SUMMARY
Background
In a recent double-blinded clinical trial the probiotic combination of L-NCFM and B-LBi07 reduced bloating symptoms in patients with functional bowel disorder; an effect more evident in those who reported abdominal pain. In mice, L-NCFM but not B-LBi07 induced colonic MOR and CB2 expression and reduced visceral sensitivity.
Aims
To determine if L-NCFM was the active component in the clinical trial and to investigate the mechanism of action in humans with mild to moderate abdominal pain.
Methods
Caucasian women (n=20) 18-70 years with mild to moderate abdominal pain were enrolled in a double-blind, two-armed, single-center study. Patients were given either L-NCFM alone or in combination with B-LBi07 for 21 days at a total dose of 2.0E10 CFU BID. Colonic biopsies were collected during un-sedated, un-prepped flexible sigmoidoscopy before and at the end of probiotic consumption. mRNA and immunostaining were then performed on these biopsies. Patients kept symptom diaries for the 7 days prior to starting probiotic therapy and for the last 7 days of therapy.
Results
L-NCFM alone, but not with B-LBi07, induced colonic MOR mRNA and protein expression, as well as downstream signaling, as measured by enterocyte STAT3-phosphorylation. In contrast, CB2 expression was decreased. Both treatment groups trended towards improvement in symptoms but the study was insufficiently powered to draw meaningful conclusions.
Conclusions
L-NCFM modulates MOR expression and activity, while the combination of L-NCFM and B-LBi07 does not. This study provides a possible mechanism of action by which probiotics modulates pain sensation in humans.
Clinical Trial Number
clinicaltrials.gov ID (NCT#): NCT01064661
Keywords: abdominal pain, probiotics, lactobacillus, opioid receptors
INTRODUCTION
The intestinal microbiota has an important role in sustaining gastrointestinal (GI) function in healthy individuals.1 This has provided opportunities for targeting the intestinal microbiota by dietary interventions and/or microbial modifications aiming to improve GI symptoms in diseases associated with disrupted intestinal microbiota, e.g., Inflammatory Bowel Diseases (IBD) 2-5 and Functional Bowel Disorders (FBD),6-8 including Irritable Bowel Syndrome (IBS).9 However, the preferred probiotic intervention for specific conditions and the mechanism/s by which probiotics exert/induce their beneficial effects is still unclear.
L-NCFM is a lactobacillus strain commercially available in the United States since the 1970s that has been investigated for numerous beneficial effects,10 including decreased incidence of pediatric diarrhea10 and enhancement of regulatory T-cell function in the T-cell transfer model of murine colitis.11 Data from animal studies suggest that daily consumption of the probiotic bacteria L-NCFM at 2×1010 CFU/day can increase expression of mu opioid receptor (MOR) and cannabinoid receptor 2 (CB2) in the intestinal mucosa and that these mucosal effects are associated with decrease in intestinal pain sensation.12 In addition, in a randomized controlled clinical trial in patients with non-constipation functional bowel disorders we have recently found that a blend of the probiotic bacteria L-NCFM and B-LBi07 BID (2×1011 CFU total probiotic bacteria per day) for 8 weeks significantly improved abdominal bloating and distention symptoms in the probiotics group (N=30) compared to the placebo group (N=27).13 Furthermore, secondary analyses of our data show that this effect was even more evident in the subgroup of patients who also reported abdominal pain. 13 This data suggest that the beneficial effect of these probiotic bacteria may be mediated through their effect on intestinal sensation mechanisms. Due to the limited available effective treatments for functional abdominal pain, as well as the recent findings regarding the beneficial effect of L-NCFM in both mice and in our human clinical trial, we conducted a pilot study in humans to investigate whether daily consumption of the probiotic bacteria L-NCFM alone or in combination with B-LBi07 is associated with increased expression of intestinal colonic mucosal MOR and/or CB2 pain receptors in subjects with functional abdominal pain.
