Introduction
Corneal collagen cross-linking (CXL) is a novel technology that utilizes a combination of riboflavin (vitamin B2) eye drops, to saturate the cornea, and UV-A light which sets off a chemical reaction to shorten the cross links between and within collagen fibers thereby increasing the biomechanical stability of the corneal stroma (1–4). The objective of this procedure is to halt progression of keratoconus, post-LASIK ectasia, and other corneal ectasias, such as pellucid marginal degeneration. In some cases, there has been improvement in visual acuity, reduction of corneal curvature and improvement in quality of life (5).
In the 1970’s Siegel discussed cross-linking reactions whereby lysyl oxidase catalyzed the formation of cross-linking aldehydes in collagen and elastin (6–7). Cross-linking is well known in material sciences and dentistry where this enzymatic process increases molecular bonds to increase the mechanical strength of tissue. Cross-linking can be induced enzymatically by means of aldehydes, chemical fixatives or by photosensitizing radiation. Of the three methods the photosensitizing radiation has been shown to be most effective. In corneal clinical practice, CXL utilizes this method where the riboflavin acts as a photosensitizer for the production of free radicals produced by the interaction of the riboflavin and UV-A light. The production of these reactive oxygen species (singlet oxygen) causes the formation of chemical bonds within the corneal stroma resulting in corneal stiffening (3).
Laboratory studies
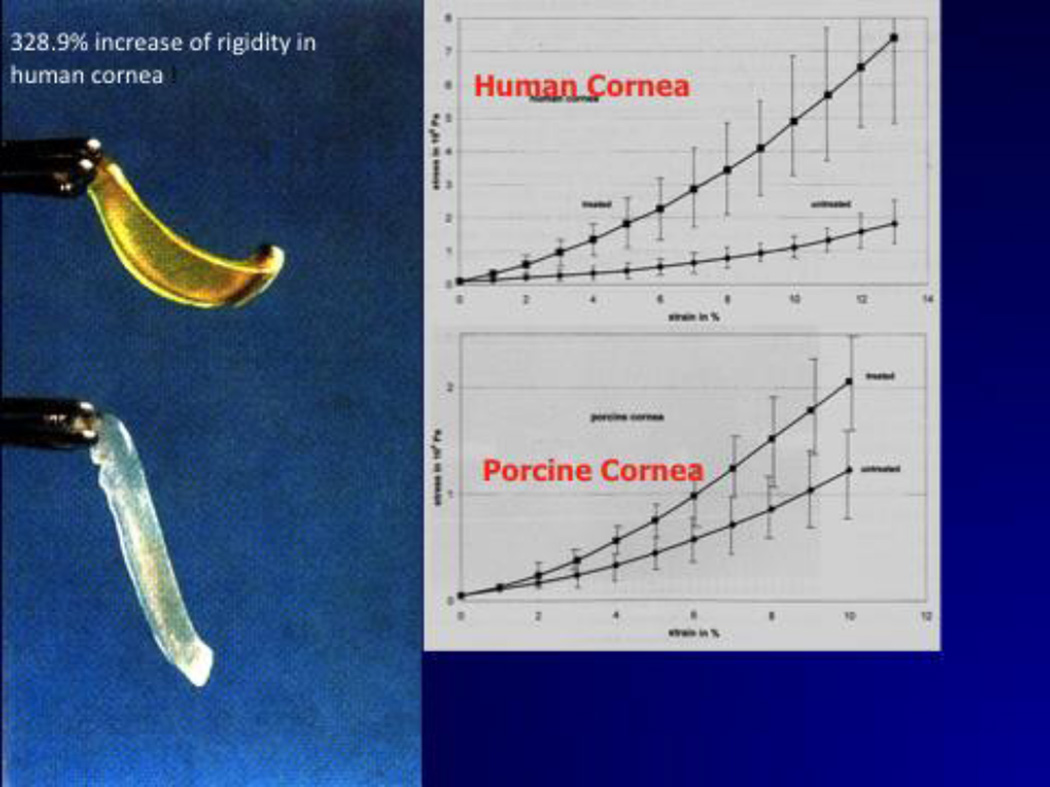
Wollensak, Spoerl and Seiler (3), using stress-strain measurements on porcine and human corneas, demonstrated a 328.9% increase in rigidity of the human cornea after being cross-linked with UV-A and riboflavin. (Fig. 1) Subsequent experiments on rabbit corneas demonstrated that the cross-linking effect could last up to 8 months. Confirmation of cross-linking was demonstrated with gel electrophoresis, demonstrated a significant increase in the diameter of collagen fibers in rabbit corneas. The presence of the cross-linking effect has also been confirmed with thermo-chemical studies on cross-linked rabbit corneas.
