Abstract
Background
This study evaluates possible effects of smoking on the following: 1) biochemical content in gingival crevicular fluid (GCF) samples from sites of gingival recession and saliva; and 2) clinical outcomes of coronally advanced flap (CAF) for root coverage.
Methods
Eighteen defects in 15 patients were included in each of the smoker and non-smoker groups. Baseline cotinine, basic fibroblast growth factor, vascular endothelial growth factor, platelet-derived growth factor, interleukin (IL)-8, IL-10, IL-12, tumor necrosis factor-α, matrix metalloproteinase (MMP)-8, MMP-9, and plasminogen activator inhibitor-1 levels were determined in GCF and saliva samples. CAF with microsurgery technique was applied. Plaque index, papilla bleeding index, recession depth (RD), recession width (RW), and root surface area were evaluated at baseline and postoperative months 1, 3, and 6. Probing depth, clinical attachment level (CAL), and keratinized gingival width (KGW) was recorded at baseline and month 6. Percentage of root coverage and complete root coverage were calculated at postoperative months 1, 3, and 6.
Results
All biochemical parameters were similar in the two groups apart from the definite difference in salivary cotinine concentrations (P = 0.000). Compared with the baseline values, RD, RW, CAL, and root surface area decreased, and KGW increased, with no significant difference between the study groups. CAL gain, percentage of root coverage, and complete root-coverage rates were similar in the study groups.
Conclusion
Similar baseline biochemical data and comparably high success rates of root coverage with CAF in systemically and periodontally healthy smokers versus non-smokers suggest lack of adverse effects of smoking on clinical outcomes.
Keywords: Gingival crevicular fluid, gingival recession, saliva, smoking, wound healing
The coronally advanced flap (CAF) is one of the most widely used surgical techniques for root coverage. 1–3 Percentage of root coverage with CAF varies from 70% to 99%, and the percentage of teeth with complete root coverage has been reported to be 24% to 95%.3 Patient-related, site-related, and technique-related factors play determining roles in the amount of root coverage obtained.4
Cigarette smoking is a patient-related factor that can affect the success rate of root-coverage procedures. Neither the nature nor the mechanisms of action of cigarette smoking on root coverage are fully understood. Various animal and human studies revealed that cigarette smoking damages vascular and immunologic systems and reduces self-healing capacity of periodontal tissues.5–11 Smokers tend to respond less favorably to periodontal treatment procedures.12,13 Smoking has been reported to affect host cytokine levels in biofluids.13,14 However, the exact mechanisms by which smoking exerts detrimental effects on periodontal tissues remain unclear.
At present, there is no clear understanding of the pathology or the molecular events occurring in the periodontal microenvironment during the tissue breakdown process15 or wound healing after periodontal treatment. Wound healing in periodontium comprises complex events orchestrated by neutrophils, platelets, and macrophages. These cells are sources for the major cytokines, such as interleukins (ILs), tumor necrosis factor-alpha (TNF-α), growth factors, and matrix metalloproteinases (MMPs), all acting in tissue remodeling.
CAF can be used alone or in combination with a connective tissue (CT) graft. Gingival thickness is the most critical determining factor for choosing the appropriate surgical technique because complete root coverage is closely related with initial thickness of gingiva. A threshold gingival thickness for complete root coverage has been suggested in some studies,1,16,17 but these studies vary in treatment procedure, measurement technique, and exact location of measurement of gingival thickness, as well as statistical handling of data. Therefore, no consensus exists so far on adequate baseline gingival thickness to achieve complete root coverage with CAF.
Few studies have been published that were specifically designed to address the possible effects of smoking on the success of root coverage with CAF.18,19 The extant data suggest that smoking is associated with greater residual recession in a small number of individuals followed for up to 2 years after surgery.18,19 Furthermore, the underlying mechanisms are, essentially, unresolved.
Therefore, the hypothesis that cigarette smoking has negative impacts on the outcomes of root coverage after CAF surgery in systemically healthy individuals with an initial gingival thickness of at least 0.8 mm and who practice optimal oral hygiene was tested. It was also hypothesized that baseline analysis of disease-related biomarkers would shed light on the underlying mechanisms of a possible effect.
MATERIALS AND METHODS
Study Population
This study was a single-centered, prospective clinical trial with an observation period of 6 months. Individuals were referred to the Periodontology Clinic, School of Dentistry, Ege University, ızmir, Turkey, from May 2010 through November 2011 for the presence of gingival recession (GR). The study was conducted in full accordance with ethical principles, including the World Medical Association’s Declaration of Helsinki, as revised in 2008. The study was approved by the Ethics Committee of the Ege University (Protocol no. 10-8/5). The study protocol was explained, and written informed consent was received from each individual before clinical periodontal examination. Exclusion criteria included the following: 1) medical disorders such as diabetes mellitus, immunologic disorders, hepatitis, or a history of previous mucogingival surgery at the GR site; 2) medications known to affect gingival tissues; 3) antibiotic treatment in the past 6 months; 4) aged <18 years; and 5) pregnancy or lactation. Smokers were those who reported smoking >10 cigarettes per day for >5 years, whereas non-smokers were those who reported that they had never smoked. At baseline, the smoking (n = 16) and non-smoking (n = 16) individuals were grouped according to self-reports, later to be confirmed biochemically.
The 32 patients (11 males and 21 females; aged 18 to 52 years; mean age for the smoker group: 33.93 years; mean age for the non-smoker group: 29 years) with Miller Class I or II recession defects were treated with CAF. Two individuals from the smoker group were excluded from the study, because they did not attend at least one control visit. The accuracy of these declarations was verified by saliva cotinine concentration analysis. One self-reported non-smoker revealed a very high saliva cotinine concentration (196.82 ng/mL) and was reassigned to the smoker group. In the reassigned smoker group, salivary cotinine level ranged from 50.14 to 978.27 ng/mL, with the reassigned non-smokers exhibiting salivary cotinine concentration ranging from 1.42 to 14.74 ng/mL. Finally, 18 defects in 15 patients were included in each of the smoker and non-smoker groups according to the cotinine data. In the smoker group, 83% of the defects were in the maxilla, and 17% were in the mandible, whereas in the non-smoker group, 89% were in the maxilla, and 11% were in the mandible. The distribution of defects in either the maxilla or mandible was similar in the study groups (P = 0.5).