MATERIALS AND METHODS
Study Population
Caucasian women 18-70 years with active mild to moderate abdominal pain (having pain in at least 3 of the 10 days at a level between 3 and 7 on a 10 point Likert scale (0=no pain through 10=very severe pain) were investigated. Subjects were excluded if they had inflammation or structural abnormality of the digestive tract, a serious or unstable medical condition (e.g., uncontrolled blood pressure, thyroid function, insulin dependent diabetes mellitus), major psychiatric diagnosis, lactase deficiency, predisposition to infection, were pregnant or lactating, received antibiotic treatment or intentionally consumed probiotics during the 4 weeks prior to enrollment, or had taken analgesics or anti-inflammatory medications in the 10 days prior to recruitment. Patients were recruited from the Chapel Hill general population by advertisement. All subjects sign an informed consent and the study protocol was approved by the UNC Institutional Review Board.
Study Design
This was a double-blind, randomized two armed, single-center study. Study involved a two week run-in phase and a four week intervention phase and included three visits (Figure 1). At the screening visit, subjects completed study questionnaires to verify eligibility and collect sociodemographic information. Subjects filled out daily diaries during the last seven days of the run-in period. At the first trial visit biological specimens were collected and subjects were randomized into one of two arms to receive either the probiotic Lactobacillus acidophilus NCFM (L-NCFM) alone BID (2×1010 CFU total bacteria per day) or a dual probiotic blend, L-NCFM and Bifidobacterium lactis Bi-07 (B-LBi07) BID (2×1010 CFU total bacteria per day) (Danisco Inc. Madison, Wisconsin, USA). Randomization was carried by the UNC Investigational Drug Service (IDS) by random numbers. Subjects and investigators were blinded throughout the study. Subjects had a daily diary to fill out from days 21-30 of the intervention and returned for a second trial visit at the end of the intervention period, for repeated questionnaires and biological specimen collection.
Figure 1. Study design.
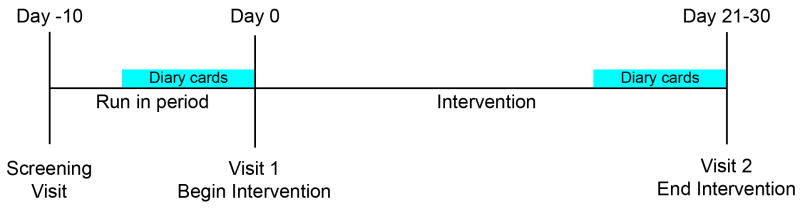
A randomized, double blind pilot study with a 7 days run-in phase and a 30 day single or dual probiotic intervention phase. Patients completed a 7-day symptom diary during the run-in period and during the last 7 days of the intervention period. Mucosal biopsies were taken from unprepped colon pre and post probiotic interventions.
Data and Sample Collection
Colonic mucosal samples were collected at baseline (study visit 1) and at the end of intervention (study visit 2). Mucosal biopsies were obtained with cold biopsy forceps from the recto-sigmoid colon 15 to 20cm above the anal verge. To avoid any possible effect of intestinal preparation on the intestinal immune function, all samples were collected during an un-prepped flexible sigmoidoscopy. Biopsies were collected for routine histology and for mRNA analysis of the opioid receptor genes MOR and CB2. Biopsies were either frozen immediately at -80°C or placed in 1 mL of RNAlater™ for 24 hours and then frozen at -80°C for subsequent immunohistochemistry and RT-PCR analysis, respectively.
Real-time polymerase chain reaction (RT-PCR)
Total RNA was isolated from mucosal biopsies using the Rneasy kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions and treated with RNase-free DNase I (Roche Diagnostics Corporation, Indianapolis, IN). 500 ng of RNA was subjected to a reverse transcription reaction to form cDNA, and real-time PCR was then performed as previously described.14 Relative fold-changes were determined using the ΔCT or ΔΔCT calculation method as previously described.14 The primer sequences used were as follows: ACTB15 : F:5’-TCACCCACACTGTGCCCATCTACG -3’ and 5’-CAGCGGAACCGCTCATTGCCAATG -3’; OPRM115 : F: 5’-ATGCCAGTGCTCATCATTAC -3’ and R: 5’-GATCCTTCGAAGATTCCTGTCCT -3’; CB2: F: 5’-GCTAAGTGCCCTGGAGAACGT -3’ and R: 5’- TCAGCCCCAGCCAAGCT -3’.
