Abstract
The evolutionarily conserved neoplastic tumor suppressor protein, Lethal (2) giant larvae (Lgl), plays roles in cell polarity and tissue growth via regulation of the Hippo pathway. In our recent study, we showed that in the developing Drosophila eye epithelium, depletion of Lgl leads to increased ligand-dependent Notch signaling. lgl mutant tissue also exhibits an accumulation of early endosomes, recycling endosomes, early-multivesicular body markers and acidic vesicles. We showed that elevated Notch signaling in lgl− tissue can be rescued by feeding larvae the vesicle de-acidifying drug chloroquine, revealing that Lgl attenuates Notch signaling by limiting vesicle acidification. Strikingly, chloroquine also rescued the lgl− overgrowth phenotype, suggesting that the Hippo pathway defects were also rescued. In this extraview, we provide additional data on the regulation of Notch signaling and endocytosis by Lgl, and discuss possible mechanisms by which Lgl depletion contributes to signaling pathway defects and tumorigenesis.
Keywords: chloroquine, Drosophila, endocytosis, Hippo, lgl, Notch
Abbreviations
- Lgl
Lethal (2) giant larvae
- aPKC
atypical Protein Kinase C
Introduction
Decades of fundamental research utilizing the vinegar fly, Drosophila melanogaster, have revealed many important genes and signaling networks that have subsequently proven to be important in human cancer (reviewed by1-4). Of particular interest for epithelial and neural cancers are the junctional neoplastic tumor suppressors, Lgl, Discs large (Dlg) and Scribbled (Scrib) (reviewed by5). These genes function in a common genetic pathway to control epithelial apical-basal cell polarity and tissue growth during Drosophila development.6-8 Lgl, Dlg and Scrib antagonize the activity of the Par apical polarity complex (consisting of Par3 (Bazooka (Baz) in Drosophila), atypical protein kinase C (aPKC), Par6 and Cdc42), which is important in polarity regulation and tissue growth control (reviewed by11,12). Conversely, aPKC can phosphorylate Lgl and thereby restrict its access to the apical membrane. aPKC also phosphorylates and activates Crumbs (Crb), a component of the Crb-Patj-Pals (Stardust) apical polarity complex, which is required for Crb-Crb extracellular domain interactions and the establishment of the apical membrane domain.13 However, our recent studies have revealed that Lgl acts distinctly from Dlg and Scrib in epithelial tissue growth control, and that this function can be separated from Lgl's role in apical-basal cell polarity.9,10 In addition to their cell polarity function, Drosophila Lgl/aPKC and Crb also regulate tissue growth via the Hippo signaling pathway, a conserved pathway that acts to phosphorylate and inhibit the activity of the Yki/Yap co-transcriptional activator (reviewed by14-16). Lgl/aPKC and Crb regulate the Hippo pathway by distinct mechanisms; Lgl/aPKC affects the localization of the Hippo core protein kinase and the negative regulator Rassf (Fig. 1), while Crb acts to control the stability of an upstream Hippo pathway activator, Expanded.15,17-21 However, Drosophila Dlg and Scrib do not directly regulate the Hippo pathway, although once cell polarity is lost the Hippo pathway is impaired, most likely due to the aberrant aPKC activity.9,16,22,23
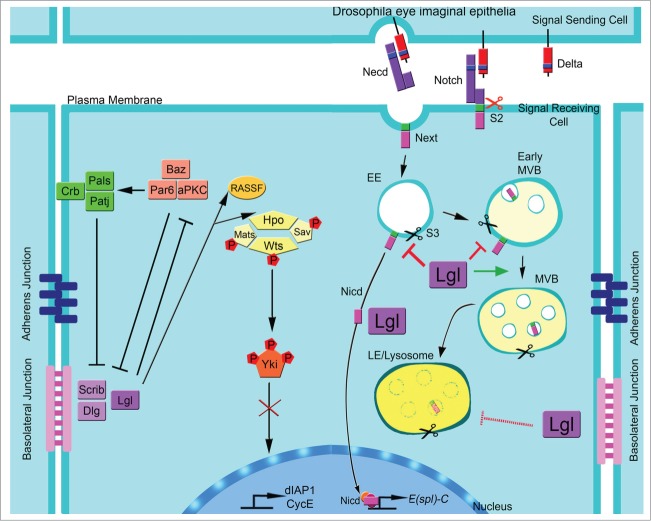
Figure 1.
Model for the regulation of Hippo and Notch by Lgl. In epithelial cells, Lgl/aPKC regulate the Hippo pathway by promoting the correct localization of Hpo/Rassf, therefore allowing the phosphorylation of Yki and preventing its translocation into the nucleus and the activation of Hippo pathway targets, dIAP1 and CycE. Lgl also regulates the ligand-dependent activation of Notch, in an aPKC-independent manner. In ligand-dependent Notch activation, the ligand (Delta) binds to the extracellular domain (N-ecd), it is cleaved by S2 protease and the Delta/Necd complex is internalized in the signal sending cell. S2 cleaved Notch (N-ext) is endocytosed and cleaved by γ-secretase (S3) in early endosomes (EE), or in early-multivesicular body (early-MVBs), releasing the active cytoplasmic N-icd fragment, which translocates into the nucleus, thereby inducing transcription of target genes, such as Enhancer of split complex (E(spl)-C). Unprocessed Notch can traffic through to the late endosome (LE)/lysosome where it undergoes proteasomal degradation. Extrapolating from our data, we propose that Lgl plays a role in controlling the activity of Notch by regulating its trafficking from the EE, early-MVB, to MVB-LE stage. Lgl also directly or indirectly limits the acidification process of the endosomes, thereby attenuating Notch signaling by preventing its cleavage by γ-secretase.
In our recent study,24 we have discovered a role for Drosophila Lgl in the regulation of the Notch signaling pathway (Fig. 1). The Notch pathway is an important cell-cell communication pathway required for cell-fate decisions during development, which is commonly deregulated in human cancer (reviewed by25-28). Engagement of the ligands (Delta or Serrate in Drosophila) in the signal-sending cell with the Notch receptor in the signal receiving cell leads to cleavage of Notch by an Adam metalloprotease (Kuzbanian in Drosophila), to result in the S2 cleavage to produce the intracellular truncated form of Notch (N-ext). N-ext is then cleaved by γ-secretase (S3 cleavage) to produce the Notch intracellular domain (N-icd) that enters the nucleus and regulates gene transcription in a complex with the transcriptional regulators CSL/Su(Hairless) and Mastermind (Mam). Recently, endocytosis has been shown to play an important role in Notch signaling (reviewed by29-33). The Notch receptor can be internalized by a ligand-dependent (where it is cleaved and activated) or a ligand-independent manner (where it is downregulated by degradation in the lysosome, or recycled to the plasma membrane). However, specific defects in endocytosis at the multivesicular body (MVB) stage (eg ESCRT II, vps25 mutants) lead to aberrant Notch cleavage and elevated Notch signaling.34,35
In our analysis, we found lgl mutant clones induced in the developing Drosophila eye, showed upregulation of Notch targets and accumulation of intracellular Notch (N-ext or N-icd), detected by an antibody to the Notch intracellular domain, but did not affect full-length or N-ecd, revealed by an antibody to the Notch extracellular domain24 (Fig. 1). Consistent with this observation, we found that upregulation of Notch activity is ligand dependent, since lgl− tissue that was also deficient for the 2 Notch ligands, Delta and Serrate, did not show ectopic expression of Notch targets. Consistent with the requirement for Notch to be internalized for activation,36 we found that in lgl− tissue also deficient in Dynamin (Shibire (Shi)) in Drosophila, by expressing a dominant negative transgene, shiDN) function (required for the initial stages of endosomal budding from the plasma membrane) or in Rab5 early endosome (EE) function (using Rab5RNAi), Notch target expression was blocked, similarly to shiDN or Rab5RNAi clones. Elevated Notch signaling in lgl− is correlated with defects in endosomal trafficking (Avl+ (Syx7) EEs, Rab11+ recycling endosomes (REs) and Hrs+ (Escrt 0) early-MVBs accumulated) and with increased acidic vesicles.24 In wild-type cells, we found that Lgl colocalizes with Notch and endocytic markers, consistent with Lgl playing a direct role in the regulation of endocytic trafficking. Based on these findings, we hypothesized that Lgl might regulate a step in endosomal maturation or in vesicle acidification that led to elevated Notch signaling. However, reducing Rab11 or Escrt 0 function did not rescue increased Notch signaling in lgl− tissue,24 suggesting that accumulation of these endocytic compartments was not responsible for the increased Notch signaling in Lgl depleted tissue. Instead, we found that reducing vesicle acidity by feeding the developing larvae the drug chloroquine restored Notch signaling to normal in lgl− tissue and dramatically rescued the lgl− mosaic adult eye phenotype. This rescue demonstrated that the increased acidity of vesicles in lgl− tissue is responsible for ectopic Notch signaling and other defects caused by Lgl depletion. This result is consistent with previous data showing that the activity of γ−secretase, which is required for S3-cleavage activation of Notch, is dependent on vesicle acidification.37,38 In contrast to many studies where the primary function of Lgl is to inhibit aPKC, we found that the ectopic Notch signaling observed upon depletion of Lgl is not dependent on aPKC activity for its effect.24 Taken together, our study has revealed a novel aPKC-independent role for Lgl in the regulation of endocytosis and Notch signaling. Here we provide additional data to probe the mechanism by which Lgl regulates endocytosis and Notch signaling. Furthermore, we compare and contrast our findings with other published studies on polarity regulators in endocytosis, and speculate upon possible mechanisms by which Lgl depletion contributes to signaling pathway defects and tumorigenesis.