Maxillary central and lateral incisors, canines, and premolars and mandibular premolars with isolated buccal recessions (≥2 mm) classified as Miller Class I or II20 are included in the present study. Study teeth had an identifiable cemento-enamel junction (CEJ) and no restoration or superficial caries lesions in the area to be treated. All individuals complained of esthetic problems and/or hypersensitivity as a result of GR, and each received initial periodontal treatment consisting of oral hygiene instructions related to the etiology of GR and supragingival and subgingival calculus removal when required.
Clinical Measurements
Clinical periodontal recordings, including plaque index (PI),21 probing depth (PD), clinical attachment level (CAL) (at six sites: mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual locations), recession depth (RD), recession width (RW), keratinized gingiva width (KGW), and papilla bleeding index (PBI)22 were recorded on each tooth present except third molars at baseline and postoperative months 1, 3, and 6. A Williams periodontal probe‡ was used for clinical periodontal measurements.
PD was measured from the gingival margin to the most apical part of the sulcus, CAL was measured from the CEJ to the bottom of the sulcus, RD was measured from the CEJ to the gingival margin, RW was measured at the CEJ from mesial to distal, and KGW was measured from the mucogingival junction to the gingival margin. RD, RW, and recession area (RA) were measured also on digital photographs using specific software.§
Gingival thickness was measured with an ultrasonic device|| that uses the pulse echo principle. Ultrasonic pulses are transmitted at intervals of 1 millisecond through the sound-permeable mucosa and reflected, in part, at the surface of the alveolar bone or tooth attributable to different acoustic impedance. When an acoustic signal is transmitted within 2 to 3 seconds, gingival thickness is digitally displayed with a sensitivity of 0.01 mm.
All measurements were performed by a single calibrated examiner (BK). The intra-examiner reliability was high, as revealed by an intraclass correlation coefficient of 0.87 and 0.85 for PD and CAL measurements, respectively.
Saliva Sampling
Expectorated 1-mL whole saliva samples with minimal stimulation were obtained in the morning after an overnight fast, during which participants were requested not to drink (except water) or chew gum, and before clinical periodontal measurements or any periodontal intervention. The method described by Navazesh23 was used for saliva sampling. The saliva samples were clarified by centrifugation (800 × g) for 10 minutes at +4°C, immediately frozen, and stored at −40°C until the sample collection period was completed and thawed immediately before assays.
Gingival Crevicular Fluid Sampling
Gingival crevicular fluid (GCF) samples were collected using filter paper strips.¶ Before GCF sampling, supragingival plaque was removed from the vestibular, mesial, and distal surfaces of the GR defect with a sterile curet; these surfaces were dried gently by an air syringe and isolated by cotton rolls. Paper strips were carefully inserted ≈1 mm into the crevice and left there for 30 seconds. Care was taken to avoid mechanical injury. Strips contaminated with blood were discarded. The absorbed GCF volume was estimated by a calibrated instrument.# Then, the three strips from vestibular, mesial, and distal sites of each GR defect were placed into one polypropylene tube and pooled before freezing at −40°C. The readings were converted to an actual volume (μL) by reference to the standard curve. All GCF samples were stored at −40°C until the laboratory analyses.
Laboratory Analyses
Cotinine analysis in saliva samples
To verify the self-reported smoking status of each individual, salivary cotinine concentrations were determined using an assay kit** according to the instructions of the manufacturer. The minimum detection limit of the kit was 0.15 ng/mL. The data were read using a microplate reader†† and reported as nanograms per milliliters.
Biochemical analyses in GCF samples
Basic fibroblast growth factor (bFGF), vascular endothelial growth factor-A (VEGF-A), IL-8, IL-10, IL-12, TNF-α, MMP-8, MMP-9, and plasminogen activator inhibitor-1 (PAI-1) levels in GCF samples were determined by multiplex enzyme-linked immunosorbent assay (ELISA) using customized assays.‡‡ The findings were reported as picograms per milliliters. The lowest detection limit was 10 pg/mL or less per analyte. All samples were assayed in duplicate. The plates were wetted first with the reading buffer solution, and then 50 μL capture antibody was added to each well. Thereafter, 50 μL sample or standard solution was added to each well. The plates were incubated for 1 hour with continuous shaking. After washing three times, the plates were incubated with biotinylated antibody for 30 minutes. After three more washes, 50 μL streptavidin–phycoerythrin was added to each well and incubated for 30 minutes. Finally, the plates were washed three times, 125 μL reading buffer solution was added, and the data were read by a specific platform.§§
Biochemical analyses in saliva samples
Salivary concentrations of bFGF, VEGF-A, platelet-derived growth factor-BB (PDGF-BB), IL-8, IL-10, IL-12, TNF-α, MMP-8, MMP-9, and PAI-1 were determined by the customized multiplex ELISA assays. The data were reported as picograms per milliliters. The minimum detection limit of the kit was 10 pg/mL or less per analyte.
Surgical Procedure
All GR defects were treated with CAF by a single experienced periodontist (BK) using microsurgical instruments, a loop with 2.5× magnification,|||| and 7-0 propylene sutures.¶¶ RD was measured to determine the starting point of the horizontal incisions, and the site was anesthetized using 0.0125 mg/mL epinephrine.## These incisions were started at a distance equal to the depth of the GR plus 1 mm from the tip of the anatomic papilla. Two horizontal incisions (at least 2 mm in length) were made from the GR defect to mesial and distal sites. Horizontal incisions were combined with a sulcular incision, and then two oblique, slightly divergent releasing incisions were made from the ends of the two horizontal incisions, extending to the mucogingival junction. A trapezoidal-shaped flap of almost full thickness was raised up to the mucogingival junction by a scalpel, preserving blood vessels within the flap. All muscle insertions were eliminated to permit the coronal advancement of the flap. Coronal mobilization of the flap was continued until the flap was able to passively reach a level 2 mm coronal to the CEJ of the tooth with a GR defect. The root surface at 1 mm coronal from the alveolar bone was treated mechanically with Gracey periodontal curets*** and rinsed with 0.9% saline solution. The anatomic interdental papillae were then carefully deepithelialized. The flap was then passively positioned 2 mm above the CEJ of the involved teeth, and light pressure was applied with wet gauze for 5 minutes to have a thin layer of clot. The suture of the flap was started at the mesial and distal papilla regions with 7-0 propylene suture material, and vertical releasing incisions were fixed. Then light pressure was applied to the flap for 5 minutes, and no periodontal dressing was applied.
