Abstract
In the ovules of most sexual flowering plants female gametogenesis is initiated from a single surviving gametic cell, the functional megaspore, formed after meiosis of the somatically derived megaspore mother cell (MMC)1,2. Because some mutants and certain sexual species exhibit more than one MMC2-4, and many others are able to form gametes without meiosis (by apomixis)5, it has been suggested that somatic cells in the ovule are competent to respond to a local signal likely to play an important function in determination6. Here we show that the Arabidopsis protein ARGONAUTE9 (AGO9) controls female gamete formation by restricting the specification of gametophyte precursors in a dosage-dependent, non-cell-autonomous manner. Mutations in AGO9 lead to the differentiation of multiple gametic cells that are able to initiate gametogenesis. The AGO9 protein is not expressed in the gamete lineage; instead, it is expressed in cytoplasmic foci of somatic companion cells. Mutations in SUPPRESSOR OF GENE SILENCING3 and RNA-DEPENDENT RNA POLYMERASE6 exhibit an identical defect to ago9 mutants, indicating that the movement of small RNA (sRNA) silencing out of somatic companion cells is necessary for controlling the specification of gametic cells. AGO9 preferentially interacts with 24 nucleotide (nt) sRNAs derived from transposable elements (TEs), and its activity is necessary to silence TEs in female gametes and their accessory cells. Our results show that AGO9-dependent sRNA silencing is crucial to specify cell fate in the Arabidopsis ovule, and that epigenetic reprogramming in companion cells is necessary for sRNA–dependent silencing in plant gametes.
Large-scale transcriptional analysis indicated that a gene encoding an ARGONAUTE (AGO) protein (At5g21150 or ARGONAUTE9) is highly expressed in ovules and anthers of Arabidopsis (Supplementary Fig. 1). The in situ pattern of expression confirmed that AGO9 mRNA is localized in ovules throughout development (Supplementary Fig. 2). In both plants and animals, AGO proteins are known to cleave endogenous mRNAs during either microRNA (miRNA) or short interfering RNA (siRNA)-guided post-transcriptional silencing7-9. To elucidate the function of AGO9 in Arabidopsis, individuals from 3 independent insertional lines harboring T-DNA elements within the coding region of the AGO9 gene were phenotypically analyzed at all stages of ovule development10 (Fig. 1a and Tabe 1). Whereas 94.2% of pre-meiotic ovules showed a single MMC in wild-type plants (Fig. 1b and Supplementary Figure 3), 5.8% exhibited 2 MMCs (Fig. 1c); however, only one of the latter underwent gametogenesis since twin female gametophytes were never observed. All ago9 insertional lines were fertile and did not show signs of ovule or seed abortion; however, in contrast to wild-type plants, the pre-meiotic ovule primordia of heterozygous ago9/+ individuals - including allele ago9-2 that was previously reported as having no defective phenotype11 - showed several abnormally enlarged sub-epidermal cells (Figure 1d,e). In ago9/+ individuals, the ovules exhibited up to 6 cells containing a conspicuous nucleus and nucleolus at a frequency of 30.29%, indicating that ago9 alleles are dominant and affect early cell differentiation in the developing ovule. In homozygous ago9/ago9 individuals, the percentage of ovule primordia showing more than one enlarged cell was of 37.16% to 47.7%, depending on the allelic variant (Table 1). Triploid (3n) individuals that had 2 wild-type and one mutant ago9-3 allele showed 14.11% to 23.49% of abnormal ovules, a value intermediate between diploid plants carrying a single ago9-3 allele and wild-type (Table 1). These results suggest that a dosage-dependent mechanism is responsible for the mutant ago9 phenotype.
Figure 1. Phenotypes of ago9 insertional mutants prior to meiosis.
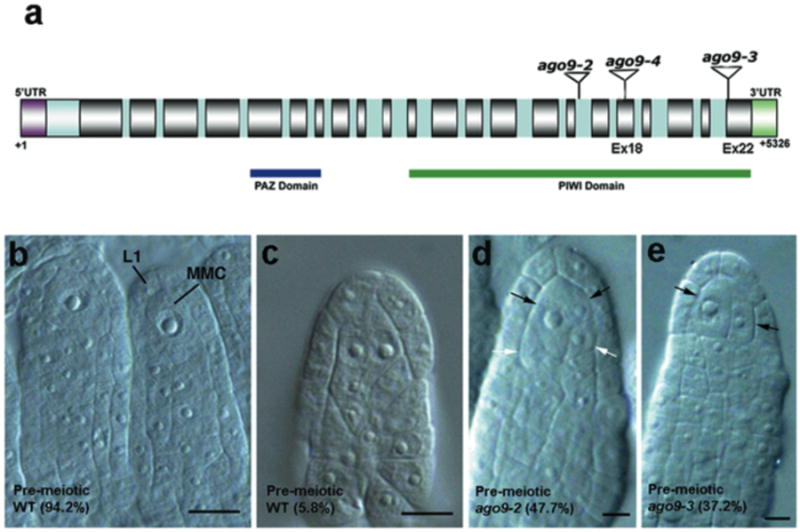
a, Genomic structure of the AGO9 gene in Arabidopsis; the location of T-DNA insertions and the gene length (nt) indicated. b, Pre-meiotic wild-type ovules showing a single sub-epidermal MMC. c, Pre-meiotic wild-type ovule showing 2 MMCs. d, Pre-meiotic ago9-2 mutant ovule showing 2 larger (black arrows) and 2 smaller (white arrows) abnormal cells. e, Pre-meiotic ago9-3 ovule showing abnormally enlarged cells (arrows); one of them has initiated a nuclear division. Scale bars: 10 μm.