Figure 1.
Wollensak et al found that CXL produced a 328.9% increase in rigidity of the human cornea.
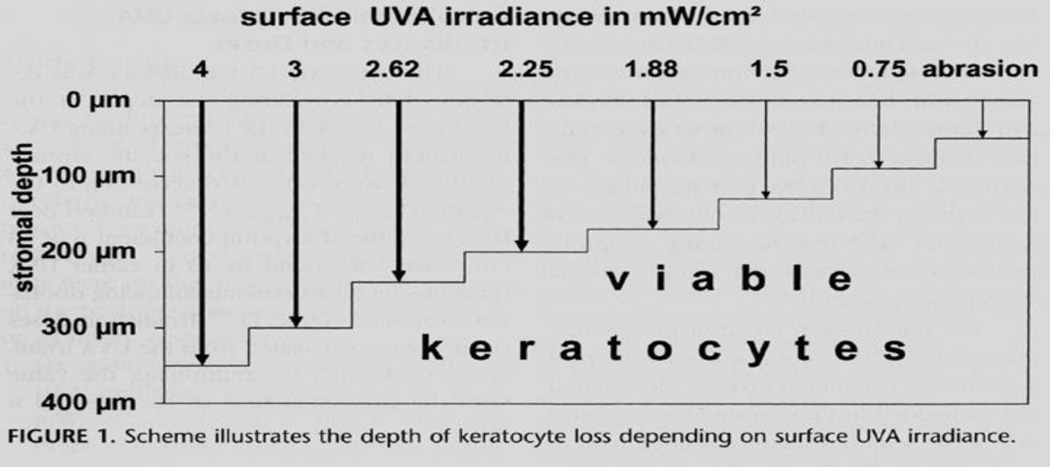
Several publications have shown the safety of CXL using the original standard Dresden technique of 30 minutes of riboflavin saturation of the cornea combined with 3 mW/cm2 of UV-A irradiation for 30 minutes while riboflavin instillation continued. Wollensak et al (8–10) studied the cytotoxicity of the riboflavin-UV-A treatment on keratocytes and endothelial cells. They found that in rabbit corneas there was keratocyte apoptosis 24 hours after standard CXL treatment as deep as 300 microns from the anterior surface of the cornea. (Fig. 2) However, deeper than 300 microns, the keratocytes were spared, and thus a cornea that was 400 microns or greater in thickness would not be at risk for endothelial cell damage from CXL.
Figure 2.
Keratocyte apoptosis occurs to a depth of 300 microns with 3 mW/cm2 of UV-A irradiance for 30 minutes.
Spoerl et al (11) confirmed these findings and found that repopulation of the keratocytes takes up to 6 months to occur, and that as long as the treated cornea had a minimum thickness of the recommended 400 microns, the endothelium, lens and retina were not at risk, if the light source was homogeneous, avoided hot spots and had a radiant exposure of 5.4 J/cm2. (Table 1)
Table 1.