The Control of Notch Signaling by Lgl
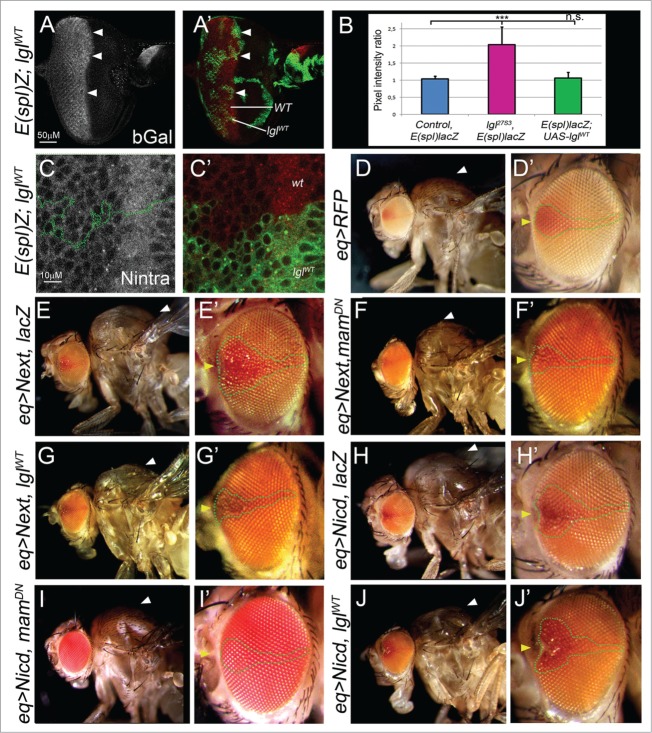
To further explore the interaction of Lgl with the Notch pathway, we sought to examine whether overexpression of Lgl (lglWT) could inhibit Notch signaling. Firstly, we overexpressed Lgl in the developing eye epithelium and examined the effect on endogenous Notch signaling in third instar larval lglWT clones relative to the surrounding wild-type tissue using the E(Spl)m8-lacZ reporter as a read out of Notch activity (Fig. 2A, B). No changes were observed relative to the control mosaic eye disc (Fig. 2A, B). Furthermore, no significant changes were observed to Notch-intra levels or localization (Fig. 2C). Since potential effects of lglWT overexpression in reducing endogenous Notch signaling might be difficult to observe, we then tested whether overexpression of Lgl could suppress ectopic Notch signaling. Accordingly, we activated Notch by expression of N-ext, which is the S2-cleaved derivative that requires γ-secretase to generate the N-icd active version, and therefore serves as a constitutive substrate for γ-secretase.38 We expressed the UAS-N-ext transgene via the equatorial (eq1)-GAL4 driver (eq > N-ext), which is expressed in a patch in the center of the developing eye, as well as in the wing disc notum that gives rise to the adult thorax. eq > N-ext resulted in a patch of roughness in the center of the adult eye and an overgrown thorax (arrowheads Fig. 2E, E’ compared with control flies Fig. 2D, D’). To test that this phenotype was sensitive to modification, we expressed a Mastermind dominant negative transgene (mamDN), which inhibits Notch activity at the promoters of its target genes. mamDN strongly suppressed the overgrown thorax and rough eye phenotypes of eq > N-ext (Fig. 2F, F’). Importantly, co-expression of Lgl with N-ext also dramatically reduced the phenotypic effects of eq > N-ext (Fig. 2G, G’). This result shows that increasing Lgl levels is sufficient to inhibit γ-secretase-mediated activation of N-ext. We then tested whether Lgl could also inhibit the phenotypic effects of expression of constitutively active Notch, N-icd (γ-secretase cleaved form) via the eq driver. eq > N-icd produced a milder phenotype in the eye and the thorax relative to eq > N-ext, perhaps due to lower expression levels (Fig. 2H, H’). While expression of mamDN could suppress the eye and thoracic defects of eq > N-icd (Fig. 2I, I’), overexpression of LglWT failed to reduce these defects (Fig J, J'). Since lglWT expression can suppress N-ext induced phenotypes but not N-icd phenotypes, this suggests that Lgl is acting at the level of γ-secretase activity rather than on N-icd function. Taken together, these results provide evidence that Lgl directly or indirectly regulates γ-secretase-mediated Notch activation.
Figure 2 (See previous page).
Overexpression of Lgl can suppress N-ext, but not N-icd, phenotypes, and does not affect endogenous Notch signaling. (A) Confocal images of β-gal staining (gray, red in merge) of lglWT mosaic third instar larval eye discs in the E(spl)m8-lacZ background. Clones are marked by the expression of GFP (green, arrowheads). (B) Quantification of E(spl)m8-lacZ expression in lglWT clones compared with wild-type clones, showing that LglWT does not significantly affect the expression of β-gal relative to wild-type tissue. The data is shown relative to the elevated expression of E(spl)m8-lacZ in lgl− clones and control mosaic eye discs. (C) Confocal images of Notch-intra staining (gray, red in merge) of lglWT mosaic third instar larval eye discs in the E(spl)m8-lacZ background. Clones are marked by the expression of GFP (green, outlined area). LglWT does not affect the levels or localization of Notch-intra. (D) Control eq-GAL4 adult thorax (D) and eye (D') (E-G) eq > N-ext crossed to UAS-lacZ (B), UAS-mamDN (C) or UAS-lglWT (D) adult thorax and eyes. Note that expression of mamDN or lglWT can suppress the adult eye roughing (outlined areas) and thoracic overgrowth phenotypes (arrowheads) of eq > N-ext, indicating that Lgl can suppress ectopic Notch signaling at the γ-secretase step. (H-J) eq > N-icd crossed to UAS-lacZ (H), UAS-mamDN (I) or UAS-lglWT (J) adult thorax and eyes. Only mamDN expression is able to rescue the adult eye roughing (outlined areas) and thoracic overgrowth phenotype (arrowheads). Genotypes: (A, C) eyFLP, UAS-GFP; FRT40, E(spl)m8-lacZ; UAS-lglWT/ tubGAL80 FRT40; tubGAL4 (D) w;; UAS-myrRFP, eq1-GAL4/TM6B (E) w;; UAS-myrRFP, eq1-GAL4, UAS-N-ext/UAS-lacZ (F) w;; UAS-myrRFP, eq1-GAL4, UAS-N-ext/UAS-mamDN (G) w;; UAS-myrRFP, eq1-GAL4, UAS-N-ext/UAS-lglWT (H) w; +/UAS-N-icd; UAS-myrRFP, eq1-GAL4, UAS-lacZ (I) w; +/UAS-N-icd; UAS-myrRFP, eq1-GAL4, UAS-mamDN (J) w; +/UAS-N-icd; UAS-myrRFP, eq1-GAL4, UAS-lglWT Flystocks: Eq1-Gal4 (H Sun), E(spl)m8-lacZ (A. Bergmann), UAS-N-ext (III) (T. Vaccari), UAS-lacZ-nls (III) (G. Baeg), UAS-mamDN (BL26672) (Bloomington stock center), UAS-lglWT (III) (J. Knoblich), UAS-N-icd (II) (S. Artavanis-Tsakonas). Antibodies: mouse β-galactosidase (Sigma, 1:500), mouse Notch-intra (Developmental Studies Hybridoma Bank, 1:50).