Postoperative Protocol and Visual Analog Scale
Patients were instructed to not brush or floss the surgical site, to consume soft foods during the first week, and to avoid mechanical trauma to the surgical site. The sutures were removed on day 7 after the surgery. The patients were recalled for control on months 1, 3, and 6 after surgery. The visual analog scale (VAS) was used to evaluate pain during the postoperative follow-up period. A 10-cm VAS, with “none” at the left end and “unbearable” at the right end as verbal endpoints, was prepared for each patient. 24 Forms were given to all patients on the day of operation, and they were asked to tick the value of pain at the end of each day. Forms were collected when patients came for removal of sutures.
Statistical Analyses
The statistical analysis was performed on the data obtained from patients who completed the study protocol. A GR defect treated with CAF, rather than a patient, was considered as the unit of observation. The sample size was calculated based on previous studies that evaluated effects of smoking on the clinical outcomes of CAF for root coverage. With a power of 99% and α = 0.05, the minimum number of defects required for the intergroup comparisons was 18 for each group.
A statistical software program††† was used for statistical analysis of all parameters measured clinically or digitally, at baseline and during the follow-up after surgery. The Mann-Whitney U test was used for intergroup comparisons, and Wilcoxon and Friedman tests were used for intragroup analysis. The Fisher exact test was used to compare the frequency of complete root coverage between the study groups. Bivariate Spearman correlations were performed on the clinical data and cotinine levels to investigate underlying relationships. All tests were performed at α= 0.05 significance level. Receiver operating characteristic (ROC) curve was used to determine a critical threshold gingival thickness for complete root coverage.
RESULTS
Demographic data of the smoker and non-smoker groups are presented in the Materials and Methods section and in Table 1. The mean age of the patients in the non-smoker group was lower than that of the smoker patients (P = 0.048).
Table 1.
Demographic Data of the Smoker and Non-Smoker Study Groups
| Demographic Variable | Smoker Group | Non-Smoker Group |
|---|---|---|
|
| ||
| n | 15 | 15 |
| Sex (males/females) | 7/8 | 3/12 |
| Age range (year) (minimum to maximum) | 24 to 50 | 18 to 52 |
| Age (years) (mean ± SD) | 33.93 ± 7.33* | 29.00 ± 9.19 |
P <0.05, significantly higher than the non-smoker group.
Clinical Periodontal Measurements
The outline of clinical periodontal parameters at baseline and follow-up visits is presented in Table 2. At baseline, no statistically significant difference was found between the smoker and non-smoker groups for PI, PBI, PD, or CAL (P >0.05). PI and PBI scores increased at month 1 compared with the baseline (P = 0.015). PI and PBI scores did not show statistically significant differences at months 3 and 6 compared with the baseline (P >0.05). PD and CAL recordings were similar in the smoker and non-smoker groups at baseline and month 6 (P >0.05).
Table 2.
Clinical Periodontal Measurements in the Study Groups (mean ± SD)
| Measurement Time | Smoker Group (n = 18 defects) | Non-Smoker Group (n = 18 defects) |
|---|---|---|
| PI | ||
| Baseline | 0.17 ± 0.38 | 0.17 ± 0.38 |
| Month 1 | 0.78 ± 0.94* | 1.00 ± 0.90* |
| Month 3 | 0.33 ± 0.68† | 0.28 ± 0.57† |
| Month 6 | 0.22 ± 0.54† | 0.28 ± 0.54† |
|
| ||
| PBI | ||
| Baseline | 0.06 ± 0.23 | 0.00 ± 0.00 |
| Month 1 | 0.94 ± 1.21* | 1.17 ± 0.92* |
| Month 3 | 0.33 ± 0.76† | 0.17 ± 0.38† |
| Month 6 | 0.33 ± 0.84† | 0.33 ± 0.68† |
|
| ||
| PD (mm) | ||
| Baseline | 1.22 ± 0.42 | 1.06 ± 0.23 |
| Month 1 | — | — |
| Month 3 | — | — |
| Month 6 | 1.11 ± 0.32 | 1.00 ± 0.00 |
|
| ||
| CAL (mm) | ||
| Baseline | 3.67 ± 0.48 | 4.11 ± 0.96 |
| Month 1 | — | — |
| Month 3 | — | — |
| Month 6 | 1.36 ± 0.66‡ | 1.28 ± 0.57‡ |
| ΔBaseline month 6 | −2.30 ± 0.68 | −2.83 ± 1.04 |
P <0.05, significantly higher than the baseline value within the same group.
P <0.05, significantly less than the month 1 value within the same group.
P <0.05, significantly less than the baseline value within the same group.
Statistical evaluation revealed that clinical measurements were not significantly different from those of the digital measurements (P >0.05). There were no significant differences between the smoker and non-smoker groups in RD, RW, KGW, or RA at baseline or follow-up measurements (P >0.05) (Table 3). In both of the groups, RD, RW, and RA decreased significantly at month 1 compared with baseline (P = 0.000), and these results were quite stable over time (Fig. 1). KGW increased significantly at 6 months in both smoker and non-smoker groups (P = 0.015). The percentages of root coverage were not significantly different between the study groups (P >0.05). Intergroup comparisons showed a smaller proportion of complete root coverage in the smoker group than in the non-smoker one, but the differences did not reach the level of statistical significance (P >0.05). No significant correlations were found between cotinine levels and clinical measurements (data not shown).
Table 3.
Measurements Related to the Recession Defect in the Study Groups (mean ± SD)
| Measurement Time | Smoker Group (n = 18 defects) | Non-Smoker Group (n = 18 defects) |
|---|---|---|
| RD (mm) | ||
| Baseline | 2.49 ± 0.53 | 2.99 ± 0.91 |
| Month 1 | 0.15 ± 0.35* | 0.03 ± 0.15* |
| Month 3 | 0.19 ± 0.48* | 0.18 ± 0.50* |
| Month 6 | 0.24 ± 0.46* | 0.24 ± 0.49* |
|
| ||
| RW (mm) | ||
| Baseline | 3.43 ± 0.46 | 3.18 ± 0.65 |
| Month 1 | 0.70 ± 1.34* | 0.12 ± 0.52* |
| Month 3 | 0.72 ± 1.42* | 0.33 ± 0.78* |
| Month 6 | 0.82 ± 1.35* | 0.46 ± 0.91* |
|
| ||
| RA (mm2) | ||
| Baseline | 7.37 ± 2.41 | 7.44 ± 3.24 |
| Month 1 | 0.66 ± 1.35* | 0.07 ± 0.30* |
| Month 3 | 0.70 ± 1.56* | 0.23 ± 0.66* |
| Month 6 | 0.83 ± 1.56* | 0.35 ± 0.72* |
|
| ||
| KGW (mm) | ||
| Baseline | 3.22 ± 1.80 | 2.28 ± 0.82 |
| Month 1 | — | — |
| Month 3 | — | — |
| Month 6 | 3.67 ± 1.49† | 2.94 ± 0.72† |
|
| ||
| Root coverage (%) | ||
| Month 1 | 92.31 ± 15.54 | 99.09 ± 3.87 |
| Month 3 | 92.31 ± 16.23 | 96.97 ± 8.02 |
| Month 6 | 90.33 ± 17.84 | 94.11 ± 12.00 |
|
| ||
| Complete root coverage (%) | ||
| Month 1 | 72.20 | 83.30 |
| Month 3 | 72.20 | 77.80 |
| Month 6 | 66.70 | 72.20 |
P <0.05, significantly less than the baseline value within the same group.
