapid Protein Kinase D Translocation in Response to G Protein-coupled Receptor Activation. Dependence on Protein Kinase C. J. Biol. Chem. 2001;276:32616–32626. doi: 10.1074/jbc.M101649200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.