The damage threshold for the corneal endothelium, anterior lens surface and retina is not reached when following the standard Dresden protocol.
| Depth | Radiant Exposure | Damage Treshold |
|---|---|---|
| Anterior corneal surface | 5.4 J/cm2 | |
| Corneal endothelium | 0.32 J/cm2 | 0.65 J/cm2 |
| Anterior lens surface | 0.27 J/cm2 | 70 J/cm2 |
| Retina | 0.22 J/cm2 | 7.7 J/cm2 |
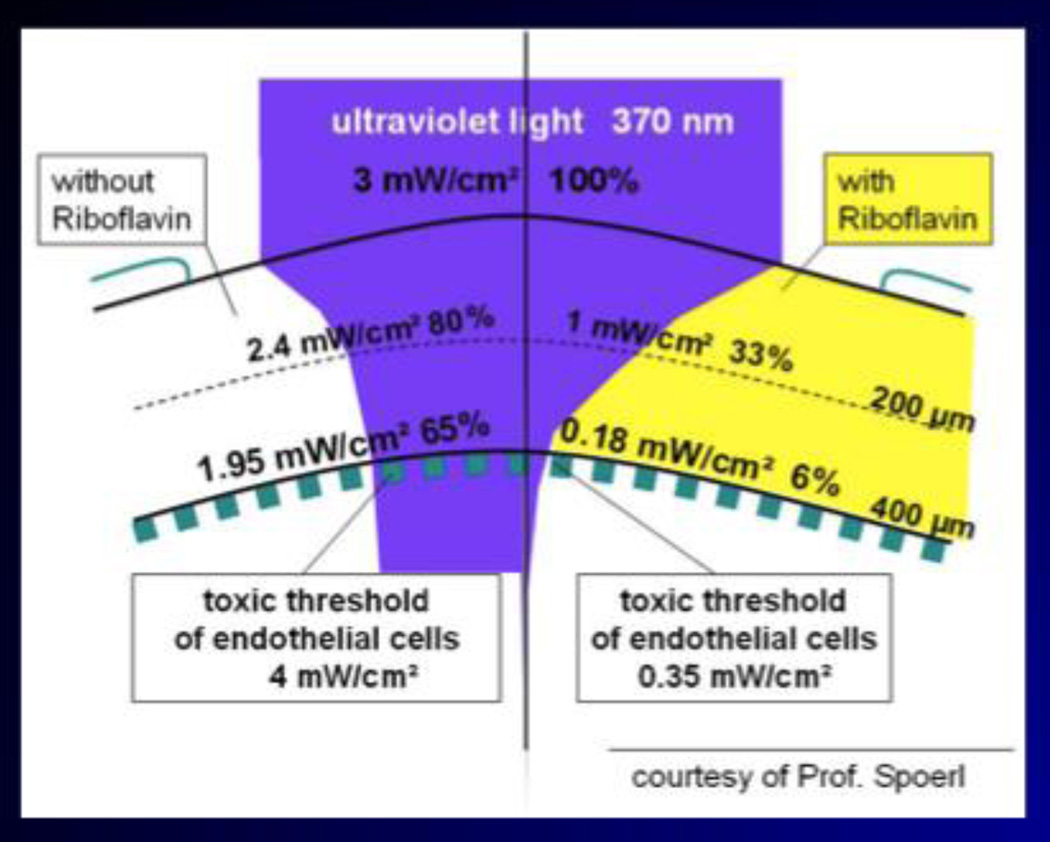
Riboflavin also acts as a shield, protecting the deeper structures of the eye (11). (Fig. 3) All structures behind the corneal stroma are exposed to a residual UV-A radiant exposure less than 1 J/cm2, which is below the safety threshold for UV-A radiation to these ocular tissues.
Figure 3.
Riboflavin acts as a shield to the UV-A irradiance and further protects the deeper structures of the eye. (Courtesy of Prof. E. Spoerl.)
Recent immunofluorescent confocal microscopy studies by Bottos et al (12) demonstrate that the epithelium is a barrier to riboflavin and very little cross-linking occurs in the presence of the epithelium. (Fig. 4) These findings suggest that for the treatment to be effective, the epithelium should always be removed as initially described by Seiler's group.
Chai, Ling, and Gaster et al (13) found a significant correlation between corneal stiffening and the intensity of collagen auto fluorescence after CXL, suggesting that assessing collagen auto fluorescence could monitor the efficacy of CXL in patients.