Table 1. Genetic analysis of insertional ago9 mutants in Arabidopsis.
| Allele | Genotype | Single MMC | Abnormally enlarged cells |
|---|---|---|---|
| ago9-3 | ago9-3/ago9-3 | 208 | 123 (37.16%) |
| ago9-3/+ | 214 | 93 (30.29%) | |
| ago9-3m/+p/+p | 286 | 47 (14.11%) | |
| +m/+m/ ago9-3p | 241 | 74 (23.49%) | |
| ago9-4 | ago9-4/ago9-4 | 139 | 118 (45.9%) |
| ago9-2 | ago9-2/ago9-2 | 162 | 148 (47.7%) |
| wild-type | +/+ | 292 | 18 (5.8%) |
No molecular marker exclusively expressed in the MMC has been reported, but the pattern of callose deposition is a reliable method to determine cell identity at pre-meiotic stages12. To determine whether one or several of the enlarged cells present in ago9-3 ovules are capable of undergoing meiosis, we analyzed callose deposition in wild-type and homozygous ago9-3 ovules. In agreement with previous descriptions, wild-type ovules showed patches of callose in the MMC prior to the initiation of meiosis (Fig. 2a). After meiosis, callose was deposited in transverse walls between the functional megaspore and its degenerated sister cells (Fig. 2b). In pre-meiotic ago9-3 ovules, less than 10% of abnormally enlarged cells showed patches of callose deposits (Fig. 2c, 2d). During meiosis, callose was only detected in the intermediate walls of a single cell and the degenerated neighboring cells, but not in the closely associated abnormally enlarged cells (Fig. 2e, 2f). This pattern persisted following meiosis (Fig. 2g, 2h). These results show that several enlarged cells differentiate before meiosis in ago9-3 ovules, but that a single one undergoes meiosis and gives rise to a functional haploid megaspore, indicating that the activity of AGO9 is necessary to restrict differentiation to a single sub-epidermal cell in the pre-meiotic ovule.
Figure 2. Meiotic and post-meiotic phenotype of the ago9 mutant.
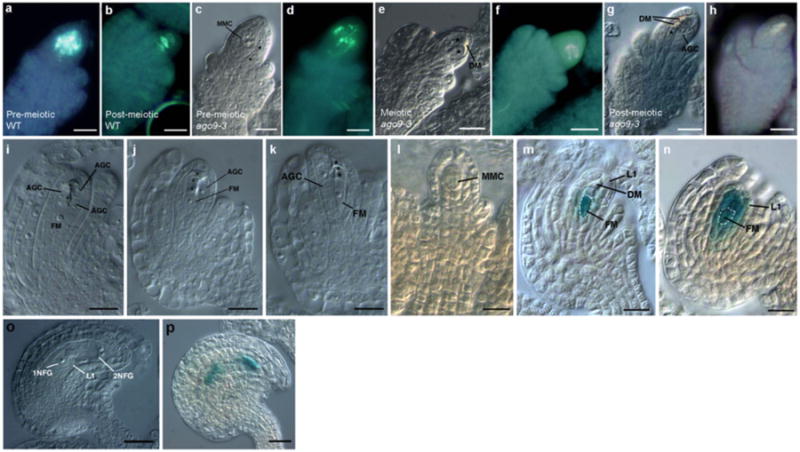
a-h, Callose deposition (a,b,d,f,h) and morphology (c,e,g) of wild-type (WT) and ago9-3 ovules. i-k, ago9-3 post-meiotic ovules showing abnormal gametic cells (AGC) adjacent to degenerated (asterisk and DM) and functional (FM) megaspores. l, Absence of pFM2-driven GUS expression in a pre-meiotic wild-type ovule. m, pFM2-driven GUS expression in the functional megaspore (dashed) of a post-meiotic wild-type ovule. n, pFM2-driven GUS expression in the functional megaspore (FM) and adjacent cells of a post-meiotic ago9-3 ovule. o, ago9-3 ovule containing a 2-nuclear (2NFG) and a 1-nuclear (1NFG) female gametophyte. P, pFM2-driven GUS expression in a ago9-3 ovule containing 2 female gametophytes (arrows). Scale bars: a to h, 5μm; i to n, 10μm; o and p, 25 μm.