Endosomal Trafficking is Affected at the Early-MVB Stage in lgl Mutant Tissue
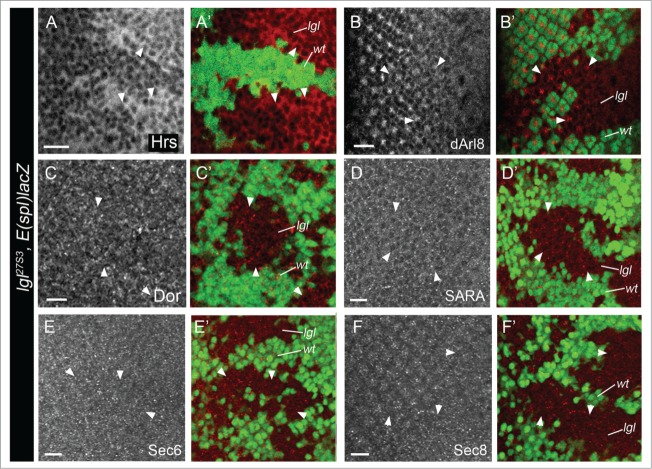
In our recent study, we showed that Avl (Syx7, EE), Hrs (Escrt 0, early-MVB) (Fig. 3A) and Rab11 (RE) were elevated in lgl− tissue, but that Rab7 (maturing late endosomes, LE) and Car (LE to lysosome fusion) remained unaffected.24 To further investigate the effect of Lgl depletion on LE-lysosomal stage, we examined the localization and levels of Dor (LE39) and Arl8 (lysosome40) in lgl− mosaic eye epithelium (Fig. 3B, C). These markers were not altered in lgl− tissue, confirming that Lgl depleted tissue leads to endosomal trafficking defects at the EE/early-MVB stages. To further explore vesicle trafficking defects in lgl− tissue, we examined SARA endosomes, which are a subclass of EE involved in Notch signaling in Drosophila neural and adult gut epithelial asymmetrically dividing cells41,42 and are also present in imaginal disc epithelial cells.43 However, SARA endosomes were not perturbed in lgl− eye disc clones (Fig. 3D), indicating that this specialized endosomal compartment was not involved in the Notch signaling defects in Lgl depleted eye epithelial tissue. Finally, we examined the exocyst, which is involved in trafficking of vesicles from REs to the plasma membrane, and is important in Notch signaling.44-47 We found that the localization and levels of Sec6 and Sec8 exocyst components were not altered in lgl mutant eye disc clones (Fig. 3E, F). Thus, although Rab11 RE accumulate in lgl− tissue,24 there is no effect on the levels or localization of Sec6 or Sec8 exocyst components. Furthermore, since the exocyst is required for E-cadherin (E-cad) trafficking,48 we investigated E-cad localization in lgl− clones and did not find any visible alterations. It is thus unlikely that exocyst function is altered in Lgl depleted eye epithelial tissue. These results, together with our recent study,24 support a specific role for Lgl in endosomal trafficking at the EE/early-MVB or RE stages.
Figure 3.
Lgl depletion affects vesicle trafficking at the early-MVB stage. lgl− third instar larval eye disc clones stained for Hrs (Early-MVB) (A), Arl8 (lysosome) (B), Dor (LE/lysosome) (C), SARA endosomes (D), Sec6 (exocyst) (E) and Sec8 (exocyst) (F) (gray or red in merges). Note that lgl− clones accumulate Hrs, but show normal levels and localization of other endosome or exocyst markers, compared with surrounding wild-type clones. lgl− clones are marked by the absence of GFP (green, arrowheads). Scale bar = 10 µM. Genotype for all panels: eyFLP; lgl27S3, FRT40, E(spl)m8-lacZ / Ubi-GFP, FRT40. Antibodies: Guinea Pig Hrs (1:500, H. Bellen), Rabbit dArl8 (1:500, I. Hofmann), Guinea pig Dor (H. Kramer, 1:100), Rabbit SARA (1:500, M. Gonzales Gaitan), Guinea pig Sec6 (1:1000, U. Tepass) and Guinea pig Sec8 (1:2000, U. Tepass).
Does Lgl Control N-Ext Cleavage Directly Via Regulation of V-ATPaseActivity or Indirectly Through Endosomal Maturation Defects?
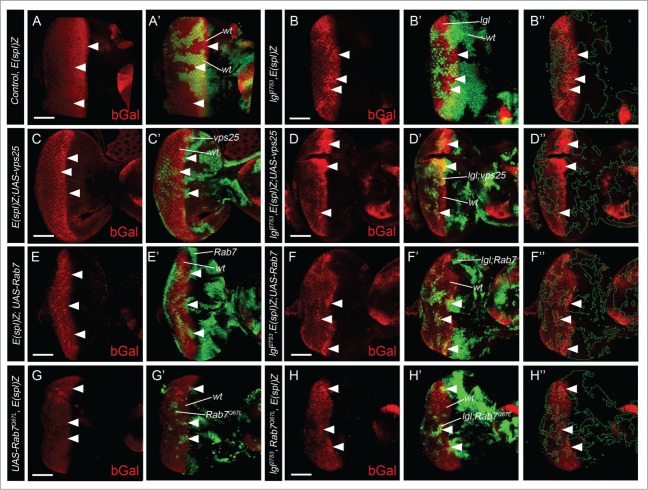
In analyzing the functional consequences of the endosomal trafficking defects upon Lgl depletion, we found that Dynamin (Shibire, initial step of vesicle budding from the plasma membrane) and Rab5 (EE) functions are required, but not Hrs/Stam (Escrt 0) or Rab11 (RE) functions, for elevated Notch signaling in lgl− tissue.24 While markers for other Escrt components in multivesicular body function are not available to determine whether they are altered in lgl− tissue, it remains possible that Lgl might regulate their activity. Since defective Escrt I, II and III activity is associated with ectopic ligand-independent Notch signaling,33-35,49 we envisioned that upregulation of Escrt I-III components might rescue elevated Notch signaling in lgl− tissue. Accordingly, we tested whether overexpression of Vps25 (Escrt II)50 could restore the Notch target reporter, E(Spl)lacZ, expression to normal in lgl− clones, however no rescue was observed (arrowheads, Fig. 4C, D compared with Fig. A, B). Although the LE protein, Rab7, was not altered in Lgl depleted tissue,24 we hypothesized that its function may be defective. Consistent with a possible role for Rab7, a recent study revealed that a Rab7 deficiency leads to accumulation of Hrs+ vesicles (early-MVBs) in the Drosophila developing eye,51 similar to Lgl depletion. We therefore tested whether overexpression of wild-type or constitutively active Rab7 (Rab7Q67L) might rescue elevated E(Spl)lacZ gene expression in lgl− clones. However, neither wild-type Rab7 nor Rab7Q67L were capable of rescuing elevated Notch signaling in Lgl depleted tissue (arrowheads, Fig. 4E-H, compared with Fig. A, B). Thus, reduced Vps25 or Rab7 function is not responsible for elevated Notch activation in lgl− tissue. However, it remains to be determined whether other Escrt I-III components are affected in lgl mutant tissue and if they are required for the effect on Notch signaling in Lgl depleted tissue. Furthermore, Lgl might regulate the activity of an unknown Rab protein, given recent evidence for a role for rat Lgl1 in the regulation of the exocytic Rab10 in neural outgrowth.52 In this study, Lgl1 was shown to act by releasing the GDP dissociation inhibitor (GDI) from Rab10, and it is therefore possible that Lgl might regulate an endocytic Rab via a similar mechanism. Since we have shown that Rab5 (EE) activity is required for elevated Notch signaling in lgl− tissue,24 Rab5 is a potential candidate for a direct target of Lgl.