In an attempt to decrease the total time for the CXL procedure, some corneal specialists have considered increasing the irradiance of the UV-A light and decreasing the time of the UV-A exposure, which maintains the same total irradiance to the cornea. Roizenblatt, Chai and Gaster et al (14) found that by increasing the UV-A treatment to 9 mW/cm2 (from 3) and decreasing the UV-A exposure time to 10 minutes (from 30), the CXL effect of stiffening the rabbit corneas was similar and showed statistically significant differences from control corneas. Clinical studies with such increased irradiance and decreased exposure times are being conducted at this time.
A recent study by Libiris et al (5) suggests that keratoconus exerts a significant negative impact on quality of life issues, even in its early stages when keratoconus patients still have normal visual acuity. This impaired quality of life issue was comparable with that of patients with advanced age-related macular degeneration. Furthermore, both CXL and CXL combined with topography-guided photorefractive keratectomy exerted a beneficial impact on these patients’ quality of life. This led them to suggest that CXL should be performed early.
Published clinical results
In 2003 Wollensak, Spoerl and Seiler (15) reported on 22 patients with progressive keratoconus upon whom they performed CXL and followed for 2 to 4 years. They demonstrated no progression of keratoconus in all eyes, improved visual acuity in 15 of 22 eyes and flattening of K-max by 2 diopters (D) in 16 of 22 eyes.
Caporossi et al (16) reported a 3.6 line increase in uncorrected visual acuity (UCVA), a 1.66 line improvement in best spectacle corrected visual acuity (BSCVA), a mean reduction in maximum keratometry (K-max) of 2.1 D (±0.13), and a 2.5 D reduction in manifest refraction spherical equivalent (MRSE) at 3 months after CXL in a series of 10 eyes in 10 patients with progressive keratoconus. There were no changes in endothelial cell density.
Raiskup-Wolf et al (17) reported on 7-year results at the University of Dresden. They noted a decrease in maximum keratometry of 2.7 D at 1 year; 2.2 D at 2 years and 4.8 D at 3 years. Visual acuity improved by one line per year in 54% of patients in the first 3 years. Two patients had continued progression and had to undergo repeat CXL procedures.
In the only randomized prospective controlled clinical trial of CXL of progressive keratoconus published to that date, Wittig-Silva et al (18) reported on 66 eyes of 49 patients with documented progression of keratoconus. Interim analysis of treated eyes showed a flattening of the steepest simulated keratometry value (K-max) by an average of 0.74 D at 3 months, 0.92 D at 6 months, and 1.45 D at 12 months. A trend toward improvement in BSCVA was also observed. In the control eyes, mean K-max steepened by 0.60 D after 3 months, by 0.60 D after 6 months, and by 1.28 D after 12 months. BSCVA decreased by logMAR 0.003 over 3 months, 0.056 over 6 months, and 0.12 over 12 months. No statistically significant changes were found for spherical equivalent or endothelial cell density.
In 2012, Goldich et al (19) reported on patients with progressive keratoconus who underwent CXL and found, after 2 years of follow-up, that these patients had stable UCVA, improved BCVA and reduced keratometry, indicating stabilization of the progression of keratoconus. Unchanged corneal pachymetry, endothelial cell density, and foveal thickness suggested long-term safety of CXL.
Recent publications with pediatric patients have shown significant and rapid functional improvement in these young patients with progressive keratoconus who underwent CXL. Caporossi et al (20) found a good functional response and stability of the keratoconus with 3 years of follow-up. Vinciguerra et al (21) reported improved UCVA and BSCVA in patients up to 18 years of age with progressive keratoconus who underwent CXL. They felt that this improvement was most likely due to significant reduction of corneal asymmetry and corneal as well as total wavefront aberrations.
Gaster and Rabinowitz (22) reported in 2012 on 31 eyes of teenagers who underwent CXL and found significant improvement in UCVA and BSCVA and decreased pachymetry along with no significant complications. They concluded that CXL in young patients is safe, efficacious and should be performed earlier rather than later.