Histology and Immunostaining
Intestinal biopsies from un-prepped flexible sigmoidoscopy samples from each patient pre- and post-treatment with probiotics were flushed with ice-cold PBS, fixed in 10% formalin for 24 hrs, and then embedded in paraffin. IHC staining for pSTAT3 (Y705) (Cell Signaling Technology Inc.; Beverly, MA, reactive to mouse, human, monkey, and rat) was performed according to the manufacturer’s specifications, at a 1:50 dilution as previously described16. Immunostaining for MOR and CB2 were performed as follows: sections were deparaffinized using Safeclear® (Fisher Protocol, Fair Lawn, NJ) for 20 minutes and rehydrated under serial ethanol concentrations. After permeabilization during 5 min in PBS containing 0.1 % triton X-100 at 4°C, sections were incubated for 15 min with 1.5 % goat normal serum and 15 min with blocking buffer (1% BSA in milk) to minimize non-specific adsorption of the antibodies. The tissues were subsequently incubated with the rabbit polyclonal primary antibody directed against human MOR (1:100, Tebu-bio, Le-Perray-en-Yvelines, France) or human CB2 (1:10, Alpha Diagnostic, San Antonio, CA) overnight at 4°C . Sections were then rinsed in 0.1M PBS containing 0.05% triton X-100 and incubated in secondary antibody for 1 h at room temperature with Alexa 488 goat anti-rabbit IgG conjugated to FITC fluorochrome (dilution 1:100; Dako Laboratories, Carpinteria, CA). Then slides were counterstained with Hoescht solution (0.125 mg/mL) and mounted with ProLong Gold antifade reagent (Invitrogen).Negative controls consisted of staining with normal rabbit serum instead of specific antibody. Immunofluorescence was visualized under a fluorescence microscope (Leica, Bensheim, Germany). Positive-staining epithelial cells were quantified by eye in all captured images in a blinded fashion.
Statistical Analysis
Two-group analysis of RT-PCR data was performed using non-parametric t-tests (Mann-Whitney). IF analysis was performed using standard two-tailed t-tests. Comparison of clinical outcomes was performed using standard, two-tailed t-tests. Correlation analysis was by Spearman’s non-parametric correlation.
RESULTS
A total of 20 Caucasian women mean age 36.6 were investigated. Ten subjects (mean age 28.9 years; mean bloating severity score 5.80; mean pain 4.0; and mean IBS-SSS score 304) received the combined intervention with L-NCFM and B-LBi07 and 10 subjects (mean age 43.6 years; mean bloating severity score 5.70; mean pain 3.0; and mean IBS-SSS score 283) received only L-NCFM.
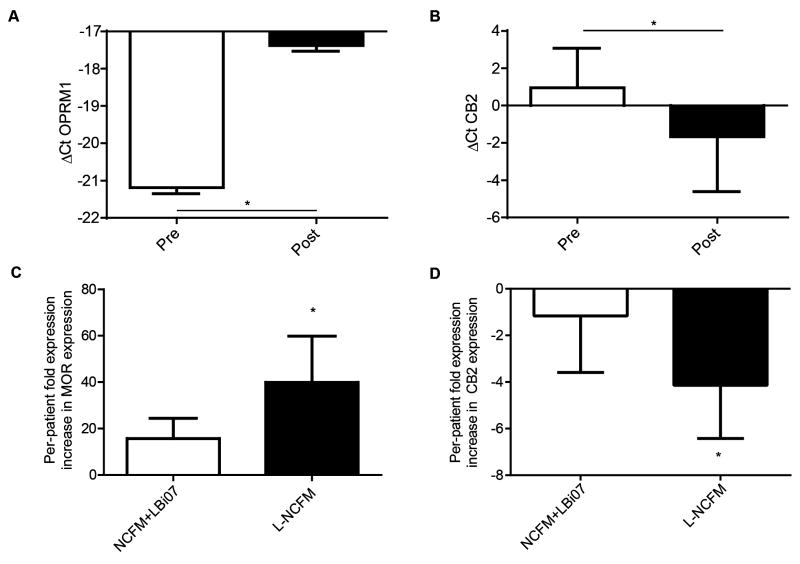
Real-time PCR analysis of pre- to post-probiotic intervention with L-NCFM and B-LBi07 or L-NCFM alone was associated with an increase in ΔCt of 3.79, demonstrating mu-opioid receptor (MOR)induction (13.8-fold, Figure 2A); in contrast, pre- to post-intervention Cannabinoid 2 (CB2) expression was found to be slightly (~2-fold) decreased (Figure 2B). Additionally, analysis of MOR expression on a per-patient-basis showed that patients treated with L-NCFM alone had a 39.9-fold pre- to post-intervention increase in the mRNA levels of the receptor while patients treated with both probiotics did not have a statistically significant increase in MOR expression (Figure 2C). Similarly, the decrease in CB2 mRNA was driven primarily by a ~4-fold decrease in expression in the NCFM group, while the dual-probiotic group showed no significant change in expression (Figure 2D). To further validate our RT-PCR findings we looked at MOR expression using immunofluorescence staining in the colonic mucosal samples of patients before and after receiving L-NCFM. Quantification of the number of positive-staining enterocytes found a significant change in mucosa-associated MOR staining (~2-fold, from 19.8 to 39.4, P = 0.0231, n = 4/group) after treatment with L-NCFM but not with dual probiotic therapy (P =0.4824) (Figure 3A&B).