Figure 4.
Vps25 or Rab7 functions are not responsible for ectopic Notch signaling in Lgl depleted tissue. (A, B) β-gal staining (red) of wild-type (A), lgl− (B) E(spl)m8-lacZ third instar larval mosaic eye discs, showing upregulation of the Notch target in lgl− clones just posterior to the Morphogenetic Furrow where ligand-dependent Notch signaling occurs (arrowheads). lgl− mutant tissue is GFP (green)-negative (arrowheads). (C, D) β-gal staining (red) of vps25 (C) and lgl− vps25 (D) E(spl)m8-lacZ third instar larval mosaic eye discs, showing no substantial rescue of the elevated β-gal expression in lgl− clones. Mutant tissue is marked by GFP (green, arrowheads). (E-H) β-gal staining (red) of Rab7WT (E) Rab7Q67L (F) lgl− Rab7WT (G) and lgl− Rab7Q67L (H) E(spl)m8-lacZ third instar larval mosaic eye discs, showing no substantial effect on the elevated β-gal expression in lgl− clones. Mutant tissue is GFP-positive (green, arrowheads). Scale bar = 50 µM. Green-dashed line in B,” D,” F” and H” outline the clones. Genotypes: (A) eyFLP; FRT40, E(spl)m8-lacZ / Ubi-GFP, FRT40 (B) eyFLP; lgl27S3, FRT40, E(spl)m8-lacZ / Ubi-GFP, FRT40 (C) eyFLP, UAS-GFP; FRT40, E(spl)m8-lacZ; UAS-vps25/ tubGAL80; tubGAL4 (D) eyFLP, UAS-GFP; lgl27S3, FRT40, E(spl)m8-lacZ; UAS-vps25/ tubGAL80,FRT40; tubGAL4 (E) eyFLP, UAS-GFP; FRT40, E(spl)m8-lacZ; UAS-Rab7-GFP/ tubGAL80,FRT40; tubGAL4 (F) eyFLP, UAS-GFP; lgl27S3, FRT40, E(spl)m8-lacZ; UAS-Rab7-GFP/ tubGAL80,FRT40; tubGAL4 (G) eyFLP, UAS-GFP; UAS-YFP-Rab7Q67L, FRT40, E(spl)m8-lacZ/ tubGAL80,FRT40; tubGAL4 (H) eyFLP, UAS-GFP; lgl27S3, UAS-YFP-Rab7Q67L, FRT40, E(spl)m8-lacZ/ tubGAL80,FRT40; tubGAL4 Flystocks: UAS-vps25–7 (T. Vaccari), UAS-Rab7-GFP (BL42706), UAS-YFP-Rab7Q67L (BL 24103, Bloomington Stock Center). Antibody: mouse β-galactosidase (Sigma, 1:500).
Alternatively, Lgl might function in Notch signaling via regulation of the vacuolar ATPase (V-ATPase), which is required for vesicular acidification and γ-secretase activity.37,38,53 Lgl could negatively regulate V-ATPase activity by affecting the localization of V-ATPase in the membrane, the composition of V-ATPase isoforms or the assembly of the V0 and V1 V-ATPase complexes. Since increased Vha44 (V-ATPase subunit C) expression results in increased Notch signaling,37 it is also possible that Lgl regulates the level of this V-ATPase component to modulate in vesicle acidification. Thus, further research is required to determine whether V-ATPase function is increased or required for elevated Notch signaling in lgl− tissue.
Vesicle Acidification is Important for the Overgrowth Phenotype of the lgl Mutant Mosaics
Our recent study revealed that lgl− clones accumulated acidic vesicles, and vesicle acidification was necessary for elevated Notch signaling and overgrowth of Lgl deficient tissue.24 Specifically, we found the deacidifying compound, chloroquine,54 rescued ectopic Notch signaling and the lgl− adult eye phenotype.24 This is not due to the elimination of lgl− tissue, since large lgl− clones (GFP-negative) were present in larval eye discs24 and in adult eyes (dark areas, arrowheads, Fig. 5A, B). Thus, lgl− tissue overgrowth, as well as morphological defects that occur during the pupal stage and contribute to the adult eye phenotype,10 are strongly rescued by feeding larvae chloroquine.24 Blocking upregulation of Notch in lgl− tissue by mamDN expression did not rescue adult eye defects to the same extent as chloroquine treatment,24 therefore we predict that chloroquine is impacting other signaling pathways or processes that are defective in the lgl− tissue. Vesicle acidification, and its inhibition by chloroquine, has been reported to affect various signaling pathways in addition to Notch, including mTorc1, Wnt/Wingless, PI3K-Akt and hypoxia pathways.55-63 Furthermore, chloroquine is being trialled as an anti-cancer agent where its mode of action has been proposed to act by blocking autophagy, which is a catabolic process where cellular proteins and organelles are targeted for degradation in the lysosome in order to generate energy (reviewed by64-67). This raised the possibility that Lgl deficient tissue might exhibit increased autophagy and chloroquine may act to suppress this. To determine whether Lgl depletion promoted autophagy, we stained lgl− mosaic eye discs for p62, a marker of autophagic flux.68 However, lgl− tissue did not exhibit changes in p62 levels (arrowheads, Fig. 5C), indicating that autophagy is not affected. This result suggests that it is unlikely that autophagy induction is contributing to the lgl− phenotype and that chloroquine rescues the Lgl deficient phenotype by blocking autophagy. Since we know that the Hippo negative growth signaling pathway is impaired in lgl− tissue and contributes to its tissue growth defects9 (see Fig. 1), this raises the question of whether the Hippo pathway might also be regulated by endocytosis or vesicle acidification and be a target of chloroquine in lgl− tissue. Interestingly, recent proteomics analysis of the Hippo pathway uncovered many endocytosis proteins as interactors with core Hippo pathway components.69 Furthermore, downregulation of endocytic regulators impairs Hippo signaling,70 although whether this occurs without affecting cell polarity is unclear. Clearly, further analysis in needed to dissect the molecular mechanism by which chloroquine acts to rescue the lgl− defects and whether this is associated with Hippo pathway restoration.
Figure 5.