Following meiosis, ago9-3 ovules showed persistent enlarged cells adjacent to meiotic products, including the 3 degenerated megaspores and the functional megaspore (Fig. 2i to 2k). To determine the identity and assess the developmental potential of extranumerary enlarged cells in mutant ovules, we examined the expression of pFM2, a marker expressed in the functional megaspore and the developing female gametophyte, but not in the MMC or in the 3 meiotically-derived degenerated megaspores (Fig. 2l, 2m). In ago9-3 ovules, pFM2 expression was initially observed following meiosis in the functional megaspore but also in a cluster of adjacent cells that forms the nucellus and includes the abnormal gamete precursors (Fig. 2n). In all ago9-3 ovules observed, more than 4 cells showed strong GUS expression at post-meiotic stages, indicating that at least some of the cells that express pFM2 have a somatic origin. pFM2 expression was absent at pre-meiotic stages, indicating that defective ago9-3 individuals differentiate additional cells that persist in the developing ovule adjacent to the meiotic products and subsequently acquire a functional megaspore identity without undergoing meiosis. At subsequent stages of development, ago9-3 individuals exhibited an unusual phenotype of 2 independent female gametophytes developing in the same ovule at a frequency of 44.03% (n=243; Fig 2o). Crosses of ago9-3 plants with individuals expressing the pFM113 or pFM2 marker revealed that both acquire a female gametophyte identity (Fig 2p and Supplementary Figure 4). These results suggest that abnormal somatic cells are able to differentiate into gametic cells and initiate gametogenesis without undergoing meiosis.
Immunoblots hybridized with a polyclonal antibody against AGO9 detected a protein of the expected 100.5 kDa size in developing wild-type gynoecia but not in 1-week old seedlings, developing rosette leaves or developing siliques (Fig. 3a). Immunolocalizations showed that the AGO9 protein was initially expressed in somatic cells of the epidermal (L1) layer located in the apical region of the pre-meiotic ovule, but not in the MMC (Figure 3b). Interestingly, we observed AGO9 in cytoplasmic foci reminiscent of P-bodies or stress granules present in the cytoplasm of animal cells (Fig. 3c to 3e). AGO9 did not localize in the haploid megaspores or the developing female gametophyte before of after cellularization. In ovules containing a female gametophyte at the 4-nuclear stage, AGO9 was localized in the outer integumentary cells, but also in the periphery of the endothelium, at the sporophyte-gametophyte cellular boundary (Fig. 3f). In anthers, AGO9 was localized in the cytoplasm of microsporocytes following meiosis, and later in the cytoplasm of the vegetative cell but not in the sperm cells (Supplementary Fig. 5a to 5d). Ovules or pollen of ago9-3 individuals did not show AGO9 expression (Supplementary Figure 5d, 5e), confirming that the antibody exclusively recognized AGO9. Overall, these results indicate that AGO9 is preferentially expressed in reproductive companion cells but not in the associated male or female gametes or their precursors.
Figure 3. AGO9 protein expression in developing ovules.

a, Immunoblot analysis of AGO9 in wild-type seedlings (1), developing gynoecia (2) leaves (3), and 7 day old siliques (4). b-d, AGO9 is expressed in cytoplasmic foci (green) of companion cells but absent from the MMC (outlined in b) or the functional megaspore (arrow in nuclei are counter-stained with DAPI (b and d). e, Diagram showing AGO9 localization (green) in a wild-type ovule at the end of meiosis. f, AGO9 expression in companion somatic cells but not within a 4-nuclear female gametophyte (FG). Scale bars: b, 5μm; c-d, 10μm; f, 20μm.
In Arabidopsis, trans-acting small interfering RNAs (ta-siRNAs) are known to move as signal molecules and cause gene silencing beyond their cellular sites of initiation14-16. Their biogenesis depends on transcription by RNA-DEPENDENT RNA POLYMERASE6 (RDR6) that converts their single-stranded RNA precursors into double-stranded RNA in a pathway that is also dependent on the function of the putative RNA binding protein SUPRESSOR OF GENE SILENCING3 (SGS3)17,18. The extent of gene silencing movement outside their site of initiation also depends upon the activity of RDR619. To determine if the function of AGO9 could be associated with a non-cell-autonomous pathway, we examined ovule development in homozygous sgs3-11 and rdr6-11 individuals. Although both sgs3 and rdr6 mutants show seedling and floral defects characterized by leaf curling and limited stamen elongation17, their possible role during gamete formation has not been investigated. Both sgs3-11 and rdr6-11 plants showed an identical phenotype to ago9 mutants with additional gametic cells differentiating in the pre-meiotic ovule (Fig. 4a to 4d). In rdr6-11 plants, post-meiotic ovules showed 2 independently developing female gametophytes at a frequency of 43.3% (n=224). Crosses of rdr6-11 plants to individuals expressing the pFM2 marker indicate that both acquire a female gametophyte identity (Fig. 4e). These results support the hypothesis that AGO9 controls gametic cell commitment by acting in a non-cell-autonomous sRNA-dependent pathway in the developing ovule of Arabidopsis.