P <0.05, significantly higher than the baseline value within the same group.
Figure 1.
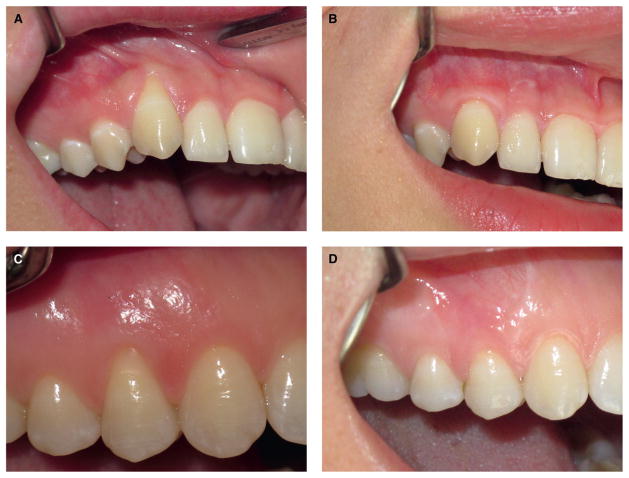
A) Baseline clinical appearance of a recession defect in a non-smoker patient. B) Clinical appearance of the same defect 6 months after CAF. C) Baseline clinical appearance of a recession defect in a smoker patient. D) Clinical appearance of the same defect 6 months after CAF.
Thickness of Gingiva
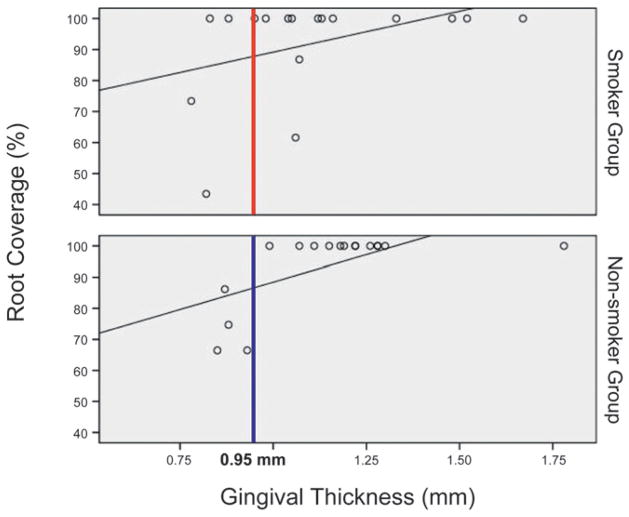
Baseline thickness of gingiva was not significantly different between the smoker and non-smoker groups (P = 0.343) (Table 4). Statistical analysis showed a significant association between gingival thickness and the percentage of root coverage (P = 0.001) (Fig. 2). Relying on the baseline thickness of gingiva, power of the predictability of complete root coverage was 81% in the smoker group, 89% in the non-smoker group, and 86% when smoker and non-smoker patients were evaluated together.
Table 4.
Baseline Gingival Thickness in the Study Groups
| Gingival Thickness (mm) | Smoker Group (n = 18 defects) | Non-Smoker Group (n = 18 defects) |
|---|---|---|
|
| ||
| Mean ± SD | 1.11 ± 0.25 | 1.15 ± 0.22 |
| Median (minimum to maximum) | 1.06 (0.80 to 1.67) | 1.18 (0.85 to 1.78) |
There was no significant difference between the study groups (P >0.05).
Figure 2.
The association between gingival thickness and root coverage at month 6 (according to the ROC curve).
A critical threshold of baseline gingival thickness for complete root coverage was determined to be 0.95 mm. In the presence of 0.95-mm baseline thickness of gingiva, the predictability of complete root coverage at month 6 was 100% in the non-smoker group and 91% in the smoker group. Complete root coverage was achieved in recession defects with a minimum gingival thickness of 0.95 mm in all non-smokers patients, whereas this was not obtained in some of the smokers (Fig. 2).
Biochemical Data in GCF and Saliva Samples
Biochemical analyses in the GCF and saliva samples are presented in Tables 5 and 6. There were no significant differences in the analyzed biochemical parameters between the smoker and non-smoker groups (P >0.05) apart from the highly significant difference in saliva cotinine levels (P = 0.000).
Table 5.
Baseline Biochemical Findings in GCF Samples of the Study Groups
| Biochemical Parameter | Smoker Group (n = 18 defects) | Non-Smoker Group (n = 18 defects) |
|---|---|---|
|
| ||
| bFGF | 1.46 ± 0.57 | 1.62 ± 0.72 |
| VEGF-A | 4.77 ± 3.33 | 4.77 ± 3.25 |
| IL-8 | 14.78 ± 11.89 | 17.38 ± 9.49 |
| IL-10 | 0.21 ± 0.06 | 0.21 ± 0.05 |
| IL-12 | 2.96 ± 1.93 | 3.44 ± 2.12 |
| TNF-α | 0.71 ± 0.22 | 0.90 ± 0.36 |
| MMP-8 | 3,020.63 ± 2,685.47 | 2,384.73 ± 1,590.02 |
| MMP-9 | 10,053.29 ± 8,152.97 | 7,171.34 ± 6,484.76 |
| PAI-1 | 135.05 ± 85.99 | 152.50 ± 83.94 |
All data are presented as mean ± SD (pg/mL). There were no significant differences between the study groups (P >0.05).
Table 6.
