Abstract
Since brown adipose tissue (BAT) dissipates energy through UCP1, BAT has garnered attention as a therapeutic intervention for obesity and metabolic diseases including type2 diabetes. As we better understand the physiological roles of classical brown and beige adipocytes, it is becoming clear that BAT is not simply a heat-generating organ. Increased beige fat mass in response to a variety of external/internal cues is associated with significant improvements in glucose and lipid homeostasis that may not be entirely mediated by UCP1. We aim to discuss recent insights regarding the developmental lineages, molecular regulation, and new functions for brown and beige adipocytes.
Keywords: Brown adipose tissue, brown adipocytes, beige adipocytes, obesity, diabetes, insulin resistance
Introduction
Thermogenic adipocytes and Ucp1
Brown and beige adipose cells have the capacity to burn glucose and fat to produce heat. This thermogenic function is mediated, in large part, by the action of Uncoupling Protein-1 (UCP1), a polypeptide that resides in the inner mitochondrial membrane of brown and beige adipocytes. UCP1, when activated, dissipates the proton gradient generated by the electron transport chain. This futile cycle of proton pump and leak reduces mitochondrial membrane potential which, in turn, drives high levels of substrate oxidation and results in the generation of heat (Cannon and Nedergaard, 2004; Lowell and Spiegelman, 2000).
Brown adipose cells develop in dedicated deposits of brown adipose tissue (BAT). In mice, the large BAT depots, including the interscapular, axillary and cervical BAT develop prenatally and provide a source of heat to protect newborns against cold-exposure. The sympathetic nervous system (SNS) is intimately involved in regulating both the growth of BAT and its thermogenic function. Brown adipocytes are innervated by sympathetic fibers, which upon cold-exposure, release norepinephrine (NE) to acutely activate thermogenesis (Cannon and Nedergaard, 2004). NE also elicits a signaling cascade via P38 MAPK and PGC-1α to increase the transcription of thermogenic genes in brown adipocytes; this allows for sustained thermogenesis during longer term cold exposure (Cao et al., 2004). Finally, cold exposure stimulates BAT expansion through activating the proliferation and differentiation of brown adipose precursor cells (Bronnikov et al., 1992; Geloen et al., 1988). Another notable feature of BAT is its dense capillary bed that supplies adipocytes with substrate and oxygen for oxidation and enables the efficient distribution of heat to the rest of the body.
Brown-like adipocytes that have a multilocular morphology and express UCP1 can also be found in white adipose tissue (WAT) depots (Cinti, 1999; Collins et al., 1997; Guerra et al., 1998; Himms-Hagen et al., 2000; Vitali et al., 2012; Young et al., 1984). These so-called “beige” (Ishibashi and Seale, 2010) or “brite” adipocytes (Petrovic et al., 2010) are only readily detected in the WAT of animals that have been exposed to cold or other inducers. This dependency on external stimuli for UCP1 induction is a distinctive feature of beige fat. By contrast, brown fat cells express relatively high amounts of UCP1 even under non-stimulated conditions. This difference is, at least, partly fat cell-autonomous since preadipocytes from BAT differentiate ex vivo into adipocytes that express UCP1 and have high levels of mitochondria; under the same conditions beige fat precursors undergo adipocyte conversion but do not activate the brown fat program unless they are treated with certain inducers, such as β-adrenergic agonists or PPARγ activators (Klaus et al., 1995; Ohno et al., 2012; Petrovic et al., 2010; Wu et al., 2012). Notably, fully stimulated beige adipocytes express similar UCP1 levels as brown adipocytes and undergo UCP1-mediated uncoupled respiration (Long et al., 2014; Okamatsu-Ogura et al., 2013; Shabalina et al., 2013; Wu et al., 2012).
The induction of beige adipocytes is highly adipose depot-dependent. In mice, the subcutaneous inguinal WAT undergoes the most profound induction of beige adipocytes, whereas the epididymal WAT of male mice is particularly resistant to “beige-ing” (Ohno et al., 2012; Vitali et al., 2012). There also exists a great deal of variability in the beige-ing response amongst inbred strains of mice as first reported by Collins et al. (Collins et al., 1997). Kozak and colleagues used classical genetic approaches to study this trait in great detail and identified several loci that affect UCP1 induction in white fat (Guerra et al., 1998; Koza et al., 2000; Xue et al., 2007). The genetic variability controlling UCP1 activation was only observed in white fat, providing some of the earliest evidence that beige and brown fat cells may belong to different lineages (see later). Importantly, the induction of UCP1 in white fat (i.e., beige-ing of WAT) is associated with a reduction in obesity in animals treated with the β3-adrenergic agonist, CL 316,243 (Guerra et al., 1998). Thus, the capacity for UCP1 induction in white fat is strongly correlated with obesity-reduction caused by β3-agonists.
Human BAT
Do humans have thermogenic brown and/or beige fat? And if so, do these tissues affect systemic metabolism in a meaningful way? Through the use of 18F-fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography computed tomography (18F-FDG-PET) imaging, it is now evident that humans have substantial depots of UCP1+ adipose cells and that these tissues are activated to take up glucose by cold or β-adrenergic agonist-treatment (Cypess et al., 2014; Cypess et al., 2009; Cypess et al., 2015; Nedergaard et al., 2007; Saito et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). In adult humans, the supraclavicular region appears to be the most enriched with UCP1+ adipocytes. While 18F-FDG-PET imaging relies on glucose uptake capacity, and may not necessarily reflect BAT mass per se, there are higher amounts of detectable BAT in young and lean subjects. This suggests that variation in BAT activity could participate in the natural regulation of body weight. Reduced BAT activity could also be a consequence of obesity and/or insulin resistance.
Various marker genes/proteins that are selectively expressed in mouse brown versus beige fat cells have been identified. The expression levels of these genes have been used as a way to classify human UCP1+ adipose depots as brown or beige. Human infants have a thin layer of interscapular BAT that shares morphological and molecular features with rodent brown fat (Heaton, 1972; Lidell et al., 2013a). This infant interscapular BAT expresses genes that are characteristic of classical brown adipocytes (Lidell et al., 2013b). In adult humans, BAT depots are generally heterogeneous, containing multiple cell types including UCP1 positive and negative adipocytes (Cypess et al., 2009; Cypess et al., 2013; Virtanen et al., 2009). At the molecular level, supraclavicular BAT possesses a molecular signature that closely resembles mouse beige adipocytes (Lee et al., 2014c; Sharp et al., 2012; Shinoda et al., 2015; Wu et al., 2012). Notably, global expression analyses of clonally-derived brown adipocytes indicate that adult human BAT depots in the supraclavicular region are largely composed of beige-like adipocytes (Shinoda et al., 2015). However, other depots, including those from the cervical and perirenal regions contain adipocytes that express classical brown markers like ZIC1 and LHX8 (Cypess et al., 2013; Nagano et al., 2015; Xue et al., 2015). In summary, the FDG-PET+ depots are heterogeneous with some composed mostly of beige-like cells while others resembling classic brown fat. In this review, we refer to the FDG-PET+ and UCP1+ human adipose depots collectively as BAT.
Of note, BAT activity is increased after prolonged cold exposure in the supraclavicular region of adult humans who had previously lacked detectable BAT depots before treatment (Lee et al., 2014b; van der Lans et al., 2013; Yoneshiro et al., 2013). Given the inducible nature of rodent beige adipocytes, it seems likely that cold can similarly promote beige fat biogenesis within these adult human depots. However, again, since these studies utilized FDG-PET, which measures glucose uptake, it will be important to determine the cellular and molecular changes in these tissues before and after chronic cold. Interestingly, it has also been shown that prevalence of FDG-PET+ human BAT is lower in elderly populations (Yoneshiro et al., 2011). This may be analagous to the reduction in beige fat mass that occurs in aging mice (Rogers et al., 2012). It will now be important to determine how aging suppresses beige and/or brown fat recruitment.
1. Developmental Lineages of Brown and Beige Adipocytes
The major classical BAT depots in mice, including the interscapular, cervical and axillary depots, are interspersed in and around deep back (epaxial) muscles and develop before WAT during embryogenesis. Most of the adipocytes in these tissues originate from precursors in the somites that also give rise to skeletal myocytes, dorsal dermis as well as a subset of white adipocytes in certain depots. The somitic multipotent precursor population is marked by the expression of certain transcription factors, including Pax7, Engrailed-1 (En1) and the myogenic factor, Myf5 (Atit et al., 2006; Lepper and Fan, 2010; Sanchez-Gurmaches et al., 2012; Seale et al., 2008; Wang et al., 2014b) (Fig. 1). These genes are almost certainly expressed at the earliest stages of BAT development, likely in multipotent cells, before adipogenic commitment factors, such as PPARγ are detectable. Through prospective analyses of different Myf5Cre lineage-traced precursor populations, Early B-Cell Factor-2 (Ebf2) was identified as a specific marker gene of embryonic brown preadipocytes (Wang et al., 2014b). EBF2 marks cells in the somitic mesoderm that do not express markers of other developmentally-related lineages, including muscle or dermis. The activation of Ebf2 is likely to be an early step in brown adipose lineage commitment (Fig. 1). Further studies are needed to determine which inductive cues initiate Ebf2 expression and adipogenic commitment. An obvious candidate is BMP7, which has been shown to play a pivotal role in BAT development. Loss of BMP7 or BMP-receptor signaling results in a near-complete absence of BAT development (Schulz et al., 2013; Tseng et al., 2008).