Figure 4. Phenotype of the rdr6 mutant and activation of transposable elements in the ago9 mutant.

a-d, Ovules of rdr6-11 showing abnormal gametic cells (AGC) adjacent to the functional megaspore (FM) and 2 female gametophytes (arrows) separated by the L1 cell layer or degenerated cells (arrowheads). e, pFM2-driven GUS expression in a rdr6-11 ovule. f, Enhancer trap ET13889 inserted in a AtLINE3 transposon shows no GUS expression in mature wild-type ovules. g-i, GUS expression conferred by enhancer traps ET13889 (g), ET11075 (h), and ET10306 (i) in the egg apparatus of ago9-3/+ ovules. Scale bars: a=10μm; b to i = 25μm.
To identify the nature of AGO9-associated sRNAs, wild-type developing gynoecia were isolated and used for total protein extraction, immunoprecipitation with the AGO9 antibody, and elution of the associated sRNA fraction. After sequencing, 2508 sRNA sequences (98% of total) could be mapped to the Arabidopsis nuclear genome and categorized based on their location and function (Supplementary Tables 1 and 2). Although the majority is 24 nt in length (79.1%), 8.9% are 21 to 22 nt long. The majority of 24 nt sequences derive from transposable elements (TEs) belonging to distinct families of retrotransposons: Gypsy (23%) Athila (9.3%), CACTA (5.5%), and less frequently LINE or Mutator. All sequences mapping to Gypsy TEs belong to the AtGP1 sub-family, and 3% of all sequences mapping to retrotransposons correspond to siRNAs shown to be dependent on RNA Polymerase IV (PolIV) for their biogenesis20. An additional 17.4% of the total maps to genomic signatures assigned to other families containing nested components of Gypsy, Athila or CACTA TEs. In contrast, 21 nt sRNAs preferentially derive from previously characterized miRNAs (3.2%) – including miR167 that is known to act in the ovule21 -, and protein-coding genes (14.5%). These results show that primary targets of AGO9-dependent silencing in the ovule of Arabidopsis are TEs.
Previous studies have shown that some TEs that are active in mature pollen grains are not expressed in developing or fully differentiated ovules of Arabidopsis22. To determine if AGO9 is necessary for the inactivation of these TEs in the ovule, we crossed lines containing enhancer traps (ET) that tagged specific TEs to homozygous ago9 individuals. In agreement with previous results, no GUS expression was observed in the ovule of ET lines present in a wild-type genetic background (Fig. 4f). By contrast, heterozygous ago9/+ individuals containing an ET within either an Athila, LINE, or Atlantys retrotransposon showed strong GUS staining in the egg and synergid cells of the mature female gametophyte prior to pollination (Fig. 4g to 4i). These results not only confirm that AGO9 is necessary for TE inactivation in the ovule, but also show that one of its targets is the egg and synergid cells (the egg apparatus) before fertilization.
The ago9 phenotype was also identified in homozygous mutants for RNA-dependent RNA polymerase2 (rdr2), dicer-like3 (dcl3), and the double mutant nrpd1a nrpd1b that is defective in the activity of both Polymerase IV and Polymerase V - but not in dicer-like1 and dicer-like4 (dcl4) that are essential for the generation of miRNAs and ta-siRNAs, respectively -, suggesting that AG09-dependent TE inactivation restricts female gametogenesis to a single gametic cell through an endogenous 24 nt siRNA biosynthetic pathway23 (Supplementary Figure 6 and 7). The consistent identification of a single cell undergoing meiosis and multiple cells acquiring a functional megaspore identity in the post-meiotic ovule, combined to the presence of two developing female gametophytes separated by several somatic cells, provides strong evidence for the initiation of female gametophytes from two non-sister cells, one of which is somatic in origin.
By preferentially interacting with sRNAs derived from TEs and silencing their activity in the female gametophyte, the function of AGO9 is reminiscent of the PIWI subclass of ARGONAUTE proteins that are necessary to maintain transposon silencing in the germline genome of invertebrates and mammals24. Some maternal siRNA sequences found in the endosperm20 and 24 nt siRNA found in pollen22 resemble AGO9-interacting sRNAs, raising the possibility that AGO9 may also contribute to these populations in a non-autonomous way. The ago9 mutant phenotype is reminiscent of apospory, a component of asexual reproduction through seeds (apomixis) prevailing in many flowering species that produce unreduced female gametes from somatic cells5. Our findings open new venues to investigate the genetic basis and molecular mechanisms that control cell fate, offering new possibilities to explore the epigenetic induction of apomixis in sexual plants.