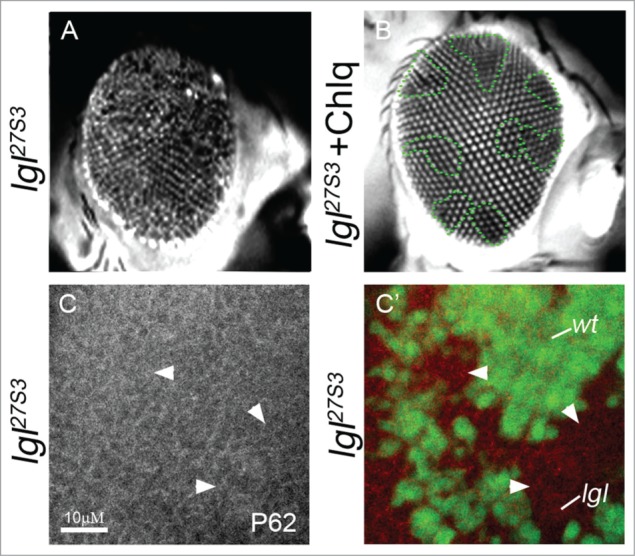
Chloroquine rescues the lgl− adult eye phenotype without affecting autophagy. (A, B) Adult eyes viewed under the fluorescent-microscope; (A) GFP-negatively marked lgl mosaic eyes from mock-treated larvae, and (B) lgl mosaic eyes from larvae fed chloroquine (at 1 mg/mL). GFP-negatively marked lgl− clones (dark) are still detected in the adult eyes when treated with chloroquine. Note, the lgl− eye phenotype is dramatically rescued by chloroquine treatment. (C) p62 staining (gray or red in merge) of lgl− mosaic third instar larval eye discs showing no obvious change between lgl− and wild-type tissue. lgl− clones are marked by the absence of GFP (green). Genotypes: (A–C) eyFLP; lgl27S3, FRT40, E(spl)m8-lacZ / Ubi-GFP, FRT40. Antibody: Rabbit p62 (1:2000, G. Juhasz).
The Regulation of Endocytosis and Notch Signaling by Apical-Basal Cell Polarity proteins during development
While our study has revealed a role for Lgl in endocytic regulation of Notch signaling, independent of aPKC, in the developing eye neuroepithelium,24 alternative regulatory mechanisms for Lgl in Notch signaling have been described in other Drosophila neural tissues.71-74 In the Drosophila neuroblasts within the central and peripheral nervous systems, Lgl is required for the basal localization of Numb (a negative regulator of Notch), which segregates into daughter cells upon cell division and inhibits Notch signaling, thereby enabling neural cell differentiation.72 In these cells, Lgl acts by sequestering aPKC, which when free phosphorylates Numb and prevents its localization to the cell cortex on one side of the cell.75,76 Lgl also prevents Sanpodo (a transmembrane protein that binds Notch and promotes its endocytosis) from localizing to the plasma membrane and thereby inhibits Notch signaling. In contrast, in another type of neural stem cell in Drosophila, the sensory organ precursors (SOPs), Lgl acts in parallel to Numb to block Notch function.71 This regulation most likely occurs via Lgl's effect on the plasma membrane localization of Sanpodo,73 by controlling the endocytosis of Sanpodo.74 The precise mechanism by which Lgl acts to control endocytosis in these cells is unknown, however this endocytic role for Lgl may be fundamental in its modus operandi in controlling Notch signaling in all neural tissues. In non-neural Drosophila tissues, whether Lgl plays a role in regulating Notch signaling and the dependency on aPKC requires further investigation. Our analysis (this study) has revealed that overexpression of Lgl represses ectopic Notch signaling in the epithelial cells of the wing notum (that generates the adult thorax), although we presently do not know whether this is aPKC-dependent. Moreover, further studies are required to determine if Lgl plays a physiological role in Notch signaling in the wing epithelial tissue. However, in another non-neural tissue, the ovarian follicle cells, functional analysis has shown that Lgl is not involved in regulating Notch signaling.36,77 Thus, there may be developmental and tissue-type requirements for Drosophila Lgl in Notch signaling and different mechanisms involved in each context.
Similarly in vertebrates, from limited information currently available, Lgl and aPKC have been shown to regulate Notch signaling via different mechanisms in different systems.78,79 During mouse brain development, the Lgl ortholog, Llgl1, is involved in the differentiation of neuro-progenitor cells.78 These Llgl1 mutant neuro-progenitor cells fail to exit the cell cycle and differentiate, which is associated with the failure to segregate Numb and inhibit Notch signaling in the progenitor cells. Whether aPKC is involved here was not investigated, however, in the chick central nervous system and myogenic precursors, an aPKC-dependent mechanism controls Notch signaling.79 In these cells, aPKCζ regulates ligand-dependent Notch1 signaling via phosphorylation of the Notch1 receptor on Ser-1791, which promotes trafficking of ligand-activated Notch1 to the nucleus, where it activates target gene expression. In the absence of ligand, aPKC instead promotes Notch1 internalization from the cell surface and from the secretory golgi-ER pathway to intracellular vesicles, where it is inactive. However, this mechanism is unlikely to occur in Drosophila, since the Drosophila Notch protein lacks the conserved Ser-1791 residue. In order to reconcile these studies, it will be important to determine whether Numb or Sanpodo are also regulated by aPKC in the chick central nervous system and myogenic precursors to contribute to Notch signaling, and in the mouse whether aPKC is involved and regulates Notch1 directly, as well as via Numb.
In our studies in the Drosophila eye epithelium, we have separated Lgl's role in tissue growth and in Hippo and Notch signaling from its cell polarity role.9,10,24 A polarity independent role has also been discovered for Lgl in the zebrafish retinal neuroepithelial cells in regulating Notch signaling.80 Here depletion of zebrafish Lgl, Llgl1, results in elevated Notch signaling and neurogenesis defects. Furthermore, blocking Notch activity in the Llgl1 depleted retina rescues the neurogenesis defects, showing that aberrant Notch signaling is critical for this occurrence. The mechanism of Notch activation by Llgl depletion in this tissue is thought to be due to the expansion of the apical cellular domain and Notch receptor accumulation, although whether endocytosis defects also occur requires further investigation. Also whether the Hippo pathway is impaired in the zebrafish Lgl1 depleted retinal neuroepithelial cells would be interesting to determine.
In our previous study, we found that Lgl acts distinctly from Scrib and Dlg in the regulation of Hippo signaling.15 However, a recent study in Drosophila epithelial cells has revealed a novel trafficking role for Lgl, together with Scrib and Dlg.81 This study showed that lgl, scrib and dlg mutants show defective trafficking of retromer cargo, such as Crb and Wntless (GPR177), to the apical membrane via aPKC-dependent and aPKC-independent mechanisms. Scrib was also found to have a role in retromer cargo trafficking in mammalian cells,82 although there are differences in the mechanism involved compared with Drosophila.81 In contrast to our system, where Lgl depleted cells do not lose polarity9,10,24 in the de Vreede study cell polarity was lost, and since Lgl regulates Dlg and Scrib localization and function,7 lgl, scrib and dlg mutant cells would be expected to behave similarly. Thus, it remains to be clarified whether Lgl is a direct regulator of the retromer trafficking pathway. However, the localization, levels or function of apical Crb complex components are not affected in lgl− mosaic larval eye epithelia.9,10 This is important in regard to Notch signaling, since Crb also regulates Notch signaling/endocytosis separate to its role in cell polarity.83 The extracellular Crb domain negatively regulates Notch signaling possibly by affecting S2 cleavage of Notch, as well as Notch and Delta endocytosis, whereas cell polarity function is coordinated by the Crb intracellular domain. Furthermore, Crb regulation of Notch signaling is distinct to that of Lgl, since Crb loss-of-function affects trafficking of full-length Notch,83 which is not observed in Lgl depleted tissue.24 Thus, Crb and Lgl affect Notch signaling by distinct mechanisms, and both have separable roles in the regulation of endocytosis/Notch signaling versus their roles in apical-basal cell polarity control.