Figure 2. Probiotic therapy in human patients induces increased levels of hMOR mRNA in human colonic mucosa, driven by L-NCFM.
A&B) Comparison by real-time PCR of expression levels pre- and post- treatment with one or both probiotics. For analysis, results from both the single- and dual-probiotic arms were pooled, and were performed without tracking per-patient changes. A) Real-time PCR shows an increase in the ΔCt and thus expression of hMOR mRNA in patients with abdominal pain receiving either one or both probiotics; -21.44 vs -17.65, P=0.0349, N=14/group. B) Real-time PCR shows a decrease in the ΔCt and thus expression of hCB2 mRNA in patients receiving either one or both probiotics; 1.287 vs. -2.396, P=0.0190, N=13/group.C) Per-patient increase in hMOR mRNA expression after treatment with one or both probiotics showed a 39.9-fold induction in patients given L-NCFM alone (P =0.0313). No significant induction was seen in patients given both L-NCFM and B-LBi07. D) Per-patient decrease in CB2 mRNA expression after treatment with L-NCFM alone showed a 4.15-fold decreased in the L-NCFM group (P=0.032). No change in expression was seen in patients given both probiotics.
Figure 3. Increase in levels of protein expression in human colonic mucosa by L-NCFM.
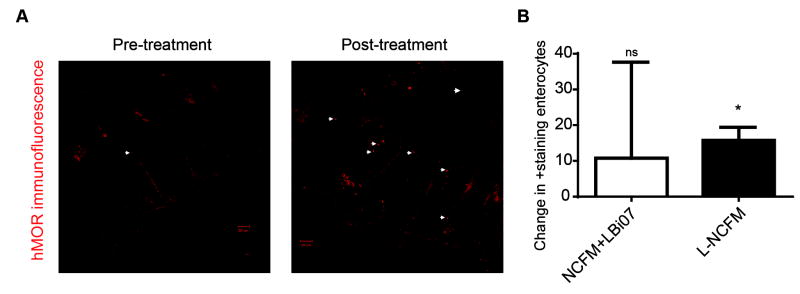
A) Immunofluorescence of colonic mucosa samples of patients given L-NCFM alone showing increased MOR expression of colonic-mucosa. Representative of 4 independent experiments. White arrow heads point to colonic-mucosa-associated MOR. Scale bars equal 50 μm. B) Bar graph shows change in positive staining enterocytes after treatment with one or both probiotics; only the L-NCFM showed a statistically significant induction; 2.17-fold, P=0.0231, n=4/group.
Together, these data demonstrate that in patients with mild to moderate abdominal pain, treatment with the probiotic L-NCFM results in increased expression of colonic MOR. However, this finding alone does not demonstrate if there is a change in downstream signaling events resulting from the increased expression of the receptor. To address this, we measured phosphorylation of mucosal STAT3 (known to be downstream of enterocyte-MOR activation)14, using samples from patients before and after treatment with L-NCFM. We found that post-treatment, patients given L-NCFM had increased levels of colonic and stromal pSTAT3 staining compared to before treatment (Figure 4).
Figure 4. Increase in post-receptor downstream signaling in human colonic mucosa by L-NCFM.