Full Methods
Plant material and growth conditions
We used A. thaliana ecotype Columbia (Col-0) for wild-type plants, chemical homozygous mutant sgs3-11 and insertional lines CS24285 (rdr6-11), SALK005512 (dcl3-1) (ref. 28), GABI160G05 (dcl4-2) (ref. 29), SAIL-1277H08 (rdr2-1) (ref. 28), a double mutant nrpd1a-2 nrpd1b-11 (SALK_128428 and SALK_029919, respectively), SAIL_34_G10 (ago9-3), SAIL_260_A03 (ago9-4), SALK_112059 (ago9-2) and A. thaliana ecotype Landsberg erecta (Ler) for Enhancer Trap lines ET13889, ET11075 and ET10306. Seeds were surface-sterilized by washing three times with 100% ethanol and plated on Murashige and Skoog (MS) medium. The pFM2 plasmid construction was generated by amplifying the pFM2 genomic regulatory region using primers 5′-GCGTGACACGCCACTACAACACACCAA-3′ (sense) and 5′-GCGGATCCAGGAAGCCATCGTCAGACAG-3′ (antisense); a 564-base-pair (bp) genomic fragment was subsequently cloned in front of the uidA gene using the pBI101.2 plasmid. Transformation was in Col-0. In all cases MS medium plates containing seeds were placed in full darkness for 3 days at 4 °C, and subsequently germinated in a growth chamber at 22 °C under a 16 h light/8 h dark photoperiod, transferred to soil, and grown in the greenhouse under long-day 16 h light/8 h dark controlled conditions.
In situ hybridization
A specific 149-bp fragment corresponding to the AGO9 3′UTR was PCR amplified by using primers ago9isS2 (5′-TCCAGTCCACACGATAGCT-3′) and ago9isAS2 (5′-ATTCTGTCGGTTTTTGTGGG-3′) and cloned in TOPO-PCRII (Invitrogen). The resulting plasmid was linearized with BamH1 (sense) and NotI (antisense) and used for generating digoxigenin-labelled RNA-probes (DIG RNA labelling kit SP6/T7; Roche). Developing flower buds were fixed in 4% paraformaldehyde and embedded in tissue-prep paraffin (Fisher Scientific). Sections of 10–12-mm thickness were generated using a Leica microtome and mounted on ProbeOnPlus slides (Fisher Biotech). Hybridization was performed as described30; for whole mount in situ hybridization, anthers and ovules were fixed, mounted in acrylamide-covered slides, and hybridized as described31.
PCR with reverse transcription (RT–PCR)
Total RNA was extracted from leaves, roots, stems, inflorescences, mature flowers, gynoecia, ovules and seedlings with TRIzol (Invitrogen). Total RNA (2 μg) from each tissue was used to synthesize first-strand cDNA by using 20mer-oligo dT (Sigma), 0.25 mM dNTPs and SuperScript III Reverse Transcriptase (Invitrogen), and incubating at 42 °C for 2 h. cDNA (100 ng) was used to amplify a 369-bp AGO9 fragment by using primers gntpS2 (5′-TCCCCAATCAAAGGAAAATGG-3′) and gntpAS2 (5′-TCTTGGAATTGTGACTCAGTGCA-3′). Amplification of a 96-bp fragment corresponding to ACTIN2 mRNA was used as a control32. PCR was performed with an initial denaturation step at 94 °C for 1 min 30 s, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C, and extension at 72 °C for 30 s.
Histological analysis
For phenotypic analysis of ovules, inflorescences from wild-type and mutant plants were fixed in FAA (formaldehyde 10%, acetic acid 5%, ethanol 50%), for 12 h and subsequently dehydrated in 70% ethanol. Gynoecia at different developing stages were dissected with hypodermic needles (1-ml insulin syringes), cleared in Herr's solution (phenol:chloral hydrate:85% lactic acid:xylene:clove oil in a 1:1:1:0.5:1 proportion), and observed using a DMR Leica microscope with Nomarski optics. Histochemical localization of GUS activity was performed by incubating dissected gynoecia in GUS staining solution (10 mM EDTA, 0.1% Triton X-100, 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide and 1 mg ml-1 5-bromo-4-chloro-3-indolyl-β-d-glucoronic acid in 50 mM sodium phosphate buffer, pH 7.0) for 24 h as described25. Callose was detected by incubating floral buds in aniline blue staining solution (0.1% aniline blue, 100 mM Na2HPO4, pH 7.4) for 12–24 h in darkness; for each developmental stage and sample, at least 100 ovules were dissected on a slide and mounted in 30% glycerol. Observations were conducted in an Olympus BX60 (Model BX60F5; Olympus Optical) microscope using epifluorescence ultraviolet filters (365 nm excitation, 420 nm emission). Micrographs were acquired using Image Pro-Plus Software, version 4.0.