A report by Hafezi et al (23) cites 10 eyes treated with cross-linking for ectasia after LASIK with 2 years follow up and no progression of the ectasia.
In another study, Kannelopolous and Binder (24) report on topography-guided PRK on a patient with progressive keratoconus after having been cross-linked with excellent results, suggesting that it might be safe to do PRK on these patients to correct their residual refractive error since the cornea is biomechanically stable after CXL.
Recently, Kymionis et al (25) described long-term results after combined transepithelial phototherapeutic keratectomy (t-PTK) and CXL as compared to mechanical epithelial debridement and CXL. They compared these 2 different methods of epithelial removal prior to CXL and found that the patients who underwent the combined t-PTK and CXL showed a statistically significant improvement in UCVA and BCVA as compared to those who underwent the standard mechanical epithelial removal. Furthermore, the patients who underwent the t-PTK and CXL had a statistically significant reduction in astigmatism post-operatively at 12 months as compared to the other group.
Based on the work of Kymionis and others, we have been performing similar t-PTK combined with CXL on our progressive keratoconus patients. We have treated over 30 such patients so far and have seen a 2- to 3-line improvement in UCVA as compared with a 1- to 2-line improvement in UCVA in patients whose epithelium was removed manually. The results of this study were presented at the 2012 ARVO meeting (26).
Corneal Collagen Cross-Linking (CXL) Trial Comparing CXL Alone to CXL and Intacs
For the past 3 years our group has conducted a clinical trial with an IDE from the FDA comparing CXL alone to CXL combined with Intacs in patients with keratoconus and post-LASIK ectasia (ClinicalTrials.Gov ID # NCT01081561). This study allows for 400 potential enrollees in each category. Keratoconus or post-LASIK ectasia patients must demonstrate 1D of progression in the prior year to be eligible. Exclusion criteria are VA 20/25 or better, central corneal scar, corneas thinner than 400 microns, or keratometry greater than 60D.
Procedure
We perform epithelium off cross-linking and our current procedure is as follows: Anesthesia is achieved by instilling topical anesthetic drops into the eye every 5 minutes for 3 doses immediately before the procedure.
The central 9 mm of corneal epithelium is removed by one of two different methods:
Alcohol is applied for 45 seconds into a 9 mm ring placed on the cornea and then washed off; the central 9 mm of epithelium is then removed by a blunt spatula, or
Using the VISX S4 excimer laser, the central 6.5 mm of epithelium is ablated in the PTK mode to a depth of 50 microns; the peripheral epithelium out to 9 mm is then removed by a blunt spatula.
Riboflavin drops are then instilled onto the cornea every 2 minutes for 30 minutes until the riboflavin has saturated the corneal stroma. The patient’s corneal pachymetry is measured to ensure that there is at least 400 microns of tissue present prior to UV-A irradiation. The patient is examined at the slit lamp to ensure that riboflavin has saturated the corneal stroma and that there is a yellow flare in the anterior chamber prior to UV-A irradiation. Pilocarpine is administered to cause pupillary miosis. The UV-X illumination system is calibrated. UV-A irradiation is applied at 3.0 mW/cm2 for 30 minutes while the riboflavin administration is continued every 2 minutes.
After the CXL treatment, a topical antibiotic drop is instilled followed by placement of a bandage soft contact lens. The patient is given topical antibiotic, non-steroidal anti-inflammatory, and frequent lubricating drops to use post-operatively. Oral analgesics are given as needed. Topical steroid drops are started at post-operative day 5 and the bandage contact lens is removed when the epithelium is healed, usually by day 4 to 7. Post-operative exams are done at day 1, week 1, and months 1, 3, 6, and 12, or as necessary in between, and then yearly.