Implications for Human Cancer
Improved cancer diagnosis relies on the establishment of tools that detect the earliest stages of cellular transformation. Our discovery that Lgl depletion in Drosophila epithelial cells results in sustained Notch activation indicates that the deregulated activity of neoplastic tumor suppressors can impact on cell signaling pathways prior to changes in cell polarity. In lgl− tissue, the effect of Notch signaling on expression of its targets, Cyclin A and Rst, is expected to contribute to the cell proliferative and survival effects observed in Lgl depleted tissue,24 together with impairment of Hippo signaling and Yki activation.9 Indeed the incomplete suppression of the lgl− adult eye phenotype by mamDN, aPKCCAAX-DN or baz-RNAi individually, suggests that aPKC-dependent (Yki activation) and Notch-dependent mechanisms contribute to the eye developmental defects of lgl− mosaic flies.24 Lgl acts as a tumor suppressor in human cancers, showing downregulation or mislocalization in many epithelial tumor types (reviewed by5). Deregulation of Lgl function might occur directly through mutation or indirectly via transcriptional regulation or polarity changes due to cells undergoing an epithelial to mesenchymal transition. Elevated Notch signaling is also associated with leukemia, breast and brain cancer (reviewed by25,84), although how this is related to Lgl function is presently unknown. Given our findings it will be important to investigate the connection between Lgl and Notch and Hippo signaling in tumorigenesis in cell lines or mouse models.
Furthermore, our discovery of a novel function for Drosophila Lgl in the regulation of endosomal acidification, and the striking suppressing effect of chloroquine treatment on the adult lgl− mosaic phenotype, reveals the importance of acidification in tumor growth. Indeed, higher acidity appears to be important for cancer progression and metastasis (reviewed by85). V-ATPase subunits are upregulated in several human cancers, and are associated with increased tumor growth and metastasis,86-88 and reducing acidity can abrogate tumorigenesis.89,90 It is therefore important to investigate whether Lgl might be linked to the regulation of acidification in human cancers.
Chloroquine is a widely-used and well-tolerated FDA-approved drug for the treatment of malaria and inflammatory conditions, such as rheumatoid arthritis and systemic lupus erythematosus (reviewed by56). More recently, chloroquine is being trialled as an anti-cancer agent with varying success (reviewed by64,67,91-93). The rationale for using chloroquine as an anti-cancer therapy has been to block autophagy, and since autophagy has context-dependent effects on tumor growth and metastasis (reviewed by67,94), chloroquine's therapeutic success may dependent on the type and grade of the cancer. Moreover, given chloroquine's effect on specific signaling pathways,55-63 the molecular profile of each patient's cancer needs to be considered to triage patients to select those who would most benefit from chloroquine therapy. Based on the dramatic rescue of the lgl− phenotype that we have observed,24 our studies suggest a novel use for this compound as an anti-cancer therapy against Notch and polarity defective cancers.
In summary, our studies in Drosophila have revealed that Lgl depletion leads to defects in endocytosis and key signaling pathways that promote tissue overgrowth. Based on our findings and given the importance of Lgl and Notch in human cancers, further investigation of the relationship between Lgl, cell polarity, endocytosis, regulation of acidification and Notch signaling in normal and malignant tissues is warranted. Moreover, the possible restoration of the Hippo pathway defects exhibited in the lgl− tissue by chloroquine highlights the need to further explore possible connections between the Hippo pathway, endocytosis and vesicle acidification. Such studies have the potential to reveal novel therapeutic avenues to treat human cancers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Patrick Humbert for critical reading of this manuscript.
Funding
HER is supported by a Senior Research Fellowship from the National Health and Medical Research Council, Australia. This work was supported by grants from the Cancer Council Victoria #APP1041817 to HER and CASS Foundation #SM/13/4847 to LMP.
References
- 1.Brumby AM, Richardson HE. Using Drosophila melanogaster to map human cancer pathways. Nat Rev Cancer 2005; 5:626-39; PMID:16034367; http://dx.doi.org/ 10.1038/nrc1671 [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez C. Drosophila melanogaster: a model and a tool to investigate malignancy and identify new therapeutics. Nat Rev Cancer 2013; 13:172-83; PMID:23388617; http://dx.doi.org/ 10.1038/nrc3461 [DOI] [PubMed] [Google Scholar]
- 3.Rudrapatna VA, Cagan RL, Das TK. Drosophila cancer models. Dev Dyn 2012; 241:107-18; PMID:22038952; http://dx.doi.org/ 10.1002/dvdy.22771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng L, Parsons LM, Richardson HE. Modelling cancer in Drosophila – The next generation. (version 2.0). Encyclopedia Life Sci (eLS Wiley) 2013; http://dx.doi.org/ 10.1002/9780470015902.a0020862.pub2 [DOI] [Google Scholar]
- 5.Elsum I, Yates L, Humbert PO, Richardson HE. The Scribble-Dlg-Lgl polarity module in development and cancer: from flies to man. Essays Biochem 2012; 53:141-68; PMID:22928514; http://dx.doi.org/ 10.1042/bse0530141 [DOI] [PubMed] [Google Scholar]
- 6.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol 2003; 5:53-8; PMID:12510194; http://dx.doi.org/ 10.1038/ncb897 [DOI] [PubMed] [Google Scholar]
- 7.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 2000; 289:113-6; PMID:10884224; http://dx.doi.org/ 10.1126/science.289.5476.113 [DOI] [PubMed] [Google Scholar]
- 8.Tanentzapf G, Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol 2003; 5:46-52; PMID:12510193; http://dx.doi.org/ 10.1038/ncb896 [DOI] [PubMed] [Google Scholar]
- 9.Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol 2010; 20:573-81; PMID:20362447; http://dx.doi.org/ 10.1016/j.cub.2010.01.055 [DOI] [PubMed] [Google Scholar]
- 10.Grzeschik NA, Amin N, Secombe J, Brumby AM, Richardson HE. Abnormalities in cell proliferation and apico-basal cell polarity are separable in Drosophila lgl mutant clones in the developing eye. Dev Biol 2007; 311:106-23; PMID:17870065; http://dx.doi.org/ 10.1016/j.ydbio.2007.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson BJ. Cell polarity: models and mechanisms from yeast, worms and flies. Development 2013; 140:13-21; PMID:23222437; http://dx.doi.org/ 10.1242/dev.083634 [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Zhang M. The Par3/Par6/aPKC complex and epithelial cell polarity. Exp Cell Res 2013; 319:1357-64; PMID:23535009; http://dx.doi.org/ 10.1016/j.yexcr.2013.03.021 [DOI] [PubMed] [Google Scholar]
- 13.Fletcher GC, Lucas EP, Brain R, Tournier A, Thompson BJ. Positive feedback and mutual antagonism combine to polarize Crumbs in the Drosophila follicle cell epithelium. Curr Biol 2012; 22:1116-22; PMID:22658591; http://dx.doi.org/ 10.1016/j.cub.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 14.Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem J 2011; 436:213-24; PMID:21568941; http://dx.doi.org/ 10.1042/BJ20110217 [DOI] [PubMed] [Google Scholar]
- 15.Grzeschik NA, Parsons LM, Richardson HE. Lgl, the SWH pathway and tumorigenesis: it's a matter of context & competition! Cell Cycle 2010; 9:3202-12; PMID:20724829; http://dx.doi.org/ 10.4161/cc.9.16.12633 [DOI] [PubMed] [Google Scholar]