Figure 1. Brown adipocyte development.
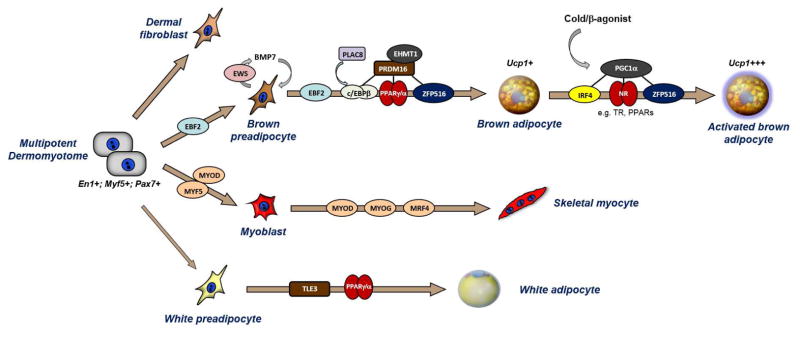
A multipotent cell population in the somitic mesoderm gives rise to: dorsal dermis, skeletal muscle, brown adipocytes and white adipocytes in certain depots. EBF2 marks committed brown preadipocytes and may also regulate the commitment process from upstream stem cells. EWS controls the expression of BMP7 by brown fat precursor cells, which in turn promotes brown adipocyte differentiation by acting in an autocrine manner. EBF2, PRDM16, C/EBP-β, and ZFP516 specifically regulate the induction of brown fat-specific genes during the differentiation process. PRDM16 binds and activates C/EBP-β, PPARγ, PPARα, Thyroid receptor (not shown) and ZFP516. Cold exposure/norepinephrine (NE) activates brown adipocytes to express high levels of thermogenic genes; this activation process is controlled, in large part, by PGC1α which interacts with IRF4 (Kong et al., 2014) and various Nuclear Receptors (NRs). MYF5 or MYOD control myoblast cell commitment, while MYOD, MYOG and MRF4 regulate myocyte differentiation and maturation. TLE3 plays an important role in driving a white fat-specific differentiation program.
The common somitic origin of brown adipocytes and muscle suggested that these lineages may be closely related in development. Consistent with this, brown preadipose cells express many muscle-specific genes and the mitochondrial proteome of brown fat and muscle are highly related to one-another (Forner et al., 2009; Timmons et al., 2007). Interestingly, several factors influence muscle versus brown fat cell fate including PRDM16, C/EBP-β, EHMT1, EWS and ZFP516 (Dempersmier et al., 2015; Kajimura et al., 2009; Ohno et al., 2013; Park et al., 2013; Seale et al., 2008) (Fig. 1). Notably, ectopic expression of PRDM16 and C/EBP-β is sufficient to convert non-adipogenic fibroblasts, such as skin fibroblasts, into functional brown adipocytes in vivo (Kajimura et al., 2009). EHMT1, an important co-regulator of PRDM16, is required to suppress the expression of muscle-specific genes in BAT. Similarly EWS was shown to be required for BAT development and its deletion causes the ectopic expression of muscle genes in BAT (Park et al., 2013). PRDM16, which can potently suppress muscle gene expression, is however not required for BAT development in mice, presumably because other related factors can compensate in its absence (Harms et al., 2014).
The embryonic origin(s) of beige fat cells (and white fat cells) is more complicated. Lineage tracing analysis showed that beige adipocytes in the inguinal WAT are not derived from Myf5-expressing cells (Sanchez-Gurmaches et al., 2012; Seale et al., 2008). However, there may be beige adipocytes traced by Myf5-activation in other WAT depots, such as the retroperitoneal WAT (Sanchez-Gurmaches et al., 2012). Through clonal analyses of adipogenic cell lines from the inguinal WAT of mice, Wu et al. discovered that beige and white adipocytes are distinct cell types that have distinctive molecular profiles (Wu et al., 2012). Importantly, only the beige clones activate UCP1 expression in response to β-adrenergic stimulation. Consistent with this finding, Ebf2-expression marks a subpopulation of adipogenic cells in WAT that are competent for Ucp1 induction, whereas Ebf2-negative adipogenic cells differentiate into UCP1-negative adipocytes (Wang et al., 2014b). Distinct molecular signatures at the precursor stage between brown/beige and white adipocytes are also found in adult humans (Shinoda et al., 2015; Xue et al., 2015).
Recent studies show that some beige adipocytes originate from smooth muscle or smooth muscle-like cells and express various smooth muscle markers (Long et al., 2014). Interestingly, the smooth muscle versus beige adipogenic fate of mesenchymal precursors is controlled by cytoskeletal tension and RhoA signaling (McDonald et al., 2015). TGFβ activates Rho-activated protein kinase (ROCK) to increase G-actin and favor smooth muscle-like differentiation whereas BMP7 inhibits ROCK activity to facilitate beige adipogenesis. Smooth muscle cells are heterogeneous and have multiple origins during development, so further studies will be needed to delineate whether a particular subset of smooth muscle or smooth muscle-like cells are beige adipogenic precursors.
Another related and debated topic is whether beige adipocytes induced by cold exposure or other stimuli arise from pre-existing mature adipocytes or via the de novo differentiation/maturation of resident precursor cells. There are compelling data to support both modes of WAT beige-ing (Fig.2). Studies by Cinti and others demonstrated that β-adrenergically-induced beige-ing occurs without significant increases in adipocyte proliferation or DNA content (Barbatelli et al., 2010; Himms-Hagen et al., 2000). This pointed to mature adipocytes as the likely precursor for the UCP1+ cells. Indeed, recent fate-mapping studies from the Granneman lab using a tamoxifen-inducible system suggest that most UCP1+ adipocytes in inguinal WAT come from mature adipocytes (Lee et al., 2015b). However, in sharp contrast to these results, the Scherer lab, using a Doxycycline-inducible (Adipochaser) system to label mature adipocytes, found that a large proportion of cold or β-agonist-induced beige cells do not come from pre-existing adipocytes (Wang et al., 2013). This implies that beige adipocytes can develop de novo from other sources, likely from resident beige precursor cells. How then does one explain these apparently conflicting fate-mapping results? First, it is important to note that the Adipochaser studies of Wang et al. demonstrate that mature adipocytes as well as non-adipocytes contribute to beige adipocytes. The inability to detect any contribution of precursor cells in the Granneman study could be due to the environmental history of animals (see next paragraph) and/or because of caveats associated with use of the tamoxifen-labeling system. Tamoxifen, given its hydrophobic properties is difficult to “wash-out” of fat tissue (Philipp Scherer, in preparation). This might then have led to the unintended labeling of newly differentiating beige adipocytes during the “chase” period after tamoxifen withdrawal.
Figure 2. Beige fat biogenesis.
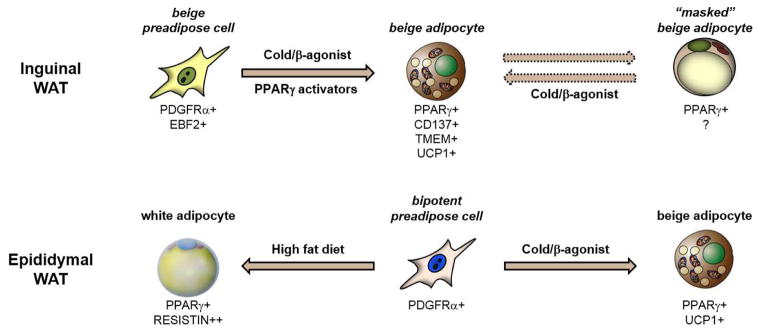
(Top panel) In inguinal WAT, β-adrenergic stimulation stimulates the de novo differentiation of EBF2+ precursor cells into beige adipocytes. In the absence of stimuli, these beige adipocytes lose their expression of UCP1 and their multilocular morphology and could be considered as “masked”. The thermogenic characteristics of these cells can be re-activated by repeated stimulation. (Bottom panel) In epididymal WAT, bipotent precursor cells can be induced to undergo beige adipocyte differentiation in response to cold/β-agonist. Under other conditions (such as high fat diet), these cells may differentiate into white adipocytes.