Baseline Biochemical Findings in Saliva Samples of the Study Groups (mean ± SD)
| Biochemical Parameter | Smoker Group (n = 15 patients) | Non-Smoker Group (n = 15 patients) |
|---|---|---|
| Cotinine (ng/mL) | 390.12 ± 287.04* | 6.43 ± 8.65 |
| bFGF (pg/mL) | 13.97 ± 15.41 | 13.61 ± 10.61 |
| VEGF-A (pg/mL) | 218.34 ± 144.45 | 215.54 ± 202.10 |
| PDGF-BB (pg/mL) | 3.87 ± 5.36 | 7.96 ± 17.33 |
| IL-8 (pg/mL) | 147.09 ± 72.86 | 173.60 ± 148.83 |
| IL-10 (pg/mL) | 2.23 ± 2.43 | 1.18 ± 1.46 |
| IL-12 (pg/mL) | 21.37 ± 17.55 | 16.77 ± 6.78 |
| TNF-α (pg/mL) | 13.49 ± 10.66 | 10.07 ± 10.08 |
| MMP-8 (pg/mL) | 12,539.63 ± 6,715.28 | 12,762.59 ± 13,228.17 |
| MMP-9 (pg/mL) | 12,860.09 ± 9,969.42 | 22,692.93 ± 21,524.92 |
| PAI-1 (pg/mL) | 515.97 ± 311.49 | 624.33 ± 556.07 |
P <0.01, significantly higher than the non-smoker group.
VAS Values
The smoker and non-smoker patients revealed similar VAS values at all time points of evaluation (P >0.05) (Table 7).
Table 7.
VAS Values in the Study Groups (mean ± SD)
| Time Point | Smoker Group (n = 15 patients) | Non-Smoker Group (n = 15 patients) |
|---|---|---|
| Day 0 (during the operation) | 0.22 ± 0.54 | 0.00 ± 0.00 |
| Day 1 | 1.78 ± 2.60* | 2.06 ± 3.22* |
| Day 2 | 0.72 ± 1.36† | 1.50 ± 2.25* |
| Day 3 | 0.44 ± 0.98† | 1.06 ± 1.98* |
| Day 4 | 0.44 ± 1.04† | 0.67 ± 1.68* |
| Day 5 | 0.28 ± 0.66† | 0.28 ± 1.17† |
| Day 6 | 0.22 ± 0.64† | 0.17 ± 0.70† |
| Day 7 | 0.11 ± 0.32† | 0.17 ± 0.70† |
P <0.05, significantly higher than the baseline value within the same study group.
P <0.05, significantly lower than the day 1 value within the same study group.
DISCUSSION
The objective of this prospective clinical study is to evaluate the possible influence of cigarette smoking on the outcomes of root coverage with CAF. The present results suggest that root coverage with CAF has high success rates in non-smoking as well as smoking systemically healthy patients. In other words, in systemically healthy patients with optimum oral hygiene and clinically healthy periodontal tissues, smoking does not seem to have an adverse effect on clinical outcomes. At month 6 after surgery, the percentage of root coverage and complete root-coverage rates were similar in the smoker and non-smoker groups.
Possible effects of smoking on the clinical outcomes of CAF have been evaluated by Silva et al.18,19 in 10 smokers and 10 non-smokers. The authors reported that root-coverage rate was 70% in the smokers and 91% in the non-smokers, whereas complete root coverage was achieved in 50% of the smokers 6 months post-surgery.18 The 2-year follow-up of the same patients showed that the long-term success of CAF was lower in smokers than in non-smokers. 19 The present study indicated similarly high success rates in the smoker versus non-smoker patients. The differences in the number of treated defects may have a role in this discrepancy because the present study had higher statistical power. The microsurgery technique and approximation of full-thickness flaps were used, which may have helped to maintain blood supply from the remaining periosteum on the bone surface and to preserve blood vessels through the flap. There was no verification of smoking status by a biochemical analysis in the study of Silva et al.,18,19 and they based their evaluation of success only on the RD measurements using a standard pressure electronic probe. Differences in the study designs may at least partially explain the discrepancy between the previous findings and those of the present study.
Optimum oral hygiene during the healing phase and follow-up are essential for the long-term success of both surgical and non-surgical periodontal treatment. 25–27 Similar to the previous studies, optimum oral hygiene was achieved and perfectly maintained in both smoker and non-smoker patients in the present study.
Silva et al.18,19 reported more reduction in gingival RD in the non-smoker group (from 2.54 to 0.22 mm) than the smoker group (from 2.74 to 0.84 mm). In the present study, position of the gingival margin was stable at month 6 in both groups. Changes in gingival RW were not evaluated by Silva et al.,18,19 but the present findings are similar to those of another study evaluating CAF success in only non-smoker patients. 17
Success of root-coverage techniques is evaluated by means of clinical parameters, such as RD, RW, KGW, and CAL. These parameters can be measured directly with periodontal probes28,29 or indirectly on digital photographs.30,31 When a periodontal probe is used, measurements are rounded to the nearest 0.529 or 1 mm.28,32,33 This rounding may lead to rather high errors in the very small distances, such as RD. In the present study, RD and RW were measured digitally on clinical photographs in an attempt to increase the sensitivity and accuracy. RA, which is difficult to measure by clinical methods, was also calculated on these photographs.
Surgical root-coverage procedures aim to cover the RA, which is a two-dimensional entity with depth and width. However, success of these surgical procedures is usually evaluated through the changes only in RD,34–36 which is one-dimensional. The RA usually has an irregular shape; its widest margin is the coronal part, whereas it is narrower in the apical border. Thus, GR usually creates a U-shaped defect in which RA defines the unit of measurement better than does the sole measurement of RD. In case of complete root coverage, RD alone can be used more safely to evaluate the outcome, whereas in case of partial coverage, measurement of RA is more realistic. Variable percentages of root coverage have been reported so far.30,37 In the present study, the RA calculated with software is the major determining parameter to evaluate the success of CAFs. The present findings could not be compared, because no publication reporting changes in RA after root-coverage procedures could be found.
The mucogingival line tends to return to its original position after apically advanced flaps or CAFs.27,38–40 Ainamo et al.38 reported that apically positioned flaps aiming to eliminate periodontal pockets resulted in similar KGW after 18 years of follow-up. Similarly, Wennström and Zucchelli39 and Zucchelli and De Sanctis27 found that the mucogingival line has a tendency to return to its original position after a CAF. Zucchelli and De Sanctis27 stated that baseline KGW positively affects the amount of gain in KGW, whereas baseline RD has a negative influence. In accordance with these studies, it was found that KGW increased at month 6 after surgery in both the smoker and non-smoker groups.