Preliminary data analysis
To date we have treated 252 patients with keratoconus and post-LASIK ectasia using the epithelium off technique; this includes 40 individuals in the pediatric age group 11 to 19 years of age. We have used both manual removal and the PTK mode, as described, to remove the epithelium, and we have categorized patients into several groups for data analysis. These groups include 1) CXL for progressive keratoconus; 2) CXL for post-LASIK ectasia; 3) CXL and Intacs for keratoconus 3 months apart; 4) Simultaneous CXL and Intacs for keratoconus; 5) CXL and Intacs for post-LASIK ectasia 3 months apart; and 6) Simultaneous CXL and Intacs for post-LASIK ectasia. It is expected that analysis of these different groups will yield valuable information for future targeted therapy for affected individuals.
In our study, when we analyzed the CXL for keratoconus only group (52 patients), the cornea flattened by 0.31D (K-value) to 0.45D (K-max) with the UCVA improving by a mean of 1.46 lines and BCVA improving by a mean of 0.57 lines. At 12 months (26 patients), the cornea had flattened by 0.06D (K-value) to 0.13D (K-max) and UCVA improved by a mean of 1.8 lines and BCVA improved by 0.57 lines. What was striking was the huge variability in response, with some patients improving by as much as 7 lines of acuity with others showing no improvement at all, despite the same treatment parameters. There was also a huge variability in the amount of haze in patients even though this was rarely visually significant. This huge variability in response and some failures, which were seen in both our study and previously reported studies, may in part be explained by genetic variability in patients with keratoconus.
Lysol oxidase (LOX) is responsible for CXL of the human cornea. Our group recently demonstrated a high association of polymorphisms for the gene encoding (LOX) in patients with both familial and non-familial keratoconus (29). Sequencing of LOX exons in a subset of keratoconus patients identified two polymorphisms, rs1800449 and rs2288393, located in LOX transcripts I and II, associated with keratoconus in case-control and family samples with a meta P value of 0.02. These results provided strong genetic evidence that LOX variants lead to increased susceptibility to developing keratoconus. Future research in this area may allow us to identify an individual patient’s response to CXL treatment both in terms of variability in effect and in identifying patients who are likely to fail and as such may not be good candidates for such treatment. This will in part fulfill the promise of individualized medicine through pharmacogenetic manipulation. Such research is ongoing in our research group.
The CXL procedure has had a very high safety profile. To date we have had the following complications: 1. One patient with corneal hydrops, which resolved after 6 months and resulted in improved vision; 2. Two patients with corneal ulcers, both of which resolved after antibiotic treatment; 3. One patient with a very significant corneal opacity resulting in decreased BCVA for the first 6 months but who later resolved with a 5 line gain in UCVA; and 4. Two patients with haze resulting in reduced acuity. Three patients have progressed after 1 year of treatment. This results in a total complication rate of 6/252 (2.4%). Fortunately, none of these complications resulted in loss of visual acuity and the fact that there was only progression in 3/252 (1.2%) speaks well of the efficacy of CXL. It remains to be seen however what the long-term progression rate will be. In Europe with 13 years’ experience, it has been quoted to be between 3 and 7%, depending on which study is read.
Epithelium-Off vs. Epithelium-On Controversy
In their initial description of the cross-linking procedure in 2003, Wollensak, Spoerl and Seiler (15), removed the epithelium prior to administration of the riboflavin. These investigators have the most experience with this procedure, have experimented with different ways of preparing the cornea prior to UV-A exposure, and have conducted many studies demonstrating the safety and efficacy of the epi-off technique. They recently reported their 6-year results, using the same technique (17).
Wollensak et al (27) experimented with the epithelium-on (epi-on) technique in the laboratory and found that it was only 20% as effective at stiffening the cornea as the epi-off procedure. Therefore, he recommended that epi-on should only be considered only in instances where corneas were not thick enough for the standard epi-off procedure.