Altogether, the available data suggest that cold/β-adrenergic signaling promotes both de novo beige fat differentiation as well as induction of UCP1/thermogenic characteristics in mature adipocytes. The latter process may or may not reflect bona fide “transdifferentiation” as it has been called in many papers. It seems more likely that there are latent or “masked” beige adipocytes that persist when the thermogenic stimulus subsides. These adipocytes may appear morphologically white but have a beige identity (lineage) and thus the capacity to quickly re-initiate the thermogenic program in response to cold (Fig. 2). Instead of “transdifferentiation” from a different cell type/lineage, cold/β-agonist treatment may simply “activate” (or unmask) the thermogenic properties of pre-existing beige cells. This concept is supported by a recent study showing that cold-induced UCP1+ cells acquire a white-like unilocular morphology after the animals are warmed and that these same cells re-activate UCP1 in response to another bout of cold (Rosenwald et al., 2013). Given this model, the extent to which beige fat cells arise by recruitment or activation may depend on the environmental history and age of the animal. For example, animals housed in warmer conditions may not acquire many beige adipocytes during normal tissue turnover and may therefore depend on higher levels of de novo beige adipogenesis when subjected to cold. This does not preclude the possibility that some white fat cells transdifferentiate to beige fat cells under unusual circumstances, perhaps very extended cold.
It is important to note that many of the studies described above have used the inguinal WAT of mice as a model of WAT beige-ing. The inguinal WAT is a major subcutaneous depot that expresses relatively high levels of PRDM16 and has an inherently high beige-ing response (Seale et al., 2011). Mechanisms of beige fat development (i.e., emergence of UCP1+ adipocytes) may differ greatly in other WAT depots. For example, the epididymal WAT of mice, a depot that is much less prone to beige-ing, contains bipotent adipogenic precursor cells that can give rise to both white and beige (UCP1+) adipocytes depending on environmental conditions (Lee et al., 2012). Ongoing and future studies will determine whether there are functionally distinct types of beige adipocytes in different WAT depots of mouse and man. In adult humans, UCP1-positive beige adipocytes emerge in subcutaneous WAT depots of severely burned subjects (Sidossis et al., 2015). The emergence of beige adipocytes appears to be highly location-dependent, such that future studies will need to establish the consensus regarding the locations of adipose tissue biopsies.
2. Molecular Regulation of Beige Adipocyte Development
Because beige adipocyte development is highly inducible, a special emphasis has been given to this cell type as an appealing cellular target of new obesity therapeutics. Given the recent reports that adult human BAT largely contains beige-like adipocytes (Lee et al., 2014c; Sharp et al., 2012; Shinoda et al., 2015; Wu et al., 2012), understanding the molecular circuits that promote WAT beige-ing may lead to new therapeutic opportunities in obese and/or elderly people who lack sufficient amounts of active BAT depots.
Over the past few years a number of studies have identified positive or negative regulators of brown and beige adipocyte development, as summarized by recent reviews (Harms and Seale, 2013; Kajimura and Saito, 2014; Wu et al., 2013). Another important advance is the development of new expression databases. As an example, independent groups have developed publically available expression databases of brown and beige adipocytes, including RNA-sequencing in mice and in humans (Alvarez-Dominguez et al., 2015; Ohno et al., 2013; Shinoda et al., 2015; Wang et al., 2014b), microarrays (Wu et al., 2012; Zhao et al., 2014), translating ribosome affinity purification (TRAP) analysis (Long et al., 2014), mitochondrial proteome (Forner et al., 2009), and ChIP-sequencing (Loft et al., 2015; Rajakumari et al., 2013). These databases serve as important resources for the scientific community to search for novel regulators and cell-type selective markers. Here, we review recent progresses on molecular circuits that control beige adipocyte development in response to a variety of external and internal cues.
Transcriptional and epigenetic regulators
While many of the transcriptional regulators act as negative regulators of brown/beige adipocyte biogenesis, a few transcriptional regulators function as powerful activators for brown and beige adipocyte development. Such regulators include Forkhead box C2 (FoxC2) (Cederberg et al., 2001), and PRDM16 and its binding partners in the PRDM16 transcriptional complex, such as PPARγ coactivator 1α (PGC1α), C/EBP-β, and Euchromatic histone-lysine N-methyltransferase 1 (EHMT1) (Fig. 1). Intriguingly, PRDM16 expression in white adipocytes not only activate the brown fat-selective gene program but also represses the white or muslce-selective gene program (Kajimura et al., 2008; Seale et al., 2008; Seale et al., 2007). Genetic ablation of these regulators leads to a substantial impairment in the determination and/or maintenance of brown adipocytes (Harms et al., 2014; Harms et al., 2015; Kajimura et al., 2009; Ohno et al., 2013; Rajakumari et al., 2013; Seale et al., 2008). Furthermore, beige adipocyte development is significantly attenuated when PRDM16 or EHMT1 are deleted in an adipose-specific manner (Cohen et al., 2014; Ohno et al., 2013; Ohno et al., 2012). Recent studies identified several new transcriptional regulators that control brown and beige adipocyte differentiation through the PRDM16 pathway. For example, ZFP516 promotes beige adipocyte development by directly interacting with PRDM16 and activating its transcriptional activity (Dempersmier et al., 2015). PLAC8 acts as an upstream activator of C/EBP-β and induces brown fat differentiation (Jimenez-Preitner et al., 2011) (Fig. 1). On the other hand, TLE3 antagonizes the function of PRDM16 and suppresses brown fat differentiation and thermogenesis (Villanueva et al., 2013). Currently, all the identified transcriptional and epigenetic regulators appear to act both in classical brown adipocytes and beige adipcoytes.
Modulation of cellular fate is associated with dynamic chromatin remodeling. Recent ChIP-sequencing analyses of histone modifications identified distinct chromatin artetectures between BAT and WAT (Loft et al., 2015; Rajakumari et al., 2013). In addition, histone modifying enzymes, such as EHMT1 and JMJD1A (also known as KDM3A), are essential for the maintenance of chromatin artitechtures that favor brown/beige adipocytes through interacting with the key transcriptional complexes. EHMT1 function as an essential lysine methyltransferase in the PRDM16 transcriptional complex and required for brown/beige adipose cell fate (Ohno et al., 2013). We have previously shown that PRDM16 potently represses the muscle-selective or WAT-selective gene programs in a EHMT1-dependent fashion (Harms et al., 2014; Ohno et al., 2013). Consistent with the clinical observations that approximately 40-50% of the EHMT1 haploinsufficiency patients exhibit an obese phenotype (Cormier-Daire et al., 2003; Willemsen et al., 2012) and that EHMT1 expression correlate well with UCP1 in adult human BAT (Nagano et al., 2015), adipose-specific deletion of EHMT1 in mice reduces BAT-mediated thermogenesis and causes obesity and insulin resistance (Ohno et al., 2013). JMJD1A forms a transcriptional complex with the SWI/SNF nucleosome remodeling complex and controls β1-adrenergic receptor (b1-AR) and it’s downstream BAT-selecive genes in BAT (Abe et al., 2015). Whole body knockout mouse for Jhjd1a gene reduces β-AR-induced fatty oxidation and oxygen consumption in BAT and confers an obese phenotype (Inagaki et al., 2009; Tateishi et al., 2009).
Non-coding RNAs
Non-coding RNAs provide another layer of regulation in the differentiation of brown and beige adipocytes. Several microRNAs (miR), including miR-133, miR-193b, and miR-365, target PRDM16 and negatively regulate brown and beige adipocyte development in mice (Liu et al., 2013; Sun et al., 2011; Trajkovski et al., 2012; Yin et al., 2013). miR-196a activates C/EBP-β expression and induces beige adipocyte differentiation through repression of HoxC8, a negative regulator of C/EBP-β (Mori et al., 2012), whereas miR-155 represses CEBP-β expression and impairs brown adipocyte differentiation (Chen et al., 2013). Additionally, miR-378 and miR-30 activate brown or beige adipocyte differentiation by targeting repressors of BAT thermogenesis, such as phosphodiesterase1b (PDE1b) and receptor-interacting protein 140 (RIP140), respectively (Hu et al., 2015; Pan et al., 2014). Similarly, long non-coding RNAs, such as Inc-BATE1 and Blinc1, are required for brown and beige adipocyte differentiation by forming a nuclear ribonucleoprotein complex to control the thermogenic gene program (Alvarez-Dominguez et al., 2015; Zhao et al., 2014). Conversely, miR-34 acts as a repressor of beige and brown adipocyte differentiation by repressing FGF21 and SIRT1 expression in mice (Fu et al., 2014). In cultured human adipocytes, miR-26 has been identified as activator of beige adipocyte differentiation and cellular respiration (Karbiener et al., 2014). While such non-coding RNAs are appealing tools to modulate BAT-thermogenesis, cell/tissue-type selectivity of the actions need be carefully examined.