Nicotine or cotinine can be analyzed in blood, urine, or saliva samples41–48 to verify smoking status. The half-life of nicotine is ≈2 hours, and its concentration reflects the last time of smoking; cotinine has a half-life of ≈17 hours, and, therefore, it is more reliable to validate the self-report of smoking.41,47,49,50 Cotinine concentration >15 ng/mL in body fluids is commonly used to differentiate smokers from non-smokers and environmentally exposed smokers.51 In the present study, patients were assigned to either the smoker or non-smoker group according to their self-reports. However, one patient had to be moved from the non-smoker group to the smoker study group because of the very high level of saliva cotinine concentration, which clearly exceeded passive smoker thresholds. This situation emphasizes the importance of the cotinine analysis to validate the patients’ smoking status, particularly in studies in which smoking is a major determinant.
In the present study, the mean age of the non-smoker group was lower than that of the smoker group. Lindhe et al.52 evaluated the possible effects of age on periodontal wound healing, and 73 patients were divided into three age groups as follows: 1) <40 years; 2) 40 to 49 years; and 3) >49 years. The authors reported no significant difference in wound-healing parameters among the age groups. Huang et al.17 reported similar results, in which the success of root-coverage procedures was reported not to be affected by patients’ age. Therefore, it is unlikely that the mean age difference of 4.93 years obtained in the present study is clinically relevant.
Gingival thickness is the most critical determining factor regarding whether to use a CT graft in combination with CAF. Baldi et al.16 reported that 0.8 mm was the critical threshold gingival thickness for complete root coverage. The authors followed 19 patients for 3 months after surgery. They used a modified Iwanson gauge to measure flap thickness after flap elevation, that is, during the surgery, at the midpoint of the distance between mucogingival junction and flap margin. It should be kept in mind that this modified Iwanson gauge has some disadvantages, such as the compression of soft tissue when the gauge is closed. Another study17 reported the critical threshold gingival thickness as 1.2 mm for complete root coverage. The researchers measured gingival thickness using a periodontal probe‡‡‡ and then rounded up to the nearest millimeter. This method may not be sensitive enough to measure gingival tissue thickness in mucogingival surgeries, because even a 0.1-mm difference can be significant for the outcome. To the best of the authors’ knowledge, this is the first study comparatively evaluating smoker and non-smoker patients in an attempt to determine critical threshold baseline gingival thickness for complete root coverage. The gingival thickness was measured with an ultrasonic device to be as sensitive as possible. Complete root coverage was achieved in GR defects with minimum gingival thickness of 0.95 mm in the non-smoker group, although some smoker patients failed to exhibit complete root coverage.
In the present study, baseline GCF and saliva samples were analyzed for content of various biochemical parameters in which no significant difference was detected between the smoker and non-smoker groups. The biochemical data provide a reference for the baseline situation, and this similarity may at least partially explain the lack of significant difference in the clinical outcomes. Moreover, the similar baseline biochemical data suggest that, in systemically healthy individuals with optimum oral hygiene and healthy periodontium, smoking alone may not have a significant impact on the evaluated growth factors, cytokines, enzymes, or their inhibitors. To the best of the authors’ knowledge, this is the first study investigating biochemical parameters in GCF and the saliva of smokers versus non-smokers regarding clinical outcomes of CAFs. The present data are in line with those previous studies reporting similar biochemical data in GCF and saliva samples of smokers and non-smokers with clinically healthy periodontium.53–56 Additional studies investigating biochemical data in smoker versus non-smoker individuals also during the healing phase may better clarify the interaction of smoking and healing outcomes.
CONCLUSIONS
The present findings suggest that root coverage with a CAF has high success rates in systemically healthy smokers with optimum oral hygiene and clinically healthy periodontal tissues. Furthermore, it is important to consider the baseline gingival thickness for the treatment plan to achieve complete root coverage. Thus, the present findings seem not to support the statement that smoking affects the biochemical content in the GCF and saliva of such healthy individuals or that it has adverse effects on the clinical outcomes of CAF. However, effects of smoking on long-term efficacy remain to be established in large-scale clinical studies.
Acknowledgments
This study was supported by Ege University Research Foundation Grant 2010 DIS 004 and the University of Louisville.
Footnotes
Hu-Friedy, Chicago, IL.
NIH ImageJ for Windows, National Institutes of Health, Bethesda, MD.
K&M Instruments, Hong Kong, China.
PerioPaper, Oraflow, Plainview, NY.
Periotron 8000, Oraflow.
High Sensitivity Salivary Cotinine Quantitative Enzyme Immunoassay Kit, Salimetrics, State College, PA.
EMax Microplate Reader, Molecular Devices, Sunnyvale, CA.
Procarta Cytokine Assay Kits, Affymetrix, Santa Clara, CA.
Luminex 100, Luminex, Austin, TX.
Seiler Instrument, St. Louis, MO.
Doğsan, Trabzon, Turkey.
Lidocaine at 20 mg/mL, Koçak Farma, ıstanbul, Turkey.
Hu-Friedy.
SPSS v.15.0 for Windows, IBM, Chicago, IL.
UNC periodontal probe, Hu-Friedy.
The authors report no conflicts of interest related to this study.