Protein analysis and immunoblots
The amino-terminal sequence of 16 N-SSRNHAGNDTNDADRK-C (16 amino acids) was selected after three-dimensional modelling (HHpred, available at http://toolkit.tuebingen.mpg.de) to generate a rabbit polyclonal peptide antibody (Invitrogen). After affinity purification, the same antibody was used for Immunoblot analysis (1:500 dilution) or immunolocalization (1:100 dilution). Immunoblots were generated with the WesternBreeze Chemiluminescent Detection Kit (Invitrogen) using 5 μg of total protein for each assay (1-week-old seedlings, developing gynoecia, developing rosette leaves, and siliques 7 days after pollination). Proteins were stained with SYPRO Ruby protein gel stain (Invitrogen) and with a Silver Staining Kit (Invitrogen).
Immunolocalization in sectioned specimens
For immunolocalization experiments, flowers at different developmental stages were fixed in 4% paraformaldehyde in PBS (10 mM KH2PO4, 150 mM NaCl, pH 7) for 12 h at room temperature, gradually dehydrated in an ethanol series (10%), and embedded in LR White Resin (Electron Microscopy Sciences). Sections (0.5 μm) were generated with a ultramicrotome (Leica Ultracut R) and placed on ProbeOnPlus (Fisher Biotech) slides. After washing twice with PBS, sections were blocked for 2 h with 5% BSA and 0.05% Tween 20 in PBS, and incubated with the AGO9 antibody (1:100 in 0.1% BSA in PBS) for 2 h at room temperature. After washing with PBS, slides were incubated with Alexa Fluor 488 goat anti-rabbit (Invitrogen) 1:50 dilution during 2 h at room temperature, washed with PBS and counterstained with 1 μg ml-1 DAPI (Sigma). The slides were mounted with ProLong Gold antifade reagent (Invitrogen). Fluorescence was visualized using a Leica DM 6000B epifluorescence microscope, using filter cubes I3 (excitation 450–490 nm, emission 510 nm) and ultraviolet filter A (excitation 340–380 nm, emission 400 nm). Images were acquired by using Leica QWin Standard V3.4.0 (Leica Microsystems).
Immunolocalization in whole-mounted specimens
Pistils and siliques at various developmental stages were fixed overnight at 4 °C in 4% paraformaldehyde:PBS:2% Triton fixative, washed three times in PBS, and dissected to isolate the ovules and early seeds. The dissected ovules and seeds were embedded in acrylamide as described33 to facilitate manipulation and maintain the three-dimensional architecture of the tissues. Samples were digested in an enzymatic solution (1% driselase, 0.5% cellulase, 1% pectolyase, 1% BSA, all from Sigma) for 25 min to 1 h at 37 °C, depending on the developmental stage, subsequently rinsed three times in PBS, and permeabilized for 2 h in PBS:2% Triton. They were then incubated overnight at 4 °C with primary antibodies used at a dilution of 1:100 for AGO9 and 1:400 otherwise. The slides were washed for a day in PBS:0.2% Triton, and coated overnight at 4 °C with secondary antibodies (Alexa Fluor 488 or 568 conjugate, Molecular Probes) used at 1:400 dilution. After washing in PBS:0.2% Triton for a minimum of 6 h, the slides were incubated with DAPI (1 μg ml-1 in PBS) for 1 h, washed for 2 h in PBS, and mounted in PROLONG medium (Molecular Probes). Complete 3D ovule or seed images were captured on a laser scanning confocal microscope (Leica SP2) equipped with 405 nm (DAPI), 488 nm (green) or 568 nm (red) excitation and either ×40 or ×63 objectives. Projections of selected optical sections were generated for this report, and edited using Graphic Converter (LemkeSOFT). At least 50 ovules were scored for each developmental stage.
Immunoprecipitation and analysis of small RNAs
Immunopurification AGO9 and its associated sRNAs was conducted as described34, with some modifications. Owing to extremely low protein yields obtained in preliminary experiments conducted with hundreds of female reproductive organs, protein extraction was conducted with a total of 12,000 wild-type developing gynoecia containing ovules at mixed developmental stages (four-nuclear stage of gametogenesis to unpollinated mature). Protein extract (0.5 ml) from 12,000 wild-type gynoecia was pre-cleared by incubation with 10 μl Protein A-Sepharose (Invitrogen, Cat. No. 10-1041) at 4 °C for 30 min. Pre-cleared extracts were then incubated either with AGO9 antibody or AGO9 pre-immune serum as a negative control, and 30 μl Protein A-Sepharose at 4 °C overnight. The immunoprecipitates were washed three times (15 min each) in extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 0.2% NP-40, 5 mM MgCl2, 5 mM dithiothreitol, one tablet of Roche Protease Inhibitor Cocktail for each 10 ml). Commercial columns (Ambion) were used to isolate sRNAs from the purified AGO9 complex. Small RNAs were resolved on a 12.5% denaturing PAGE 7 M urea gel, and stained with SYBR-gold (Invitrogen). Before cloning, gel slices within the range of 18–30 nucleotides were excised, and the RNAs were eluted and purified using DTR Gel Filtration Cartridges (EdgeBio). A detailed protocol of the immunoprecipitation and elution procedure is available on request.