3. Environmental Cues that Control Beige Adipocyte Recruitment
Table 1 summarizes recent findings regarding the molecular mechanisms of environmental cue-induced beige adipocyte biogenesis. Chronic cold exposure is a well-known stimulus that powerfully promotes brown and beige adipocyte development. Human studies using18F-FDG-PET/CT scans found that adult human BAT activity increases after chronic cold exposure even in subjects who do not possess appreciable amounts of BAT before cold exposure (Lee et al., 2014b; van der Lans et al., 2013; Yoneshiro et al., 2013). Upon cold exposure, NE may be released from the sympathetic nerve or M2 macrophages to activate the BAT/beige fat thermogenic program (Nguyen et al., 2011; Young et al., 1982). These pathways also stimulate Ucp1 transcription through enhancing phosphorylation of transcriptional regulators including PGC1α, CREB, and ATF2 (Collins, 2011). While the β-adrenergic signaling pathway is clearly a dominant circuit, recent observations indicate that alternative pathways play roles in regulating beige adipocyte biogenesis. In many cases, it remains unclear whether these non-canonical stimuli that affect beige fat development also affect the recruitment and/or thermogenic activity of brown fat. Given the inducible nature of beige adipocytes as compared to classical brown adipocytes, it has been easier to identify factors that regulate UCP1 levels in the beige compartment. A key question for future studies will be to determine if increased beige fat development and UCP1-induction in white fat in response to various stimuli is associated with increases in local thermogenesis.
Table 1.
Cues and mediators that promote beige adipocyte recruitment.
Recent studies, as summarized below, report new regulatory circuits of beige adipocyte development, some of which do not necessarily demand thermogenesis per se (e.g., exercise and bariatric surgery). These studies imply that biological significance of beige adipocytes may go beyond simply generating heat in response to sympathetic nerve stimulation.
Exercise
Irisin and Meteorin-like (METRNL) are recently identified exercise-induced myokines that are induced in muscle through the PGC1α pathway. Administration of Irisin and METRNL via adenoviral vectors powerfully activates beige adipocyte development in mice (Bostrom et al., 2012; Rao et al., 2014). In adult humans, circulating irisin is also increased during cold exposure (Lee et al., 2014a). Mechanistically, irisin induces the beige adipocyte-gene program in a cell-autonomous manner, presumably through a selective receptor. Human irisin has recently been shown to employ a non-canonical initiator codon (ATA) for its translation, suggesting that it might be subject to complex regulation in muscle and/or other tissues (Jedrychowski et al., 2015). On the other hand, METRNL promotes an eosinophil-dependent activation of M2 macrophages to elicit beige adipocyte biogenesis (Rao et al., 2014). IL6 is a well-known exercise-induced myokine that has been shown to activate beige adipocyte development and also be required for exercise-induced WAT beige-ing in mice (Knudsen et al., 2014). In addition, metabolites derived from skeletal muscle after exercise, such as lactate and ß-aminoisobutyric acid (BAIBA), promote beige adipocyte biogenesis in the subcutaneous WAT depots in mice (Carriere et al., 2014; Roberts et al., 2014). Intriguingly, a recent study also showed that transplantation of the subcutaneous WAT from exercised-trained mice into the visceral cavity of sedentary mice significantly improves systemic glucose homeostasis. This improvement is associated with an enhanced insulin-stimulated glucose uptake in the skeletal muscle and BAT, suggesting a role for secreted molecules/metabolites from the subcutaneous WAT (Stanford et al., 2015).
Cancer Cachexia
Cancer cachexia, characterized by weight loss, chronic inflammation and muscle/adipose atrophy, is often associated with higher resting energy expenditure. This increased energy expenditure may be partly due to enhanced WAT beige-ing as has been seen in mouse models of K-ras induced pancreatic and lung cancer (Petruzzelli et al., 2014). For instance, blockade of IL6 or BAT denervation significantly impaired cachexia-associated beige adipocyte biogenesis (Petruzzelli et al., 2014). In addition, Parathyroid hormone related protein (PTHrP) derived from the Lewis lung carcinoma potently promotes beige adipocyte biogenesis (Kir et al., 2014). High level of serum PTHrP is associated with lean body mass in cachectic mice and humans. Treatment of cachectic mice with neutralizing antibody for PTHrP blocked WAT beige-ing and the loss of muscle mass and strength, indicating that PTHrP is the major lung-tumour-derived factor stimulating beige adipocyte biogenesis and hypermetabolism, at least in this experimental model of cachexia.
Burn-induced cachexia
In addition to cancer cachexia, a recent study by Sidossis’s group reported that beige adipocyte development is highly induced in the human subcutaneous WAT of severely burned subjects. In the burn patients, significantly higher levels of urinary epinephrine and norepinephrine excretion are observed. In addition, several inflammatory cytokines, including IL-6, IL-8, and IL-10, are high. While the underlying mechanisms here require further study, these circulating factors may contribute to the burn-induced WAT beige-ing in humans (Sidossis et al., 2015).
Environmental enrichment
Mice in an enriched environment with complex physical and social stimuli exhibit higher whole body energy expenditure and are more resistant to diet-induced obesity. Hypothalamic brain-derived neurotrophic factor (BDNF) mediates the environment-associated activation of the sympathetic nerve system (SNS) and promotes beige adipocyte differentiation. Notably, hypothalamic overexpression of BDNF is sufficient to promote beige adipocyte biogenesis and increased energy expenditure (Cao et al., 2011).
Innate immunity
Innate immune pathways are known to control adipose tissue function and systemic glucose homeostasis (Molofsky et al., 2013; Wu et al., 2011). Interestingly, decreased group 2 innate lymphoid cells (ILC2) in WAT are associated with obesity in humans and mice (Brestoff and Artis, 2015). IL-33 is required for the maintenance of ILC2s in WAT and also for beige adipocyte development in mice (Brestoff et al., 2015; Lee et al., 2015a). Conversely, IL-33 treatment is sufficient to promote beige adipocyte biogenesis and increased whole body energy expenditure. IL33-action in WAT involves multiple mechanisms including: (1) increasing the production of Met-enkephalin peptides by ILC2s which acts on adipocytes (Brestoff et al., 2015); and (2) stimulating IL4 secretion by eosinophils which acts to promote the proliferation and beige differentiation of adipogenic precursor cells (Lee et al., 2015a). In addition, alternatively activated (type2/M2) macrophages are recruited to the subcutaneous WAT and secrete catecholamines to activate BAT and induce beige adipocyte development (Nguyen et al., 2011). Mice that lacked IL4 signaling or the capacity to produce catecholamines selectively in macrophages appeared to have an impaired beige adipocyte development under cold (Qiu et al., 2014). However, relative contribution of M2 macrophage versus sympathetic nerve-mediated beige adipocyte development and whole body energy expenditure awaits further investigation.
Bariatric surgery
Bariatric surgery is an effective approach to reduce body weight and ameliorate type 2 diabetes. In a human study using FDG-PET scans, higher BAT activity (i.e., glucose uptake) was observed in morbidly obese subjects one year after laparoscopic adjustable gastric banding surgery (Vijgen et al., 2012). Similarly, body weight reduction by the Roux-en-Y gastric bypass (RYGB) was associated with an increase in BAT activity under cold. This increase in BAT activity levels appears to be independent of hypothalamic activity (Rachid et al., 2015). In mice, higher expression of UCP1 was observed in the gonadal WAT but not in the inguinal WAT after RYGB surgery. This increase in UCP1 expression is associated with an increase in the gene expression of b3-adrenoceptor, natriuretic peptides, ANP, and BNP (Neinast et al., 2015). The underlying mechanisms by which bariatric surgery promotes beige adipocyte biogenesis remain elusive.
Extrinsic regulators
Studies over the past few years have identified a variety of secreted factors and hormones that control beige adipocyte development. Such factors include Bone Morphogenetic Protein (BMP)4 (Qian et al., 2013), BMP7 (Schulz et al., 2011; Tseng et al., 2008), BMP8b (Whittle et al., 2012), Fibroblast Growth Factor (FGF)21 (Emanuelli et al., 2014; Fisher et al., 2012), Growth Differentiation Factor (GDF)5 (Hinoi et al., 2014), natriuretic peptides (Bordicchia et al., 2012), prostaglandins (Madsen et al., 2010; Vegiopoulos et al., 2010), and Vascular Endothelial Growth Factor (VEGF) (During et al., 2015; Sun et al., 2012). When treated with each factor, mice exhibit increase whole body energy expenditure and are protected from diet-induced body weight gain and insulin resistance. Conversely, Transforming Growth Factor (TGF)-β and Notch are up-regulated under obesity and blocks beige adipocyte differentiation. Blockade of TGF-β signaling by genetic ablation of Smad3 or by neutralizing antibody for TGF-β promote beige adipocyte development and protects animals from diet-induced obesity and insulin resistance (Koncarevic et al., 2012; Yadav et al., 2011). Similarly, genetic or pharmacological inactivation of Notch signaling induces beige adipocyte development (Bi et al., 2014). Therapeutic application of these endocrine regulators in obese humans would be an important topic in the near future.