References
- 1.Allen EP, Miller PD., Jr Coronal positioning of existing gingiva: Short term results in the treatment of shallow marginal tissue recession. J Periodontol. 1989;60:316–319. doi: 10.1902/jop.1989.60.6.316. [DOI] [PubMed] [Google Scholar]
- 2.Harris RJ, Harris AW. The coronally positioned pedicle graft with inlaid margins: A predictable method of obtaining root coverage of shallow defects. Int J Periodontics Restorative Dent. 1994;14:228–241. [PubMed] [Google Scholar]
- 3.Wennström JL. Mucogingival therapy. Ann Periodontol. 1996;1:671–701. doi: 10.1902/annals.1996.1.1.671. [DOI] [PubMed] [Google Scholar]
- 4.Wennström JL, Pini Prato GP. Mucogingival therapy-periodontal plastic surgery. In: Lindhe J, Karring T, Lang NP, editors. Clinical Periodontology and Implant Dentistry. 4. Copenhagen: Munksgaard; 2003. pp. 576–649. [Google Scholar]
- 5.Benatti BB, César-Neto JB, Gonçalves PF, Sallum EA, Nociti FH., Jr Smoking affects the self-healing capacity of periodontal tissues. A histological study in the rat. Eur J Oral Sci. 2005;113:400–403. doi: 10.1111/j.1600-0722.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 6.Bergström J, Boström L. Tobacco smoking and periodontal hemorrhagic responsiveness. J Clin Periodontol. 2001;28:680–685. doi: 10.1034/j.1600-051x.2001.028007680.x. [DOI] [PubMed] [Google Scholar]
- 7.Nair P, Sutherland G, Palmer RM, Wilson RF, Scott DA. Gingival bleeding on probing increases after quitting smoking. J Clin Periodontol. 2003;30:435–437. doi: 10.1034/j.1600-051x.2003.20039.x. [DOI] [PubMed] [Google Scholar]
- 8.Morozumi T, Kubota T, Sato T, Okuda K, Yoshie H. Smoking cessation increases gingival blood flow and gingival crevicular fluid. J Clin Periodontol. 2004;31:267–272. doi: 10.1111/j.1600-051X.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 9.Morozumi T, Kubota T, Sugita N, Itagaki M, Yoshie H. Alterations of gene expression in human neutrophils induced by smoking cessation. J Clin Periodontol. 2004;31:1110–1116. doi: 10.1111/j.1600-051X.2004.00612.x. [DOI] [PubMed] [Google Scholar]
- 10.Söder B, Jin LJ, Wickholm S. Granulocyte elastase, matrix metalloproteinase-8 and prostaglandin E2 in gingival crevicular fluid in matched clinical sites in smokers and non-smokers with persistent periodontitis. J Clin Periodontol. 2002;29:384–391. doi: 10.1034/j.1600-051x.2002.290502.x. [DOI] [PubMed] [Google Scholar]
- 11.Rezavandi K, Palmer RM, Odell EW, Scott DA, Wilson RF. Expression of ICAM-1 and E-selectin in gingival tissues of smokers and non-smokers with periodontitis. J Oral Pathol Med. 2002;31:59–64. doi: 10.1046/j.0904-2512.2001.joptest.doc.x. [DOI] [PubMed] [Google Scholar]
- 12.Ah MK, Johnson GK, Kaldahl WB, Patil KD, Kalkwarf KL. The effect of smoking on the response to periodontal therapy. J Clin Periodontol. 1994;21:91–97. doi: 10.1111/j.1600-051x.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 13.Boström L, Linder LE, Bergström J. Smoking and cervicular fluid levels of IL-6 and TNF-alpha in periodontal disease. J Clin Periodontol. 1999;26:352–357. doi: 10.1034/j.1600-051x.1999.260604.x. [DOI] [PubMed] [Google Scholar]
- 14.Rawlinson A, Grummitt JM, Walsh TF, Ian Douglas CW. Interleukin 1 and receptor antagonist levels in gingival crevicular fluid in heavy smokers versus non-smokers. J Clin Periodontol. 2003;30:42–48. doi: 10.1034/j.1600-051x.2003.300107.x. [DOI] [PubMed] [Google Scholar]
- 15.Buduneli N, Kinane DF. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol. 2011;38(Suppl 11):85–105. doi: 10.1111/j.1600-051X.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 16.Baldi C, Pini-Prato G, Pagliaro U, et al. Coronally advanced flap procedure for root coverage. Is flap thickness a relevant predictor to achieve root coverage? A 19-case series. J Periodontol. 1999;70:1077–1084. doi: 10.1902/jop.1999.70.9.1077. [DOI] [PubMed] [Google Scholar]
- 17.Huang LH, Neiva RE, Wang HL. Factors affecting the outcomes of coronally advanced flap root coverage procedure. J Periodontol. 2005;76:1729–1734. doi: 10.1902/jop.2005.76.10.1729. [DOI] [PubMed] [Google Scholar]
- 18.Silva CO, Sallum AW, de Lima AF, Tatakis DN. Coronally positioned flap for root coverage: Poorer outcomes in smokers. J Periodontol. 2006;77:81–87. doi: 10.1902/jop.2006.77.1.81. [DOI] [PubMed] [Google Scholar]
- 19.Silva CO, de Lima AF, Sallum AW, Tatakis DN. Coronally positioned flap for root coverage in smokers and non-smokers: Stability of outcomes between 6 months and 2 years. J Periodontol. 2007;78:1702–1707. doi: 10.1902/jop.2007.070068. [DOI] [PubMed] [Google Scholar]
- 20.Miller PD., Jr A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 1985;5(2):8–13. [PubMed] [Google Scholar]
- 21.Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 22.Saxer UP, Mühlemann HR. Motivation and education (in German) SSO Schweiz Monatsschr Zahnheilkd. 1975;85:905–919. [PubMed] [Google Scholar]
- 23.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 24.Gracely RH. Studies of pain in human subjects. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5. Philadelphia: Elsevier Churchill Livingstone; 2006. pp. 267–289. [Google Scholar]
- 25.Caffesse RG, Alspach SR, Morrison EC, Burgett FG. Lateral sliding flaps with and without citric acid. Int J Periodontics Restorative Dent. 1987;7(6):42–57. [PubMed] [Google Scholar]
- 26.Miller PD. Root coverage grafting for regeneration and aesthetics. Periodontol 2000. 1993;1:118–127. [PubMed] [Google Scholar]
- 27.Zucchelli G, De Sanctis M. Long-term outcome following treatment of multiple Miller class I and II recession defects in esthetic areas of the mouth. J Periodontol. 2005;76:2286–2292. doi: 10.1902/jop.2005.76.12.2286. [DOI] [PubMed] [Google Scholar]
- 28.Caffesse RG, De LaRosa M, Garza M, Munne-Travers A, Mondragon JC, Weltman R. Citric acid demineralization and subepithelial connective tissue grafts. J Periodontol. 2000;71:568–572. doi: 10.1902/jop.2000.71.4.568. [DOI] [PubMed] [Google Scholar]
- 29.Tatakis DN, Trombelli L. Gingival recession treatment: Guided tissue regeneration with bioabsorbable membrane versus connective tissue graft. J Periodontol. 2000;71:299–307. doi: 10.1902/jop.2000.71.2.299. [DOI] [PubMed] [Google Scholar]