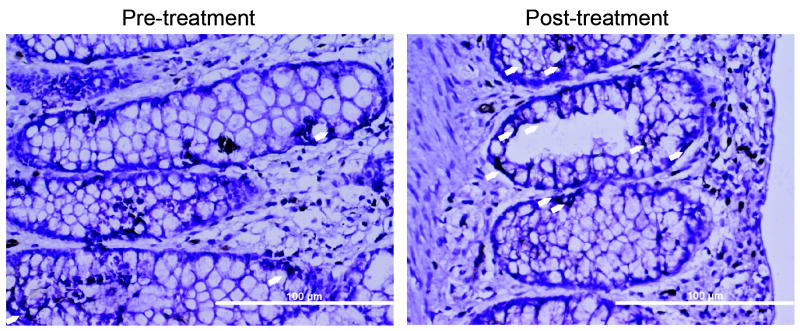
Immunostaining is for pSTAT3 (Y705), with staining indicated by white arrow heads. Representative of 4 patients. Scale bars are 100 μm. Mean pre- to post-intervention increase in staining was 18 more cells after treatment (19.8 to 39.4 post-treatment, P=0.0469, n=4).
Although this study was designed to look at the effect(s) of the probiotic interventions on the mucosal opioid receptor gene and was not powered to investigate potential clinical effects, we still performed analysis to see if there were any pre- to post-intervention changes in the clinical outcomes we measured and if changes in any of these clinical outcomes correlated with changes in MOR expression. Both intervention groups tended to have improvement in several clinical measures following probiotic interventions including in FBD symptoms severity scores (IBS-SSS)17, bloating severity, number of daily bowel movements (BMs), daily wellbeing, and number of days with abdominal pain (Table 1). Interestingly, the L-NCFM-alone group did have a statistically significant decrease in the number of daily BMs, while this was not observed in the dual-probiotic group. There were no significant differences in clinical outcomes between the two treatment groups (Table 2). Analysis on the entire study population (including patients from both interventional arms) did not reveal significant correlations between MOR mRNA levels and/or changes in probiotic-mediated MOR induction after therapy and clinical outcomes measures (data not shown).
Table 1. Clinical outcome measures after probiotic administration.
Analysis by paired t-tests.
| Outcome | NCFM+B-LBi07 (N = 10) | NCFM (N = 7) | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| IBS-SSS | 304 | 182** | 283 | 173 |
| Bloating severity | 5.80 | 3.30* | 6.29 | 2.57 (P=0.1) |
| Number of daily BMs | 1.29 | 1.40 | 1.61 | 1.44* |
| Daily wellbeing | 3.28 | 3.61 | 3.04 | 3.38 |
| Number of days with abdominal pain | 7.00 | 3.80* | 4.57 | 3.86 |
Significant differences (P<0.05) pre to post intervention are in italics, with * = P<0.05,
P <0.01,
P<0.001
Table 2. Clinical outcome measure comparisons between treatment groups.
Values are reported as mean±SEM. Analysis is by two-tailed t-test
| Outcomes (Post-Pre) | NCFM+B-LBi07 | NCFM | P-value |
|---|---|---|---|
| IBS-SSS | -122±33 | -100±36 | 0.81 |
| Bloating severity | -2.5±0.9 | -3.7±0.8 | 0.36 |
| Number of daily BMs | 0.11±0.15 | -1.7±0.07 | 0.35 |
| Daily wellbeing | 0.33±0.16 | 0.34±0.17 | 0.99 |
| Number of days with abdominal pain | -3.2±1.1 | -0.71±0.27 | 0.15 |
DISCUSSION
Our study has several strengths: the design included a seven day run-in period that enabled careful selection of study subjects, we focused on abdominal pain as the primary clinical outcome, and the pre- and post- intervention mucosal samples were collected from un-prepped colons.
Looking at the physiological data, we found that administration of L-NCFM but not the combination of L-NCFM and B-LBi07 induced increased levels of MOR mRNA expression. We were also able to establish that induction of MOR mRNA was correlated with enhanced protein expression by IHC, as well as STAT3 downstream enterocyte signaling, which is not always the case.18, 19 Interestingly, our study found that administration of L-NCFM alone was associated with decreased CB2 expression, unlike what was seen in mice, while there was no significant change in CB2 expression in patients given both probiotics. The lack of effect on MOR mRNA expression and STAT3 signaling in the group receiving the probiotic combination can be explained by the lower dose of L-NCFM in this group or by a possible competitively inhibiting effect of B-LBi07 on colonization or the physiologic effect of the bio-active L-NCFM strain.12
Previous clinical work established that administration of L-NCFM and B-LBi07 improved bloating symptoms, particularly in patients with abdominal pain13.The goal of our study was to investigate the physiological effects of L-NCFM and B-LBi07 and it was not powered to investigate potential clinical effects. However, we did find that both intervention groups tended to improve in several clinical measures following probiotic interventions. The statistically significant decrease in the number of daily bowel movements in the L-NCFM group is consistent with our physiological findings since the L-NCFM-alone group was the only treatment group to show an induction of MOR, which is known to suppress gut motility20.Together with the effects of L-NCFM on MOR expression our physiological findings suggest that L-NCFM is the primary inducer of physiological changes as related to mucosal opioid receptor expression and signaling.