Neuronal circuits
BAT thermogenesis is highly regulated by the core thermoregulatory neural network in the central nerve system (CNS). Series of neuroanatomical studies developed a neuroanatomical network model for thermoregulation in the interscapular BAT in response to cold and warm temperature (Morrison et al., 2014). Recent studies further identified new neuronal circuits that control brown and beige adipocyte thermogenesis. For example, activation of hepatic glucokinase leads to impaired thermogenesis in BAT through the BAT-liver connection between the afferent vagues from the liver and sympathetic efferents from the medulla to BAT (Tsukita et al., 2012). More recently, Elmquist’s group reported that deletion of PPARγ in the Phox2b-positive vagal sensory neurons promotes beige adipocyte biogenesis (Liu et al., 2014).
Activation of hypothalamic POMC neurons by leptin and insulin treatment promotes beige adipocyte biogenesis (Dodd et al., 2015), whereas inhibition of AgRP neurons by genetic deletion of O-linked β-N-acetylglucosamine promotes beige adipocyte biogenesis (Ruan et al., 2014). On the other hand, depletion of serotonergic neurons in the CNS attenuates the thermogenesis in the interscapular BAT as well as WAT beige-ing (McGlashon et al., 2015). These studies indicate new neuronal circuits that control brown and/or beige adipocyte thermogenesis in rodents. It would be important to address if such neuronal regulatory circuits are conserved in adult humans.
4. Therapeutic Opportunities in Adult Humans
A major goal in this field is to determine if activation of brown/beige fat thermogenesis can prevent body weight gain and reverse metabolic abnormalities in adult humans. Several studies clearly demonstrate that chronic cold acclimation is able to recruit higher levels of activated BAT depots in the supraclavicular region of adult humans (Lee et al., 2014b; van der Lans et al., 2013; Yoneshiro et al., 2013). Importantly, this chronic cold-stimulated BAT recruitment is associated with an improvement in post-prandial insulin sensitivity (Lee et al., 2014b) or with an increase in cold induced energy expenditure (van der Lans et al., 2013; Yoneshiro et al., 2013). Hence, promoting BAT recruitment is an important aspect to be considered for those who do not possess sufficient amounts of active BAT, such as obese and elderly population.
The β3-AR pathway is a dominant signaling pathway to activate BAT thermogenesis in rodents and in humans, however, adverse effects on the cardiovascular system hampered the use of β-AR agonists in the clinic (Villarroya and Vidal-Puig, 2013). Recently, Cypess and colleagues reported that oral administration of a selective β3-AR agonist Mirabegron robustly stimulated glucose uptake in the BAT depots in healthy adult humans, in parallel with an increase in resting metabolic rate (Cypess et al., 2015). On the other hand, minimal efficacy on BAT activity was observed in obese subjects after administration of a pan-adrenergic agonist Ephedrine (Carey et al., 2013). The lack of efficacy of β-AR agonists in obese subjects appears to be due to an impaired β-AR signaling and/or development of resistance to the β-AR agonists in adipose tissues. The underlying mechanisms for the β-AR resistance remain poorly understood and should be further investigated.
Saito’s group demonstrated that six weeks treatment of with capsinoids, non-pungent capsaicin analogs, led to an increase in cold-induced thermogenesis that may have been associated with the recruitment of new BAT in subjects with undetectable BAT before the treatment (Yoneshiro et al., 2013). More recently, Saito’s group found several dietary substances, such as catechins are able to increase BAT recruitment (In Preparation). These data indicate that new BAT can be recruited (and/or activated) by dietary supplements as well as cold-exposure in humans.
In addition to the above compounds that have been tested in adult humans, new candidates are identified through a variety of approaches and shown to be effective at least in rodent models. For example, we have recently developed a mouse model, termed ThermoMouse in which luciferase expression is regulated by the Ucp1 gene regulatory elements. A cell based high-throughput screen platform using brown adipocytes from ThermoMouse identified a small compound WWL113 that induces UCP1 expression and whole body energy expenditure in vivo (Galmozzi et al., 2014). This study provides an important proof of concept that targeting UCP1 using small molecules can be a plausible approach for enhancing whole body energy expenditure in vivo. Additionally, a number of synthetic agonists or inhibitors, such as the PPARγ agonists (Ohno et al., 2012; Petrovic et al., 2010; Qiang et al., 2012), FXR agonist fexaramine (Fang et al., 2015), soluble guanylyl cyclase (Hoffmann et al., 2015), GLP-1 agonist (Beiroa et al., 2014), Notch inhibitor (Bi et al., 2014) and amlexanox, an inhibitor of TBK1 and IKK-e (Reilly et al., 2013), potently increase whole body energy expenditure by activating thermogenesis in BAT and/or by promoting beige adipocyte biogenesis. Clinical applications of the above compounds await further investigation.
5. New Function of Beige Fat in Energy Homeostasis
The biomedical interest in brown and beige fat cells is centered on the capacity of these cells to counteract obesity and metabolic disease. Classical experiments by Rothwell and Stock first demonstrated that brown fat is activated by various high-calorie diets and that this provided a potential mechanism to limit weight/fat gain (Rothwell and Stock, 1979). In support to this, BAT and beige fat-deficient mice expressing a Ucp1-driven toxygene, develop obesity and insulin resistance in the absence of hyperphagia (Lowell et al., 1993). Further evidence supporting a natural role for BAT in regulating body weight/composition came from recent studies of UCP1-knockout mice. These animals display an increase in metabolic efficiency and gain more weight than wild-type controls when housed under thermoneutral conditions (Feldmann et al., 2009). Importantly, high fat diet increases the thermogenic capacity of BAT in wild-type but not in UCP1-knockout animals. Of note, UCP1-knockout mice are not obese when housed under standard room temperature conditions. This suggests that alternative thermogenic mechanisms, which are engaged in response to cold, also suppress weight gain and can conceal the role of UCP1 in body weight regulation.
Genetic or pharmacological elevation of brown and/or beige fat activity in mice provides further protection against metabolic disease and obesity. Many mouse models have increased brown/beige fat function and a correlated resistance to high fat diet-induced obesity and insulin resistance. Importantly, these results indicate that the ectopic increases in brown fat are not necessarily counteracted by increases in metabolic efficiency or higher food intake.
Currently there is no experimental system that allows for quantitative estimation of brown vs. beige in rodents and in humans. However, recent results, as discussed below, indicate that beige adipocytes contribute significantly to the regulation of whole-body energy expenditure and systemic glucose/lipid homeostasis.
Anti-diabetic role of beige fat
Perhaps the most obvious area of interest is in type 2 diabetes and insulin resistance. In fact, improvement in systemic glucose homeostasis and insulin sensitivity is associated with glucose uptake activity in adult human BAT (Chondronikola et al., 2014; Lee et al., 2014b). Loss of fat mass would be expected to be accompanied by improvements in insulin resistance and indeed that is usually observed. However, the improvements in glucose tolerance and/or insulin resistance often seem well out of proportion to the anti-obesity effects. For example, fat selective expression of PRDM16 in obese transgenic mice causes a modest but significant decrease in total adipose mass, accompanied by a small improvement in insulin resistance (Seale et al., 2011). On the other hand, glucose tolerance was vastly improved. This data strongly suggested that expansion and activation of (in this case) beige adipocytes improved glucose tolerance by mechanisms that may not be limited to insulin sensitivity. Perhaps the beige adipocytes clear glucose from the blood through mechanisms that are not totally insulin-dependent or perhaps systemic glucose tolerance is improved via some as yet unknown mechanisms. Conversely, adipose-specific deletion of PRDM16 (Cohen et al., 2014) or its co-activator EHMT1 (Ohno et al., 2013) significantly impaired adipocyte development and caused insulin resistance before the development of obesity. While this stimulates a mild obesity, with increased fat accumulation in the subcutaneous depot, a striking metabolic phenotype in the adipose-specific knockout mice of PRDM16 or EHMT1 is hepatic steatosis. Taken together, these data strongly suggest (but do not prove) that brown and beige fat play some important function in glucose homeostasis that goes beyond the dissipation of calories stored as fat. A specific role for beige fat in directly lowering circulating glucose and blood fatty acids via oxidation could explain a “kick-on” effect on other aspects of glucose homeostasis if the liver is forced to absorb this energy and become steatotic when beige fat is lost. In fact, BAT functions as a major “metabolic sink” not only for glucose but also for lipoproteins and fatty acids (Bartelt et al., 2011), and presumably for other metabolites. Hence, therapeutic potential of brown and beige fat may not simply be limited to anti-obesity therapy, but can also be explored in the treatment of fatty liver diseases.