- 16.Parsons LM, Grzeschik NA, Allott ML, Richardson HE. Lgl/aPKC and Crb regulate the Salvador/Warts/Hippo pathway. Fly (Austin) 2010; 4:288-93; PMID:20798605; http://dx.doi.org/ 10.4161/fly.4.4.13116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci U S A 2010; 107:10532-7; PMID:20498073; http://dx.doi.org/ 10.1073/pnas.1004279107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, Halder G. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A 2010; 107:15810-5; PMID:20798049; http://dx.doi.org/ 10.1073/pnas.1004060107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol 2010; 20:582-90; PMID:20362445; http://dx.doi.org/ 10.1016/j.cub.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribeiro P, Holder M, Frith D, Snijders AP, Tapon N. Crumbs promotes expanded recognition and degradation by the SCF(Slimb/beta-TrCP) ubiquitin ligase. Proc Natl Acad Sci U S A 2014; 111:E1980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsons LM, Grzeschik NA, Richardson HE. lgl regulates the hippo pathway independently of Fat/Dachs, Kibra/Expanded/Merlin and dRASSF/dSTRIPAK. Cancers (Basel) 2014; 6:879-96; PMID:24743776; http://dx.doi.org/ 10.3390/cancers6020879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doggett K, Grusche FA, Richardson HE, Brumby AM. Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling. BMC Dev Biol 2011; 11:57; PMID:21955824; http://dx.doi.org/ 10.1186/1471-213X-11-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong GR, Goulding KR, Amin N, Richardson HE, Brumby AM. scribble mutants promote aPKC and JNK-dependent epithelial neoplasia independently of Crumbs. BMC Biol 2009; 7:62; PMID:19778415; http://dx.doi.org/ 10.1186/1741-7007-7-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons LM, Portela M, Grzeschik NA, Richardson HE. Lgl regulates Notch signaling via endocytosis, independently of the apical aPKC-Par6-Baz polarity complex. Curr Biol 2014; 24:2073-84; PMID:25220057; http://dx.doi.org/ 10.1016/j.cub.2014.07.075 [DOI] [PubMed] [Google Scholar]
- 25.Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev 2007; 17:52-9; PMID:17178457; http://dx.doi.org/ 10.1016/j.gde.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 26.Louvi A, Artavanis-Tsakonas S. Notch and disease: a growing field. Semin Cell Dev Biol 2012; 23:473-80; PMID:22373641; http://dx.doi.org/ 10.1016/j.semcdb.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Artavanis-Tsakonas S, Muskavitch MA. Notch: the past, the present, and the future. Curr Top Dev Biol 2010; 92:1-29; PMID:20816391; http://dx.doi.org/ 10.1016/S0070-2153(10)92001-2 [DOI] [PubMed] [Google Scholar]
- 28.Ntziachristos P, Lim JS, Sage J, Aifantis I. From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer Cell 2014; 25:318-34; PMID:24651013; http://dx.doi.org/ 10.1016/j.ccr.2014.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musse AA, Meloty-Kapella L, Weinmaster G. Notch ligand endocytosis: mechanistic basis of signaling activity. Semin Cell Dev Biol 2012; 23:429-36; PMID:22306180; http://dx.doi.org/ 10.1016/j.semcdb.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kandachar V, Roegiers F. Endocytosis and control of Notch signaling. Curr Opin Cell Biol 2012; 24:534-40; PMID:22818956; http://dx.doi.org/ 10.1016/j.ceb.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto S, Charng WL, Bellen HJ. Endocytosis and intracellular trafficking of Notch and its ligands. Curr Top Dev Biol 2010; 92:165-200; PMID:20816395; http://dx.doi.org/ 10.1016/S0070-2153(10)92005-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furthauer M, Gonzalez-Gaitan M. Endocytic regulation of notch signalling during development. Traffic 2009; 10:792-802; PMID:19416471; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00914.x [DOI] [PubMed] [Google Scholar]
- 33.Fortini ME, Bilder D. Endocytic regulation of Notch signaling. Curr Opin Genet Dev 2009; 19:323-8; PMID:19447603; http://dx.doi.org/ 10.1016/j.gde.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaccari T, Rusten TE, Menut L, Nezis IP, Brech A, Stenmark H, Bilder D. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J Cell Sci 2009; 122:2413-23; PMID:19571114; http://dx.doi.org/ 10.1242/jcs.046391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell 2005; 9:687-98; PMID:16256743; http://dx.doi.org/ 10.1016/j.devcel.2005.09.019 [DOI] [PubMed] [Google Scholar]
- 36.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol 2008; 180:755-62; PMID:18299346; http://dx.doi.org/ 10.1083/jcb.200708127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petzoldt AG, Gleixner EM, Fumagalli A, Vaccari T, Simons M. Elevated expression of the V-ATPase C subunit triggers JNK-dependent cell invasion and overgrowth in a Drosophila epithelium. Dis Model Mech 2013; 6:689-700; PMID:23335205; http://dx.doi.org/ 10.1242/dmm.010660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaccari T, Duchi S, Cortese K, Tacchetti C, Bilder D. The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development 2010; 137:1825-32; PMID:20460366; http://dx.doi.org/ 10.1242/dev.045484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sriram V, Krishnan KS, Mayor S. Deep-orange and carnation define distinct stages in late endosomal biogenesis in Drosophila melanogaster. J Cell Biol 2003; 161:593-607; PMID:12743107; http://dx.doi.org/ 10.1083/jcb.200210166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofmann I, Munro S. An N-terminally acetylated Arf-like GTPase is localised to lysosomes and affects their motility. J Cell Sci 2006; 119:1494-503; PMID:16537643; http://dx.doi.org/ 10.1242/jcs.02958 [DOI] [PubMed] [Google Scholar]
- 41.Coumailleau F, Furthauer M, Knoblich JA, Gonzalez-Gaitan M.. Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nature 2009; 458:1051-5; PMID:19295516; http://dx.doi.org/ 10.1038/nature07854 [DOI] [PubMed] [Google Scholar]
- 42.Montagne C, Gonzalez-Gaitan M. Sara endosomes and the asymmetric division of intestinal stem cells. Development 2014; 141:2014-23; PMID:24803650; http://dx.doi.org/ 10.1242/dev.104240 [DOI] [PubMed] [Google Scholar]
- 43.Bokel C, Schwabedissen A, Entchev E, Renaud O, Gonzalez-Gaitan M. Sara endosomes and the maintenance of Dpp signaling levels across mitosis. Science 2006; 314:1135-9; PMID:17110576; http://dx.doi.org/ 10.1126/science.1132524 [DOI] [PubMed] [Google Scholar]
- 44.Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell 2005; 9:351-63; PMID:16137928; http://dx.doi.org/ 10.1016/j.devcel.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 45.Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J Cell Biol 2005; 169:635-46; PMID:15897260; http://dx.doi.org/ 10.1083/jcb.200410081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol 2005; 12:879-85; PMID:16155582; http://dx.doi.org/ 10.1038/nsmb987 [DOI] [PubMed] [Google Scholar]
- 47.Murthy M, Ranjan R, Denef N, Higashi ME, Schupbach T, Schwarz TL. Sec6 mutations and the Drosophila exocyst complex. J Cell Sci 2005; 118:1139-50; PMID:15728258; http://dx.doi.org/ 10.1242/jcs.01644 [DOI] [PubMed] [Google Scholar]
- 48.Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell 2005; 9:355-76; PMID:16224820; http://dx.doi.org/ 10.1016/j.devcel.2005.07.013 [DOI] [PubMed] [Google Scholar]
- 49.Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, Cohen SM. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell 2005; 9:711-20; PMID:16256745; http://dx.doi.org/ 10.1016/j.devcel.2005.09.020 [DOI] [PubMed] [Google Scholar]