Interestingly, while the clinical outcome data suggests similar outcomes for single and dual-probiotic therapy, the physiological data showed significant differences between the two groups. This could suggest that MOR induction is not the only explanation for the beneficial effects of the probiotic intervention. Indeed, recent studies have shown that certain probiotics can affect brain activity in regions related to central processing of visceral sensation21 and that certain drugs can prevent opioid-induced bowel dysfunction without interfering with analgesia.22 It is also possible that any enhancement of clinical outcomes by the L-NCFM-alone group compared to the dual probiotic group were masked by the low power of the study. Nevertheless, our study findings provides a solid basis for pursuing further clinical trials to investigate the clinical relevance of the L-NCFM-induced increase in MOR expression and the clinical efficacy of L-NCFM in treating abdominal pain. Future studies should consider longer period of intervention and including another arm with B-LBi07 alone to help clarify the significance of enhancing the mu-opioid receptor and diminishing the cannabinoid receptors.
Another implication of this work is the potential beneficial effect of L-NCFM in patients with IBD. Studies in zebrafish23 and mice14, 24 have demonstrated that activation of MOR signaling is protective against intestinal injury and helps restore the epithelial barrier. This property of MOR signaling has recently been shown to be of clinical benefit in a case report25 of a patient with immunosuppressive IBD. Furthermore, a recent murine study has shown that L-NCFM is protective against T-cell transfer colitis in mice,11 a model known to be ameliorated by activation of MOR signaling.26 Thus, L-NCFM-mediated induction of MOR could be an excellent modality to induce targeted, colonic epithelial MOR in human patients for the treatment of IBD, warranting further clinical studies outside of the scope of the work presented here focusing on abdominal pain.
In conclusion, we replicated in humans the animal model observation of increased MOR mRNA expression and demonstrated that this increase in MOR mRNA expression is associated with increased mucosal protein expression and post-receptor STAT3 activation. Our data provides the first evidence for a probiotic effect on opioid-mediated pathways in humans. The clinical implications of these physiological findings require further investigation.
Acknowledgments
FUNDING
Y.R acknowledges support from the NIHK23 DK075621, RO3 DK084294, and research/educational support from Danisco Inc. to UNC. C.J acknowledges support from the NIH RO1DK047700 and RO1DK073338. J.G acknowledges support from the NIH F30DK085906. T.R.K acknowledges research/educational support from Danisco Inc. to UNC.
List of Abbreviations
- B-LBi07
Bifidobacterium lactis Bi-07
- CB2
cannabinoid receptor 2
- FBD
Functional Bowel Disorders
- IBD
Inflammatory bowel diseases
- L-NCFM
Lactobacillus acidophilus NCFM
- MOR
Mu opioid receptors
- STAT3
Signal Tranducer and Activator of Transcription 3
- UNC
University of North Carolina
Footnotes
Authors’ Contributions
TRK – study design, funding acquisition, implementation, interpretation of results and preparation of manuscript
JG – molecular techniques, data analysis, interpretation of results and preparation of manuscript
IMC – molecular techniques, data analysis, interpretation of results and preparation of manuscript
SPB – IHC analysis, interpretation of results and input to the manuscript
OP – statistical analysis, interpretation of results and input to the manuscript
CJ – interpretation of results and input to the manuscript
YR – study design, funding acquisition, implementation, and interpretation of the results and preparation of manuscript
COMPETING INTERESTS/DISCLOSURE
J.G is a paid technical consultant for Protagonist Therapeutics (Milpitas, CA).
The other authors declare that they have no competing interests.