Endocrine Function
Since white fat cells secrete many adipokines, it is not far fetched to think that brown and beige fat cells might secrete “batokines” with various functions. Along the same lines, endurance exercise has been shown to stimulate beige-ing of the subcutaneous adipose tissue in rodents (Bostrom et al., 2012). Transplantation of the inguinal WAT depots from exercised mice improves various aspects of glucose homeostasis much more than equivalent fat transplantations from sedentary mice (Stanford et al., 2015). Hence, the notion that classical brown and/or beige fat may secrete other molecules that improve glucose homeostasis must be considered and studied. Regarding this hypothesis, two polypeptides secreted by muscle with exercise, irisin and METRNL, have also shown to be secreted by beige fat when challenged with cold. FGF21 and BMP8b have also been shown to be secreted by adipose tissues and induced in the cold, suggesting a paracrine or autocrine “batokine” function. More recently, it has been shown that neuregulin 4 (NRG4), a member of the epidermal growth factor (EGF) family of extracellular ligands, is secreted from BAT and regulates hepatic lipid metabolism by inhibiting lipogenesis in the liver (Wang et al., 2014a). The substrate depletion and “batokine” models are of course not mutually exclusive.
Adipose tissue remodeling
WAT beige-ing is associated with dynamic adipose remodeling in the subcutaneous WAT. For instance, increased microcapillary formation and tyrosine hydroxylase (TH)-positive nerve (i.e., sympathetic nerve) innervation are tightly coupled with the clusters of beige adipocytes within WAT (Cinti, 1999). Xue et al., reported that chronic cold exposure activates angiogenesis in the inguinal WAT both in wild type and UCP1 null mice (Xue et al., 2009). Neutralizing antibody for VEGF-R2, but not VEGF-R1, significantly blocked the cold-induced angiogenesis in WAT. Conversely, adipose tissue-selective VEGF expression promotes beige adipocyte biogenesis in WAT (During et al., 2015; Sun et al., 2012), indicating that VEGF controls not only angiogenesis but also the beige adipocyte differentiation program. Nerve growth factor (NGF) is also abundantly expressed in BAT and regulated by cold and obesity (Nisoli et al., 1996). However, whether NGF is required for nerve innervation during WAT beige-ing is unknown.
In addition to angiogenesis and innervation, innate and adaptive immune cells, including eosinophil, macrophages, T-regs, and ILC2, are recruited within adipose tissues and controls not only inflammatory responses but also beige adipocyte biogenesis (Brestoff and Artis, 2015), providing an alternative pathway to regulate adipose tissue composition. Another intriguing aspect during the WAT beige-ing may be adipose fibrosis. TGF-β expression in the WAT is highly increased under obesity and powerfully drives adipose fibrosis, while blocking the TGF-β signaling pathway promotes beige adipocyte development (Koncarevic et al., 2012; Yadav et al., 2011). Given the recent studies showing that a population of beige adipocytes arises from a SMA+ smooth muscle lineage (Long et al., 2014) and that expression of SRF-target genes, such as SMA and Collagen1a1 and 3a1, are down-regulated during beige adipocyte differentiation (McDonald et al., 2015), it is conceivable that beige adipocyte recruitment is coupled with inhibition of adipose fibrosis.
UCP1-independent thermogenesis
UCP1 dissipates the proton gradient in the form of heat by uncoupling cellular respiration from mitochondrial ATP synthesis. Because UCP1 null mice are cold sensitive and fail to maintain body temperature in response to prolong cold exposure (Enerback et al., 1997; Golozoubova et al., 2001), UCP1 is considered to be the major “thermogenin” that is responsible for adaptive non-shivering thermogenesis (Nedergaard et al., 2001). On the other hand, Granneman et al. reported that chronic stimulation of β3-AR significantly increased metabolic rate in UCP1 null mice, although the induction was lower than what was observed in wild type mice (Granneman et al., 2003). This UCP1-independent increase in metabolic rate is accompanied by an increase in mitochondrial biogenesis and lipid oxidation in the WAT of UCP1 null mice, indicating that beige adipocyte may possess UCP1-independent thermogenesis. Intriguingly, two recent papers showed that FGF21 treatment was able to reduce body weight gain and improved glucose and lipid homeostasis even in UCP1 null mice (Samms et al., 2015; Veniant et al., 2015). While the FGF21’s effects appear to be partly due to reduced food intake (Samms et al., 2015), FGF21 also potently increases PGC1α expression in the inguinal WAT of UCP1 null mice (Veniant et al., 2015).
Recent data indicates that beige adipocytes contain a second thermogenic pathway that relies on futile cycling of creatine and creatine phosphate. It is noteworthy that this pathway is thermogenic but depends on coupled rather than uncoupled respiration. Interestingly, several components of this pathway are elevated in UCP1 KO mice, and chemical inhibition of this pathway suggests that it is indeed involved in thermal defense when the UCP1 pathway is ablated (Kazak et al., 2015). These data indicate the existence of UCP1-independent mechanisms that control thermogenesis and/or metabolism in adipose tissues and maybe in other organs.
6. Emerging Questions
A major function of BAT remains thermogenesis via UCP1. However, as we learn more about the two types of thermogenic adipocytes, i.e., classical brown adipocytes and beige adipocytes, it is important to ask whether these cells have additional functions in regulating systemic metabolism that may go beyond UCP1-mediated thermogenesis. In this regard, it is likely that BAT is not simply a heat-generating organ: some of the metabolic improvements observed through increasing beige fat mass are not mediated by UCP1 (Fig.3). In particular, brown/beige fat secrete several “batokines” that may function in an endocrine, autocrine, and/or paracrine manner to control systemic glucose and lipid homeostasis. Furthermore, the metabolic significance of brown/beige fat acting as “metabolic sink” for toxic substances should be investigated. Finally, WAT beige-ing is associated with large-scale tissue remodeling, including increased micro-capillary formation, nerve innervation, and modulation of immune cell populations. These changes in other tissue components likely affect the local and systemic metabolism. Determining how the various cell types in adipose interact with one another is an important area for future study.
Figure 3. Physiological roles of brown and beige fat in energy metabolism.
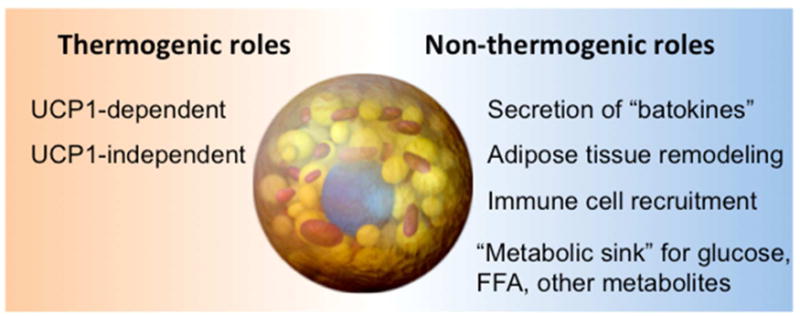
Thermogenic and non-thermogenic functions of brown and beige adipocytes are listed.
Another important goal is to establish reliable quantitative methods to measure human BAT mass regardless of environmental conditions. While 18F-FDG-PET based measurement has recently been substantially improved, the assessment is highly variable depending on the conditions of measurement, such as outside temperature and length of cold exposure (Sidossis and Kajimura, 2015). Furthermore, FDG measurements are based on glucose uptake, which represents only one function of BAT. It will also be important to development new devices/techniques that have enough sensitivity and resolution to detect beige adipocytes that sporadically reside in subcutaneous WAT and other adipose depots. The identification of specific cell surface markers and circulating markers that reflect brown fat mass would be a huge advance.
Lastly, an improved understanding of the beige adipocyte-selective signaling pathways will allow us to selectively target WAT beige-ing for therapeutic effect. We also need to understand the mechanisms of beige adipocyte maintenance, as beige adipocytes gradually lose their thermogenic characteristics after removing the appropriate external cues. These studies will lead to a plausible approach to increase whole body energy expenditure and improve glucose/lipid homeostasis in human populations who have little or no existing active BAT, such as obese and/or elderly populations.