- 30.Rosetti EP, Marcantonio RA, Rossa C, Jr, Chaves ES, Goissis G, Marcantonio E., Jr Treatment of gingival recession: Comparative study between subepithelial connective tissue graft and guided tissue regeneration. J Periodontol. 2000;71:1441–1447. doi: 10.1902/jop.2000.71.9.1441. [DOI] [PubMed] [Google Scholar]
- 31.Kerner S, Borghetti A, Katsahian S, et al. A retrospective study of root coverage procedures using an image analysis system. J Clin Periodontol. 2008;35:346–355. doi: 10.1111/j.1600-051X.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 32.Aichelmann-Reidy ME, Yukna RA, Evans GH, Nasr HF, Mayer ET. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J Periodontol. 2001;72:998–1005. doi: 10.1902/jop.2001.72.8.998. [DOI] [PubMed] [Google Scholar]
- 33.Cordioli G, Mortarino C, Chierico A, Grusovin MG, Majzoub Z. Comparison of 2 techniques of subepithelial connective tissue graft in the treatment of gingival recessions. J Periodontol. 2001;72:1470–1476. doi: 10.1902/jop.2001.72.11.1470. [DOI] [PubMed] [Google Scholar]
- 34.Borghetti A, Glise JM, Monnet-Corti V, Dejou J. Comparative clinical study of a bioabsorbable membrane and subepithelial connective tissue graft in the treatment of human gingival recession. J Periodontol. 1999;70:123–130. doi: 10.1902/jop.1999.70.2.123. [DOI] [PubMed] [Google Scholar]
- 35.Harris RJ. Root coverage with a connective tissue with partial thickness double pedicle graft and an acellular dermal matrix graft: A clinical and histological evaluation of a case report. J Periodontol. 1998;69:1305–1311. doi: 10.1902/jop.1998.69.11.1305. [DOI] [PubMed] [Google Scholar]
- 36.Trombelli L, Scabbia A, Tatakis DN, Checchi L, Calura G. Resorbable barrier and envelope flap surgery in the treatment of human gingival recession defects. Case reports. J Clin Periodontol. 1998;25:24–29. doi: 10.1111/j.1600-051x.1998.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 37.Bouchard P, Nilveus R, Etienne D. Clinical evaluation of tetracycline HCl conditioning in the treatment of gingival recessions. A comparative study J Periodontol. 1997;68:262–269. doi: 10.1902/jop.1997.68.3.262. [DOI] [PubMed] [Google Scholar]
- 38.Ainamo A, Bergenholtz A, Hugoson A, Ainamo J. Location of the mucogingival junction 18 years after apically repositioned flap surgery. J Clin Periodontol. 1992;19:49–52. doi: 10.1111/j.1600-051x.1992.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 39.Wennström JL, Zucchelli G. Increased gingival dimensions. A significant factor for successful outcome of root coverage procedures? A 2-year prospective clinical study. J Clin Periodontol. 1996;23:770–777. doi: 10.1111/j.1600-051x.1996.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 40.Gürgan CA, Oruç AM, Akkaya M. Alterations in location of the mucogingival junction 5 years after coronally repositioned flap surgery. J Periodontol. 2004;75:893–901. doi: 10.1902/jop.2004.75.6.893. [DOI] [PubMed] [Google Scholar]
- 41.Dabbs JM., Jr Salivary testosterone measurements: Collecting, storing, and mailing saliva samples. Physiol Behav. 1991;49:815–817. doi: 10.1016/0031-9384(91)90323-g. [DOI] [PubMed] [Google Scholar]
- 42.Etter JF, Perneger TV, Ronchi A. Collecting saliva samples by mail. Am J Epidemiol. 1998;147:141–146. doi: 10.1093/oxfordjournals.aje.a009426. [DOI] [PubMed] [Google Scholar]
- 43.Foulds J, Bryant A, Stapleton J, Jarvis MJ, Russell MA. The stability of cotinine in unfrozen saliva mailed to the laboratory. Am J Public Health. 1994;84:1182–1183. doi: 10.2105/ajph.84.7.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glasgow RE, Mullooly JP, Vogt TM, et al. Biochemical validation of smoking status: Pros, cons, and data from four low-intensity intervention trials. Addict Behav. 1993;18:511–527. doi: 10.1016/0306-4603(93)90068-k. [DOI] [PubMed] [Google Scholar]
- 45.Greeley DA, Valois RF, Bernstein DA. Stability of salivary cotinine sent through the U.S. mail for verification of smoking status. Addict Behav. 1992;17:291–296. doi: 10.1016/0306-4603(92)90034-s. [DOI] [PubMed] [Google Scholar]
- 46.Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health. 1987;77:1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider SJ, Singer H. Validating reports of non-smoking with breath and saliva samples: Your checkup is in the mail. Addict Behav. 1983;8:187–191. doi: 10.1016/0306-4603(83)90013-8. [DOI] [PubMed] [Google Scholar]
- 48.Valois RF, Adams KG, Kammermann SK. One-year evaluation results from CableQuit: A community cable television smoking cessation pilot program. J Behav Med. 1996;19:479–499. doi: 10.1007/BF01857680. [DOI] [PubMed] [Google Scholar]
- 49.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 50.Etter JF, Vu Duc T, Perneger TV. Saliva cotinine levels in smokers and nonsmokers. Am J Epidemiol. 2000;151:251–258. doi: 10.1093/oxfordjournals.aje.a010200. [DOI] [PubMed] [Google Scholar]
- 51.Fraser HS, Palmer RM, Wilson RF, Coward PY, Scott DA. Elevated systemic concentrations of soluble ICAM-1 (sICAM) are not reflected in the gingival crevicular fluid of smokers with periodontitis. J Dent Res. 2001;80:1643–1647. doi: 10.1177/00220345010800070901. [DOI] [PubMed] [Google Scholar]
- 52.Lindhe J, Socransky S, Nyman S, Westfelt E, Haffajee A. Effect of age on healing following periodontal therapy. J Clin Periodontol. 1985;12:774–787. doi: 10.1111/j.1600-051x.1985.tb01403.x. [DOI] [PubMed] [Google Scholar]
- 53.Buduneli N, Kardeşler L, Işik H, et al. Effects of smoking and gingival inflammation on salivary antioxidant capacity. J Clin Periodontol. 2006;33:159–164. doi: 10.1111/j.1600-051X.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 54.Ozçaka Ö, Biçakci N, Pussinen P, Sorsa T, Köse T, Buduneli N. Smoking and matrix metalloproteinases, neutrophil elastase and myeloperoxidase in chronic periodontitis. Oral Dis. 2011;17:68–76. doi: 10.1111/j.1601-0825.2010.01705.x. [DOI] [PubMed] [Google Scholar]
- 55.Gürsoy UK, Könönen E, Pradhan-Palikhe P, et al. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J Clin Periodontol. 2010;37:487–493. doi: 10.1111/j.1600-051X.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- 56.Giannopoulou C, Cappuyns I, Mombelli A. Effect of smoking on gingival crevicular fluid cytokine profile during experimental gingivitis. J Clin Periodontol. 2003;30:996–1002. doi: 10.1034/j.1600-051x.2003.00416.x. [DOI] [PubMed] [Google Scholar]