The purported advantages of epi-on CXL include reduction of postoperative pain, less chance of infection or scarring, and faster return to hard contact lens use. However, in the absence of published, solid scientific data demonstrating its safety and efficacy, it is necessary to be skeptical of this method of CXL. One study by Leccisotti and Islam (28) suggests that the epi-on technique is as efficacious as epi-off. However, the eyes were subjected to instillation of various eye drops for 3–4 hours before the instillation of riboflavin and then the CXL procedure. The results of this CXL procedure were not as good as other published epi-off studies, according to their paper. In the United States, a group conducting a study with the epi-on approach cites excellent results, but none of the data has been published to date. The UV-A light source and the riboflavin these investigators use are “proprietary,” so thus far no researchers are able to reproduce the technique. In addition, there are no long-term data showing that this technique is safe or efficacious.
During the past 5 years, our practice has seen over 20 patients who have undergone an epi-on procedure called C3R, performed locally. In all 20 patients there was significant progression of their ectasia despite undergoing this epi on technique.. Several of these patients have had to undergo a repeat CXL procedure with epi-off with good results and no further progression to date.
Future Developments in CXL
The most important next development in the United States will be the approval by the FDA of this major advance in the treatment of patients with progressive keratoconus and post-LASIK ectasia. Studies demonstrating the safety and efficacy of CXL are being submitted to the FDA. Hopefully, the knowledge of the benefits of CXL will be dispersed to all eye care professionals and pediatricians so that early detection can lead to decreased visual loss. Advances in delivery of the riboflavin into the cornea, such as iontophoresis and shorter UVA exposure time, are currently being investigated. Combining CXL with either PRK or LASIK to help strengthen the cornea and avoid post-LASIK ectasia is also being studied at this time. In the near future, we should have better methods to perform CXL to help this select group of patients
Acknowledgments
Supported in part by a Grant from the National Eye Institutes of Health NEI R01-09052 and the Eye Defects Research Foundation Inc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no proprietary or commercial interest in the medical devices discussed in this chapter.
References
- 1.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 2.Spoerl E, Seiler T. Techniques for stiffening the cornea. J Refract Surg. 1999;15:711–713. doi: 10.3928/1081-597X-19991101-21. [DOI] [PubMed] [Google Scholar]
- 3.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine cornea after riboflavin/ultraviolet-A-induced crosslinking. J Refract Surg. 2003;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 4.Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29:35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 5.Labiris G, Giarmoukakis A, Sideroudi H, et al. Impact of keratoconus, cross-linking and cross-linking combined with photorefractive keratectomy on self-reported quality of life. Cornea. 2012;31:734–739. doi: 10.1097/ICO.0b013e31823cbe85. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RC, Pinnell SR, Martin GR. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry. 1970;9:4486–4492. doi: 10.1021/bi00825a004. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RC. Biosynthesis of collagen crosslinks: increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proc Nat Acad Sci USA. 1974;71:4826–4830. doi: 10.1073/pnas.71.12.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wollensak G, Spoerl E, Reber F, et al. Corneal endothelial cytotoxicity of riboflavin/UVA treatment in vitro. Ophthalmic Res. 2003;35:324–328. doi: 10.1159/000074071. [DOI] [PubMed] [Google Scholar]
- 9.Wollensak G, Spoerl E, Wilsch M, et al. Keratocyte apoptosis after collagen cross-linking using riboflavin/UVA treatment. Cornea. 2004;23:43–49. doi: 10.1097/00003226-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Wollensak G, Spoerl E, Reber F, et al. Keratocyte cytotoxicity of riboflavin/UVA treatment in vitro. Eye. 2004;18:718–722. doi: 10.1038/sj.eye.6700751. [DOI] [PubMed] [Google Scholar]