Acknowledgments
We apologize for our inability to cite a number of papers that contributed to the advance of this field. We acknowledge supported from the NIH grant DK087853 and support from the JST-PRESTO to S.K., DK103008 to P.S., and DK314905 and a grant from the JPB Foundation to B.M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe Y, Rozqie R, Matsumura Y, Kawamura T, Nakaki R, Tsurutani Y, Tanimura-Inagaki K, Shiono A, Magoori K, Nakamura K, et al. JMJD1A is a signal-sensing scaffold that regulates acute chromatin dynamics via SWI/SNF association for thermogenesis. Nature communications. 2015;6:7052. doi: 10.1038/ncomms8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dominguez JR, Bai Z, Xu D, Yuan B, Lo KA, Yoon MJ, Lim YC, Knoll M, Slavov N, Chen S, et al. De Novo Reconstruction of Adipose Tissue Transcriptomes Reveals Long Non-coding RNA Regulators of Brown Adipocyte Development. Cell metabolism. 2015;21:764–776. doi: 10.1016/j.cmet.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, Niswander L, Conlon RA. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Developmental biology. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The Emergence of Cold-Induced Brown Adipocytes in Mouse White Fat Depots Is Predominantly Determined by White to Brown Adipocyte Transdifferentiation. American journal of physiology Endocrinology and metabolism. 2010;298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. Brown adipose tissue activity controls triglyceride clearance. Nature medicine. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Ferno J, Salvador J, Escalada J, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- Bi P, Shan T, Liu W, Yue F, Yang X, Liang XR, Wang J, Li J, Carlesso N, Liu X, et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nature medicine. 2014;20:911–918. doi: 10.1038/nm.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. The Journal of clinical investigation. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronnikov G, Houstek J, Nedergaard J. Beta-adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via beta 1 but not via beta 3 adrenoceptors. The Journal of biological chemistry. 1992;267:2006–2013. [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell metabolism. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Molecular and cellular biology. 2004;24:3057–3067. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, Kalff V, Duffy SJ, Cherk MH, Kingwell BA. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013;56:147–155. doi: 10.1007/s00125-012-2748-1. [DOI] [PubMed] [Google Scholar]
- Carriere A, Jeanson Y, Berger-Muller S, Andre M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63:3253–3265. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Siegel F, Kipschull S, Haas B, Frohlich H, Meister G, Pfeifer A. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nature communications. 2013;4:1769. doi: 10.1038/ncomms2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. The adipose organ. Editrice Kurtis; Milano, Italy: 1999. [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and Beige Adipose Causes Metabolic Dysfunction and a Subcutaneous to Visceral Fat Switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. beta-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure. Frontiers in endocrinology. 2011;2:102. doi: 10.3389/fendo.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Daniel KW, Petro AE, Surwit RS. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology. 1997;138:405–413. doi: 10.1210/endo.138.1.4829. [DOI] [PubMed] [Google Scholar]
- Cormier-Daire V, Molinari F, Rio M, Raoul O, de Blois MC, Romana S, Vekemans M, Munnich A, Colleaux L. Cryptic terminal deletion of chromosome 9q34: a novel cause of syndromic obesity in childhood? Journal of medical genetics. 2003;40:300–303. doi: 10.1136/jmg.40.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown fat in humans: consensus points and experimental guidelines. Cell metabolism. 2014;20:408–415. doi: 10.1016/j.cmet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Weiner LS, Roberts-Toler C, Elia EF, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, et al. Activation of Human Brown Adipose Tissue by a beta3-Adrenergic Receptor Agonist. Cell Metab. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature medicine. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempersmier J, Sambeat A, Gulyaeva O, Paul SM, Hudak CS, Raposo HF, Kwan HY, Kang C, Wong RH, Sul HS. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Molecular cell. 2015;57:235–246. doi: 10.1016/j.molcel.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Munzberg H, Zhang ZY, Kahn BB, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Liu X, Huang W, Magee D, Slater A, McMurphy T, Wang C, Cao L. Adipose VEGF Links the White-to-Brown Fat Switch With Environmental, Genetic, and Pharmacological Stimuli in Male Mice. Endocrinology. 2015;156:2059–2073. doi: 10.1210/en.2014-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, Michael MD, Adams AC, Kharitonenkov A, Kahn CR. Interplay between FGF21 and insulin action in the liver regulates metabolism. The Journal of clinical investigation. 2014;124:515–527. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nature medicine. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell metabolism. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell metabolism. 2009;10:324–335. doi: 10.1016/j.cmet.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Fu T, Seok S, Choi S, Huang Z, Suino-Powell K, Xu HE, Kemper B, Kemper JK. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Molecular and cellular biology. 2014;34:4130–4142. doi: 10.1128/MCB.00596-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmozzi A, Sonne SB, Altshuler-Keylin S, Hasegawa Y, Shinoda K, Luijten IH, Chang JW, Sharp LZ, Cravatt BF, Saez E, et al. ThermoMouse: an in vivo model to identify modulators of UCP1 expression in brown adipose tissue. Cell reports. 2014;9:1584–1593. doi: 10.1016/j.celrep.2014.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geloen A, Collet AJ, Guay G, Bukowiecki LJ. Beta-adrenergic stimulation of brown adipocyte proliferation. Am J Physiol. 1988;254:C175–182. doi: 10.1152/ajpcell.1988.254.1.C175. [DOI] [PubMed] [Google Scholar]
- Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. American journal of physiology. 2003;285:E1230–1236. doi: 10.1152/ajpendo.00197.2003. [DOI] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. The Journal of clinical investigation. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, Kurokawa M, Won KJ, Seale P. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell metabolism. 2014;19:593–604. doi: 10.1016/j.cmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MJ, Lim HW, Ho Y, Shapira SN, Ishibashi J, Rajakumari S, Steger DJ, Lazar MA, Won KJ, Seale P. PRDM16 binds MED1 and controls chromatin architecture to determine a brown fat transcriptional program. Genes & development. 2015;29:298–307. doi: 10.1101/gad.252734.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton JM. The distribution of brown adipose tissue in the human. Journal of anatomy. 1972;112:35–39. [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. American journal of physiology Cell physiology. 2000;279:C670–681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Nakamura Y, Takada S, Fujita H, Iezaki T, Hashizume S, Takahashi S, Odaka Y, Watanabe T, Yoneda Y. Growth differentiation factor-5 promotes brown adipogenesis in systemic energy expenditure. Diabetes. 2014;63:162–175. doi: 10.2337/db13-0808. [DOI] [PubMed] [Google Scholar]
- Hoffmann LS, Etzrodt J, Willkomm L, Sanyal A, Scheja L, Fischer AW, Stasch JP, Bloch W, Friebe A, Heeren J, et al. Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue. Nature communications. 2015;6:7235. doi: 10.1038/ncomms8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Wang M, Xiao T, Yin B, He L, Meng W, Dong M, Liu F. miR-30 Promotes Thermogenesis and the Development of Beige Fat by Targeting RIP140. Diabetes. 2015;64:2056–2068. doi: 10.2337/db14-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Tachibana M, Magoori K, Kudo H, Tanaka T, Okamura M, Naito M, Kodama T, Shinkai Y, Sakai J. Obesity and metabolic syndrome in histone demethylase JHDM2a-deficient mice. Genes to cells : devoted to molecular & cellular mechanisms. 2009;14:991–1001. doi: 10.1111/j.1365-2443.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- Ishibashi J, Seale P. Medicine. Beige can be slimming. Science. 2010;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, JS, Robinson MM, Nair KS, Gygi S, Spiegelman BM. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell metabolism. 2015 doi: 10.1016/j.cmet.2015.08.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, Thorens B. Plac8 is an inducer of C/EBPbeta required for brown fat differentiation, thermoregulation, and control of body weight. Cell metabolism. 2011;14:658–670. doi: 10.1016/j.cmet.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annual review of physiology. 2014;76:225–249. doi: 10.1146/annurev-physiol-021113-170252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL, Chin S, Tempst P, Lazar MA, Spiegelman BM. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes & development. 2008;22:1397–1409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbiener M, Pisani DF, Frontini A, Oberreiter LM, Lang E, Vegiopoulos A, Mossenbock K, Bernhardt GA, Mayr T, Hildner F, et al. MicroRNA-26 family is required for human adipogenesis and drives characteristics of brown adipocytes. Stem cells (Dayton, Ohio) 2014;32:1578–1590. doi: 10.1002/stem.1603. [DOI] [PubMed] [Google Scholar]
- Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss S, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015 doi: 10.1016/j.cell.2015.09.035. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour–derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus S, Ely M, Encke D, Heldmaier G. Functional assessment of white and brown adipocyte development and energy metabolism in cell culture. Dissociation of terminal differentiation and thermogenesis in brown adipocytes. Journal of cell science. 1995;108(Pt 10):3171–3180. doi: 10.1242/jcs.108.10.3171. [DOI] [PubMed] [Google Scholar]
- Knudsen JG, Murholm M, Carey AL, Bienso RS, Basse AL, Allen TL, Hidalgo J, Kingwell BA, Febbraio MA, Hansen JB, et al. Role of IL-6 in exercise training-and cold-induced UCP1 expression in subcutaneous white adipose tissue. PloS one. 2014;9:e84910. doi: 10.1371/journal.pone.0084910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Davies M, Sako D, Liu J, Kumar R, Burton R, et al. A novel therapeutic approach to treating obesity through modulation of TGFβ signaling. Endocrinology. 2012;153:3133–3146. doi: 10.1210/en.2012-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, Wang X, Yu S, Lo JC, Tseng YH, et al. IRF4 is a key thermogenic transcriptional partner of PGC-1alpha. Cell. 2014;158:69– 83. doi: 10.1016/j.cell.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koza RA, Hohmann SM, Guerra C, Rossmeisl M, Kozak LP. Synergistic gene interactions control the induction of the mitochondrial uncoupling protein (Ucp1) gene in white fat tissue. The Journal of biological chemistry. 2000;275:34486–34492. doi: 10.1074/jbc.M002136200. [DOI] [PubMed] [Google Scholar]