After elution and gel-purification, sRNAs were ligated with adaptors at their 5′ and 3′ ends, converted to cDNA products, and subsequently cloned and sequenced by Sanger methods. Whereas immunopurifications conducted with the pre-immune serum did not yield any bacterial clones containing endogenous Arabidopsis sequences, we obtained a total 2,552 sequences representing 344 distinct small RNAs with the AGO9 antibody. Cloning of small RNAs was performed with the miRCat Small RNA Cloning Kit (Integrated DNA Technologies) following manufacturer instructions. Individually cloned products were sequenced with the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems) in a 3730xl DNA Analyzer (Applied Biosystems,). Sequences were quality-checked with sequence Scanner 1.0 (Applied Biosystems). Sequences were filtered and mapped to the Arabidopsis genome (http://www.arabidopsis.org). Annotation of sRNAs was performed using information from TAIR9 (ftp://ftp.arabidopsis.org/Sequences/blast_datasets/TAIR9_blastsets), and miRBase (http://microrna.sanger.ac.uk/sequences).
Supplementary Material
Acknowledgments
We thank Nidia Sánchez for sharing the pFM2 marker, E. Demunck for technical assistance during cloning and sequencing, S. Poethig, J. Carrington, T. Lagrange and the Arabidopsis Stock Center for providing mutants, J. Mendiola and C. Alvarez for help with genetic and bioinformatic analysis, R. Jorgensen for critically reading the manuscript, and an anonymous reviewer for suggestions. This work was supported by IRD-France and ANR (D.A., D.G.), NIH (R.K.S.) Consejo Nacional de Ciencia y Tecnología (V.O., N.D., M.A., E.D., J.P.V.), Consejo Estatal de Ciencia y Tecnología de Guanajuato (J.P.V.), and the Howard Hughes Medical Institute (J.P.V.).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions. J.P.V. and V.O. designed the research, V.O. generated the phenotypic analysis, performed the histological and expression analysis, and conducted the genetic experiments, N.D. designed the antibody and performed the immunoprecipitations and sRNA analysis, M.A. conducted the bioinformatic expression analysis, E.D. performed immunolocalization experiments, D.G. contributed pertinent ideas and performed immunolocalization experiments, K.S., and D.A provided unpublished materials, R.M contributed pertinent ideas J.P.V. wrote the paper.
References
- 1.Evans MM, Walbot V. Unique features of the plant life cycle and their consequences. Nat Rev Genet. 2003;4:369–379. doi: 10.1038/nrg1064. [DOI] [PubMed] [Google Scholar]
- 2.Maheswari P. An introduction to the embryology of the angiosperms. Published by McGraw-Hill Book Company; New York: 1950. [Google Scholar]
- 3.Sheridan WF, Avalkina NA, Shamrov II, Batygina TB, Golubovskaya IN. The mac1 gene: controlling the commitment to the meiotic pathway in maize. Genetics. 1999;153:933–941. doi: 10.1093/genetics/142.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nonomura K, Miyoshi K, Eiguchi M, Suzuki T, Miyao A, Hirochika H, Kurata N. The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell. 2003;15:1728–1739. doi: 10.1105/tpc.012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bicknell RA, Koltunow AM. Understanding apomixis: recent advances and remaining conundrums. Plant Cell. 2004;16(Suppl 1):S228–S245. doi: 10.1105/tpc.017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossniklaus U, Schneitz K. The molecular and genetic basis of ovule and megagametophyte development. Sem Cell Dev Biol. 1998;9:227–238. doi: 10.1006/scdb.1997.0214. [DOI] [PubMed] [Google Scholar]
- 7.Baumberger N, Baulcombe DC. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. PNAS. 2005;102:11928–11933. doi: 10.1073/pnas.0505461102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz DS, Hutvágner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 9.Pham JW, Pellino JL, Lee YS, Carthew RW, Sontheimer EJ. A Dicer-2-dependent 80s complex cleaves targeted mRNAs during RNAi in Drosophila. Cell. 2004;117:83–94. doi: 10.1016/s0092-8674(04)00258-2. [DOI] [PubMed] [Google Scholar]
- 10.Alonso JM, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 11.Takeda A, Iwasaki S, Watanabe T, Utsumi M, Watanabe Y. The mechanism selecting the guide strand from small RNA duplexes is different among argonaute proteins. Plant Cell Physiol. 2008;49:493–500. doi: 10.1093/pcp/pcn043. [DOI] [PubMed] [Google Scholar]
- 12.Webb MC, Gunning BES. Embryo sac development in Arabidopsis thaliana: Megasporogenesis, including the microtubular cytoskeleton. Sex Plant Reprod. 1990;3:244–258. [Google Scholar]