References
- 1.Backhed F, Fraser CM, Ringel Y, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell host & microbe. 2012;12(5):611–22. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Bibiloni R, Fedorak RN, Tannock GW, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100(7):1539–46. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 3.Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104(2):437–43. doi: 10.1038/ajg.2008.118. [DOI] [PubMed] [Google Scholar]
- 4.Whelan K, Quigley EM. Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29(2):184–9. doi: 10.1097/MOG.0b013e32835d7bba. [DOI] [PubMed] [Google Scholar]
- 5.Sartor RB. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology. 2008;134(2):577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 6.Saulnier DM, Ringel Y, Heyman MB, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut microbes. 2013;4(1):17–27. doi: 10.4161/gmic.22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringel Y, Ringel-Kulka T. The rationale and clinical effectiveness of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2011;45(Suppl):S145–8. doi: 10.1097/MCG.0b013e31822d32d3. [DOI] [PubMed] [Google Scholar]
- 8.Ringel-Kulka T. Targeting the intestinal microbiota in the pediatric population: a clinical perspective. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2012;27(2):226–34. doi: 10.1177/0884533612439895. [DOI] [PubMed] [Google Scholar]
- 9.Ringel Y, Maharshak N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2013;305(8):G529–41. doi: 10.1152/ajpgi.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders ME, Klaenhammer TR. Invited review: the scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. Journal of dairy science. 2001;84(2):319–31. doi: 10.3168/jds.S0022-0302(01)74481-5. [DOI] [PubMed] [Google Scholar]
- 11.Petersen ER, Claesson MH, Schmidt EG, et al. Consumption of probiotics increases the effect of regulatory T cells in transfer colitis. Inflamm Bowel Dis. 2012;18(1):131–42. doi: 10.1002/ibd.21709. [DOI] [PubMed] [Google Scholar]
- 12.Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13(1):35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 13.Ringel-Kulka T, Palsson OS, Maier D, et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011;45(6):518–25. doi: 10.1097/MCG.0b013e31820ca4d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldsmith JR, Uronis JM, Jobin C. Mu Opioid Signaling Protects Against Acute Murine Intestinal Injury in a Manner Involving Stat3 Signaling. Am J Pathol. 2011;179(2):673–683. doi: 10.1016/j.ajpath.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philippe D, Chakass D, Thuru X, et al. Mu opioid receptor expression is increased in inflammatory bowel diseases: implications for homeostatic intestinal inflammation. Gut. 2006;55(6):815–823. doi: 10.1136/gut.2005.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS ONE. 2009;4(6):e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 18.Law P-Y, Wong YH, Loh HH. Molecular Mechanisms and Regulation of Opioid Receptor Signaling. Annual Review of Pharmacology and Toxicology. 2000;40(1):389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- 19.Pan Y-X. Diversity and Complexity of the Mu Opioid Receptor Gene: Alternative Pre-mRNA Splicing and Promoters. DNA and Cell Biology. 2005;24(11):736–750. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- 20.Bueno L, Fioramonti J. Action of opiates on gastrointestinal function. Baillieres Clin Gastroenterol. 1988;2(1):123–39. doi: 10.1016/0950-3528(88)90024-3. [DOI] [PubMed] [Google Scholar]
- 21.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–401. 1401e1–4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Current opinion in anaesthesiology. 2010;23(5):616–22. doi: 10.1097/ACO.0b013e32833c3473. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith JR, Cocchiaro JL, Rawls JF, Jobin C. Glafenine-induced intestinal injury in zebrafish is ameliorated by mu-opioid signaling via enhancement of Atf6-dependent cellular stress responses. Dis Model Mech. 2012;6(1):146–59. doi: 10.1242/dmm.009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldsmith JR, Perez-Chanona E, Yadav PN, Whistler J, Roth B, Jobin C. Intestinal epithelial cell-derived mu-opioid signaling protects against ischemia reperfusion injury through PI3K signaling. Am J Pathol. 2013;182(3):776–85. doi: 10.1016/j.ajpath.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldsmith JR, Kelly M, Freeman KB, Duro D. Combined Restitutive Therapy For Treatment of Immunosuppressive Refractory Crohn's Disease. Journal of pediatric gastroenterology and nutrition. 2013 doi: 10.1097/MPG.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philippe D, Dubuquoy L, Groux H, et al. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. The Journal of clinical investigation. 2003;111(9):1329–38. doi: 10.1172/JCI16750. [DOI] [PMC free article] [PubMed] [Google Scholar]