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015a;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell metabolism. 2014a;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, Werner CD, Chen KY, Celi FS. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014b;63:3686–3698. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. International journal of obesity (2005) 2014c;38:170–176. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of coldinduced brown adipocytes in adult mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015b;29:286–299. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell metabolism. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013a;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Leinhard OD, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nature medicine. 2013b;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- Liu C, Bookout AL, Lee S, Sun K, Jia L, Lee C, Udit S, Deng Y, Scherer PE, Mangelsdorf DJ, et al. PPARgamma in vagal neurons regulates high-fat diet induced thermogenesis. Cell metabolism. 2014;19:722–730. doi: 10.1016/j.cmet.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Bi P, Shan T, Yang X, Yin H, Wang YX, Liu N, Rudnicki MA, Kuang S. miR-133a regulates adipocyte browning in vivo. PLoS genetics. 2013;9:e1003626. doi: 10.1371/journal.pgen.1003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft A, Forss I, Siersbaek MS, Schmidt SF, Larsen AS, Madsen JG, Pisani DF, Nielsen R, Aagaard MM, Mathison A, et al. Browning of human adipocytes requires KLF11 and reprogramming of PPARgamma superenhancers. Genes & development. 2015;29:7–22. doi: 10.1101/gad.250829.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. A smooth muscle-like origin for beige adipocytes. Cell metabolism. 2014;19:810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- Lowell BB, VSS, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Madsen L, Pedersen LM, Lillefosse HH, Fjaere E, Bronstad I, Hao Q, Petersen RK, Hallenborg P, Ma T, De Matteis R, et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PloS one. 2010;5:e11391. doi: 10.1371/journal.pone.0011391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor a regulates conversion of progenitors to beige adipocytes. Cell. 2015;160:105–118. doi: 10.1016/j.cell.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashon JM, Gorecki MC, Kozlowski AE, Thirnbeck CK, Markan KR, Leslie KL, Kotas ME, Potthoff MJ, Richerson GB, Gillum MP. Central serotonergic neurons activate and recruit thermogenic brown and beige fat and regulate glucose and lipid homeostasis. Cell metabolism. 2015;21:692–705. doi: 10.1016/j.cmet.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. The Journal of experimental medicine. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS biology. 2012;10:e1001314. doi: 10.1371/journal.pbio.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell metabolism. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano G, Ohno H, Oki K, Kobuke K, Shiwa T, Yoneda M, Kohno N. Activation of classical brown adipocytes in the adult human perirenal depot is highly correlated with PRDM16-EHMT1 complex expression. PloS one. 2015;10:e0122584. doi: 10.1371/journal.pone.0122584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. American journal of physiology. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Shabalina I, Ohba K, Ohlson K, Jacobsson A, Cannon B. Life without UCP1: mitochondrial, cellular and organismal characteristics of the UCP1-ablated mice. Biochemical Society transactions. 2001;29:756–763. doi: 10.1042/0300-5127:0290756. [DOI] [PubMed] [Google Scholar]
- Neinast MD, Frank AP, Zechner JF, Li Q, Vishvanath L, Palmer BF, Aguirre V, Gupta RK, Clegg DJ. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Molecular metabolism. 2015;4:427–436. doi: 10.1016/j.molmet.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Benarese M, Liberini P, Carruba MO. Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology. 1996;137:495–503. doi: 10.1210/endo.137.2.8593794. [DOI] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;504:163– 167. doi: 10.1038/nature12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARgamma agonists Induce a White-to-Brown Fat Conversion through Stabilization of PRDM16 Protein. Cell metabolism. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamatsu-Ogura Y, Fukano K, Tsubota A, Uozumi A, Terao A, Kimura K, Saito M. Thermogenic ability of uncoupling protein 1 in beige adipocytes in mice. PloS one. 2013;8:e84229. doi: 10.1371/journal.pone.0084229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Mao C, Quattrochi B, Friedline RH, Zhu LJ, Jung DY, Kim JK, Lewis B, Wang YX. MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nature communications. 2014;5:4725. doi: 10.1038/ncomms5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Kang HJ, Kang SI, Lee JE, Hur J, Ge K, Mueller E, Li H, Lee BC, Lee SB. A multifunctional protein, EWS, is essential for early brown fat lineage determination. Developmental cell. 2013;26:393–404. doi: 10.1016/j.devcel.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. The Journal of biological chemistry. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell metabolism. 2014 doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E798–807. doi: 10.1073/pnas.1215236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachid B, van de Sande-Lee S, Rodovalho S, Folli F, Beltramini GC, Morari J, Amorim BJ, Pedro T, Ramalho AF, Bombassaro B, et al. Distinct regulation of hypothalamic and brown/beige adipose tissue activities in human obesity. International journal of obesity (2005) 2015 doi: 10.1038/ijo.2015.94. [DOI] [PubMed] [Google Scholar]
- Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, Seale P. EBF2 Determines and Maintains Brown Adipocyte Identity. Cell metabolism. 2013;17:562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nature medicine. 2013;19:313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LD, Bostrom P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell metabolism. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NH, Landa A, Park S, Smith RG. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging cell. 2012;11:1074–1083. doi: 10.1111/acel.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159:306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samms RJ, Smith DP, Cheng CC, Antonellis PP, Perfield JW, 2nd, Kharitonenkov A, Gimeno RE, Adams AC. Discrete Aspects of FGF21 In Vivo Pharmacology Do Not Require UCP1. Cell reports. 2015;11:991–999. doi: 10.1016/j.celrep.2015.04.046. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell metabolism. 2012;16:348–362. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng YH. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. The Journal of clinical investigation. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell metabolism. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell reports. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS one. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nature medicine. 2015;21:389–394. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. The Journal of clinical investigation. 2015;125:478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell metabolism. 2015;22:219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2002–2014. doi: 10.2337/db14-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Xie H, Mori MA, Alexander R, Yuan B, Hattangadi SM, Liu Q, Kahn CR, Lodish HF. Mir193b-365 is essential for brown fat differentiation. Nature cell biology. 2011;13:958–965. doi: 10.1038/ncb2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K, Okada Y, Kallin EM, Zhang Y. Role of Jhdm2a in regulating metabolic gene expression and obesity resistance. Nature. 2009;458:757–761. doi: 10.1038/nature07777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4401– 4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovski M, Ahmed K, Esau CC, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nature cell biology. 2012;14:1330–1335. doi: 10.1038/ncb2612. [DOI] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Ishihara H, et al. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell metabolism. 2012;16:825–832. doi: 10.1016/j.cmet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jorgensen JA, Wu J, Mottaghy FM, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. The Journal of clinical investigation. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nusing RM, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science (New York, NY. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- Veniant MM, Sivits G, Helmering J, Komorowski R, Lee J, Fan W, Moyer C, Lloyd DJ. Pharmacologic Effects of FGF21 Are Independent of the "Browning" of White Adipose Tissue. Cell metabolism. 2015;21:731–738. doi: 10.1016/j.cmet.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P, van Marken Lichtenbelt WD. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. The Journal of clinical endocrinology and metabolism. 2012;97:E1229– 1233. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- Villanueva CJ, Vergnes L, Wang J, Drew BG, Hong C, Tu Y, Hu Y, Peng X, Xu F, Saez E, et al. Adipose Subtype-Selective Recruitment of TLE3 or Prdm16 by PPARgamma Specifies Lipid Storage versus Thermogenic Gene Programs. Cell metabolism. 2013;17:423–435. doi: 10.1016/j.cmet.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell metabolism. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53:619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nature medicine. 2014a;20:1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, Seale P. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proceedings of the National Academy of Sciences of the United States of America. 2014b;111:14466–14471. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871– 885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen MH, Vulto-van Silfhout AT, Nillesen WM, Wissink-Lindhout WM, van Bokhoven H, Philip N, Berry-Kravis EM, Kini U, van Ravenswaaij-Arts CM, Delle Chiaie B, et al. Update on Kleefstra Syndrome. Molecular syndromology. 2012;2:202–212. doi: 10.1159/000335648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science (New York, N Y. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & development. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. Journal of lipid research. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- Xue R, Lynes MD, Dreyfuss JM, Shamsi F, Schulz TJ, Zhang H, Huang TL, Townsend KL, Li Y, Takahashi H, et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nature medicine. 2015;21:760–768. doi: 10.1038/nm.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell metabolism. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, et al. Protection from obesity and diabetes by blockade of TGF–beta/Smad3 signaling. Cell metabolism. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, Seale P, Fernando P, van Ijcken W, Grosveld F, et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell metabolism. 2013;17:210–224. doi: 10.1016/j.cmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. The Journal of clinical investigation. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, Miyagawa M, Tsujisaki M, Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring, Md. 2011;19:1755–1760. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- Young JB, Saville E, Rothwell NJ, Stock MJ, Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. The Journal of clinical investigation. 1982;69:1061–1071. doi: 10.1172/JCI110541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167:10–14. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Li S, Wang GX, Yu Q, Lin JD. A long noncoding RNA transcriptional regulatory circuit drives thermogenic adipocyte differentiation. Molecular cell. 2014;55:372–382. doi: 10.1016/j.molcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