- 11.Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385–389. doi: 10.1097/ICO.0b013e3180334f78. [DOI] [PubMed] [Google Scholar]
- 12.Bottos KM, Dreyfuss JL, Regatieri CV, et al. Immunoflourescence confocal microscopy of porcine corneas following collagen cross-linking treatment with riboflavin and Ultraviolet A. J Refract Surg. 2008 Sep;24:S715–S719. doi: 10.3928/1081597X-20080901-14. [DOI] [PubMed] [Google Scholar]
- 13.Chai D, Gaster RN, Roizenblatt R, et al. Quantitative assessment of UVA-riboflavin corneal cross-linking using nonlinear optical microscopy. Invest Ophthalmol Vis Sci. 2011;52:4231–4238. doi: 10.1167/iovs.10-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roizenblatt R, Chai D, Gaster RN, et al. Comparison study of ultraviolet A irradiance of 3mW/cm2 versus 9 mW/cm2 with riboflavin on corneal collagen cross-linking efficacy in rabbit eyes. Invest. Ophthalmol. Vis. Sci. 2010;51 E-Abstract 4979. [Google Scholar]
- 15.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 16.Caporossi A, Baiocchi S, Mazzotta C, et al. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen. J Cataract Refract Surg. 2006;32:837–845. doi: 10.1016/j.jcrs.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 17.Raiskup-Wolf F, Hoyer A, Spoerl E, et al. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 18.Wittig-Silva C, Whiting M, Lamoureux E, et al. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J Refract Surg. 2008;24:S720–S725. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 19.Goldich Y, Marcovich AL, Barkana Y, et al. Clinical and corneal biomechanical changes after collagen cross-linking with riboflavin and UV irradiation in patients with progressive keratoconus: results after 2 years of follow-up. Cornea. 2012;31:609–614. doi: 10.1097/ICO.0b013e318226bf4a. [DOI] [PubMed] [Google Scholar]
- 20.Caporossi A, Mazzotta C, Baiocchi S, et al. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 2012;31:227–231. doi: 10.1097/ico.0b013e31822159f6. [DOI] [PubMed] [Google Scholar]
- 21.Vinciguerra P, Albe E, Frueh BE, et al. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol. 2012 doi: 10.1016/j.ajo.2012.03.020. in press. [DOI] [PubMed] [Google Scholar]
- 22.Gaster RN, Rabinowitz YS, Canedo AL. Corneal cross-linking in teenagers. Presented at the Cross-Linking Congress; July 29, 2012; Deer Valley, Utah. [Google Scholar]
- 23.Hafezi F, Kanellopoulos J, Wiltfang R, et al. Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33:2035–2040. doi: 10.1016/j.jcrs.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Kanellopoulos AJ, Binder PS. Collagen cross-linking (CCL) with sequential topography-guided PRK: a temporizing alternative for keratoconus to penetrating keratoplasty. Cornea. 2007;26:891–895. doi: 10.1097/ICO.0b013e318074e424. [DOI] [PubMed] [Google Scholar]
- 25.Kymionis GD, Grentzelos MA, Kounis GA, et al. Combined transepithelial phototherapeutic keratectomy and corneal cross-linking for progressive keratoconus. Ophthalmology. 2012 doi: 10.1016/j.ophtha.2012.03.038. in press. [DOI] [PubMed] [Google Scholar]
- 26.Rabinowitz YS, Gaster RN. Early results of laser-assisted collagen cross-linking (laser CXL) suggest that patients achieve better uncorrected acuity at 6 months compared to mechanical debridement of the epithelium in patients with progressive keratoconus. Presented at the 2012 ARVO Meeting; May 10, 2012; Ft. Lauderdale, FL. [Google Scholar]
- 27.Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refract Surg. 2009;35:540–546. doi: 10.1016/j.jcrs.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 28.Leccisotti A, Islam T. Transepithelial corneal cross-linking in keratoconus. J Refract Surg. 2010;26:942–948. doi: 10.3928/1081597X-20100212-09. [DOI] [PubMed] [Google Scholar]
- 29.Bykhovskaya Y, Li X, Epifantseva I, Haritunians T, Siscovick D, Aldave A, Szczotka-Flynn L, Iyengar SK, Taylor KD, Rotter JI, Rabinowitz YS. Variation in the Lysyl Oxidase (LOX) Gene Is Associated with Keratoconus in Family-Based and Case-Control Studies. Invest Ophthalmol Vis Sci. 2012 Jun 28;53(7):4152–4157. doi: 10.1167/iovs.11-9268. [DOI] [PMC free article] [PubMed] [Google Scholar]