- 13.Huanca-Mamani W, Garcia-Aguilar M, León-Martínez G, Grossniklaus U, Vielle-Calzada JP. CHR11, a chromatin-remodeling factor essential for nuclear proliferation during female gametogenesis in Arabidopsis thaliana. PNAS. 2005;102:17231–17236. doi: 10.1073/pnas.0508186102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chitwood DH, Nogueira FT, Howell MD, Montgomery TA, Carrington JC, Timmermans MC. Pattern formation via small RNA mobility. Genes Dev. 2009;23:549–554. doi: 10.1101/gad.1770009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwab R, Maizel A, Ruiz-Ferrer V, Garcia D, Bayer M, Crespi M, Voinnet O, Martienssen RA. Endogenous tasiRNAs mediate non-cell autonomous effects on gene regulation in Arabidopsis thaliana. PLoS One. 2009;19:e5980. doi: 10.1371/journal.pone.0005980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voinnet O. Non-cell autonomous RNA silencing. FEBS Lett. 2005;579:5858–5871. doi: 10.1016/j.febslet.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 17.Peragine A, Yoshikawa M, Wu G, Albrecht HL, Poethig RS. SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes Dev. 2004;18:2368–2379. doi: 10.1101/gad.1231804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshikawa M, Peragine A, Park MY, Poethig RS. A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev. 2005;15:2164–2175. doi: 10.1101/gad.1352605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Himber C, Dunoyer P, Moissiard G, Ritzenthaler C, Voinnet O. Transitivity-dependent and -independent cell-to-cell movement of RNA silencing. EMBO J. 2003;22:4523–4533. doi: 10.1093/emboj/cdg431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosher RA, Melnyk CW, Kelly KA, Dunn RM, Studholme DJ, Baulcombe DC. Uniparental expression of PolIV-dependent siRNAs in developing endosperm of Arabidopsis. Nature. 2009;460:283–286. doi: 10.1038/nature08084. [DOI] [PubMed] [Google Scholar]
- 21.Wu MF, Tian Q, Reed JW. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development. 2006;133:4211–4218. doi: 10.1242/dev.02602. [DOI] [PubMed] [Google Scholar]
- 22.Slotkin RK, Vaughn M, Borges F, TanurdziĆ M, Becker JD, Feijó JA, Martienssen RA. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell. 2009;136:461–472. doi: 10.1016/j.cell.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X. Small RNAs and their roles in plant development. Annu Rev Cell Dev Biol. 2009;25:21–44. doi: 10.1146/annurev.cellbio.042308.113417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.C Klattenhoff C, Theurkauf W. Biogenesis and germline functions of piRNAs. Development. 2008;135:3–9. doi: 10.1242/dev.006486. [DOI] [PubMed] [Google Scholar]
- 25.Vielle-Calzada JP, Baskar R, Grossniklaus U. Delayed activation of the paternal genome during seed development. Nature. 2000;404:91–94. doi: 10.1038/35003595. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqi I, Ganesh G, Grossniklaus U, Subbiah V. The dyad gene is required for progression through female meiosis in Arabidopsis. Development. 2000;127:197–207. doi: 10.1242/dev.127.1.197. [DOI] [PubMed] [Google Scholar]
- 27.Qi Y, Denli AM, Hannon GJ. Biochemical specialization within Arabidopsis RNA silencing pathways. Mol Cell. 2005;19:421–428. doi: 10.1016/j.molcel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC. Genetic and functional diversification of RNA pathways in plants. PLoS Biol. 2004;2:e104. doi: 10.1371/journal.pbio.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie Z, Allen E, Wilken A, Carrington JC. DICER-LIKE 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2005;102:12984–12989. doi: 10.1073/pnas.0506426102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vielle-Calzada JP, Thomas J, Spillane C, Coluccio A, Hoepner Ma, Grossniklaus U. Maintenance of genomic imprintimg at the Arabidopsis medea locus requires zygotic DDM1 activity. Genes & Development. 1999;13:2971–2982. doi: 10.1101/gad.13.22.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García-Aguilar M, Dorantes-Acosta A, Pérez-España V, Vielle-Calzada JP. Whole-Mount in situ mRNA localization in developing ovules and seeds of Arabidopsis. Plant Molecular Biology Reporter. 2005;23:1–11. [Google Scholar]
- 32.Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN. MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell. 2005;17:2981–2992. doi: 10.1105/tpc.105.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bass HW, Riera-Lizarazu O, Ananiev EV, Bordoli SJ, Rines HW, Phillips RL, Sedat JW, Agard DA, Cande WZ. Evidence for the coincident initiation of homolog pairing and synapsis during the telomere-clustering (bouquet) stage of meiotic prophase. J Cell Sci. 2000;Pt 6:1033–42. doi: 10.1242/jcs.113.6.1033. [DOI] [PubMed] [Google Scholar]
- 34.Qi Y, Denli AM, Hannon GJ. Biochemical specialization within Arabidopsis RNA silencing pathways. Mol Cell. 2005;19(3):421–8. doi: 10.1016/j.molcel.2005.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