- 50.Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, Bergmann A. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development 2006; 133:1871-80; PMID:16611691; http://dx.doi.org/ 10.1242/dev.02356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cherry S, Jin EJ, Ozel MN, Lu Z, Agi E, Wang D, Jung WH, Epstein D, Meinertzhagen IA, Chan CC, Hiesinger PR. Charcot-Marie-Tooth 2B mutations in rab7 cause dosage-dependent neurodegeneration due to partial loss of function. eLIFE 2013; 2:1-22; ; http://dx.doi.org/ 10.7554/eLife.01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T, Liu Y, Xu XH, Deng CY, Wu KY, Zhu J, Fu XQ, He M, Luo ZG. Lgl1 activation of rab10 promotes axonal membrane trafficking underlying neuronal polarization. Dev Cell 2011; 21:431-44; PMID:21856246; http://dx.doi.org/ 10.1016/j.devcel.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 53.Yan Y, Denef N, Schupbach T. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell 2009; 17:387-402; PMID:19758563; http://dx.doi.org/ 10.1016/j.devcel.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Homewood CA, Warhurst DC, Peters W, Baggaley VC. Lysosomes, pH and the anti-malarial action of chloroquine. Nature 1972; 235:50-2; PMID:4550396; http://dx.doi.org/ 10.1038/235050a0 [DOI] [PubMed] [Google Scholar]
- 55.Kobia F, Duchi S, Deflorian G, Vaccari T. Pharmacologic inhibition of vacuolar H+ ATPase reduces physiologic and oncogenic Notch signaling. Mol Oncol 2014; 8:207-20; PMID:24309677; http://dx.doi.org/ 10.1016/j.molonc.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thome R, Lopes SC, Costa FT, Verinaud L. Chloroquine: modes of action of an undervalued drug. Immunol Lett 2013; 153:50-7; PMID:23891850; http://dx.doi.org/ 10.1016/j.imlet.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 57.Rives AF, Rochlin KM, Wehrli M, Schwartz SL, DiNardo S. Endocytic trafficking of Wingless and its receptors, Arrow and DFrizzled-2, in the Drosophila wing. Dev Biol 2006; 293:268-83; PMID:16530179; http://dx.doi.org/ 10.1016/j.ydbio.2006.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim JH, Park JW, Kim MS, Park SK, Johnson RS, Chun YS. Bafilomycin induces the p21-mediated growth inhibition of cancer cells under hypoxic conditions by expressing hypoxia-inducible factor-1alpha. Mol Pharmacol 2006; 70:1856-65; PMID:16940187; http://dx.doi.org/ 10.1124/mol.106.028076 [DOI] [PubMed] [Google Scholar]
- 59.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 2010; 327:459-63; PMID:20093472; http://dx.doi.org/ 10.1126/science.1179802 [DOI] [PubMed] [Google Scholar]
- 60.Hermle T, Saltukoglu D, Grunewald J, Walz G, Simons M. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol 2010; 20:1269-76; PMID:20579879; http://dx.doi.org/ 10.1016/j.cub.2010.05.057 [DOI] [PubMed] [Google Scholar]
- 61.Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, Niehrs C, Boutros M. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol 2010; 20:1263-8; PMID:20579883; http://dx.doi.org/ 10.1016/j.cub.2010.05.028 [DOI] [PubMed] [Google Scholar]
- 62.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012; 150:1196-208; PMID:22980980; http://dx.doi.org/ 10.1016/j.cell.2012.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011; 334:678-83; PMID:22053050; http://dx.doi.org/ 10.1126/science.1207056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kimura T, Takabatake Y, Takahashi A, Isaka Y. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res 2013; 73:3-7; PMID:23288916; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-2464 [DOI] [PubMed] [Google Scholar]
- 65.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 2012; 11:709-30; PMID:22935804; http://dx.doi.org/ 10.1038/nrd3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol 2013; 15:713-20; PMID:23817233; http://dx.doi.org/ 10.1038/ncb2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Cell 2013; 155:1216-9; PMID:24315093; http://dx.doi.org/ 10.1016/j.cell.2013.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeVorkin L, Gorski SM. Monitoring autophagic flux using Ref(2)P, the Drosophila p62 ortholog. Cold Spring Harb Protoc 2014; 2014:959-66; PMID:25183816 [DOI] [PubMed] [Google Scholar]
- 69.Kwon Y, Vinayagam A, Sun X, Dephoure N, Gygi SP, Hong P, Perrimon N. The Hippo signaling pathway interactome. Science 2013; 342:737-40; PMID:24114784; http://dx.doi.org/ 10.1126/science.1243971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson BS, Moberg KH. Drosophila endocytic neoplastic tumor suppressor genes regulate Sav/Wts/Hpo signaling and the c-Jun N-terminal kinase pathway. Cell Cycle 2011; 10:4110-8; PMID:22101275; http://dx.doi.org/ 10.4161/cc.10.23.18243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Justice N, Roegiers F, Jan LY, Jan YN. Lethal giant larvae acts together with numb in notch inhibition and cell fate specification in the Drosophila adult sensory organ precursor lineage. Curr Biol 2003; 13:778-83; PMID:12725738; http://dx.doi.org/ 10.1016/S0960-9822(03)00288-4 [DOI] [PubMed] [Google Scholar]
- 72.Ohshiro T, Yagami T, Zhang C, Matsuzaki F. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 2000; 408:593-6; PMID:11117747; http://dx.doi.org/ 10.1038/35046087 [DOI] [PubMed] [Google Scholar]
- 73.Langevin J, Le Borgne R, Rosenfeld F, Gho M, Schweisguth F, Bellaiche Y. Lethal giant larvae controls the localization of notch-signaling regulators numb, neuralized, and Sanpodo in Drosophila sensory-organ precursor cells. Curr Biol 2005; 15:955-62; PMID:15916953; http://dx.doi.org/ 10.1016/j.cub.2005.04.054 [DOI] [PubMed] [Google Scholar]
- 74.Roegiers F, Jan LY, Jan YN. Regulation of membrane localization of Sanpodo by lethal giant larvae and neuralized in asymmetrically dividing cells of Drosophila sensory organs. Mol Biol Cell 2005; 16:3480-7; PMID:15901829; http://dx.doi.org/ 10.1091/mbc.E05-03-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wirtz-Peitz F, Nishimura T, Knoblich JA. Linking cell cycle to asymmetric division: aurora-A phosphorylates the Par complex to regulate Numb localization. Cell 2008; 135:161-73; PMID:18854163; http://dx.doi.org/ 10.1016/j.cell.2008.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haenfler JM, Kuang C, Lee CY. Cortical aPKC kinase activity distinguishes neural stem cells from progenitor cells by ensuring asymmetric segregation of Numb. Dev Biol 2012; 365:219-28; PMID:22394487; http://dx.doi.org/ 10.1016/j.ydbio.2012.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian AG, Deng WM. Lgl and its phosphorylation by aPKC regulate oocyte polarity formation in Drosophila. Development 2008; 135:463-71; PMID:18094021; http://dx.doi.org/ 10.1242/dev.016253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev 2004; 18:559-71; PMID:15037549; http://dx.doi.org/ 10.1101/gad.1178004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sjoqvist M, Antfolk D, Ferraris S, Rraklli V, Haga C, Antila C, Mutvei A, Imanishi SY, Holmberg J, Jin S, et al.. PKCzeta regulates Notch receptor routing and activity in a Notch signaling-dependent manner. Cell Res 2014; 24:433-50; PMID:24662486; http://dx.doi.org/ 10.1038/cr.2014.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clark BS, Cui S, Miesfeld JB, Klezovitch O, Vasioukhin V, Link BA. Loss of Llgl1 in retinal neuroepithelia reveals links between apical domain size, Notch activity and neurogenesis. Development 2012; 139:1599-610; PMID:22492354; http://dx.doi.org/ 10.1242/dev.078097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Vreede G, Schoenfeld JD, Windler SL, Morrison H, Lu H, Bilder D. The Scribble module regulates retromer-dependent endocytic trafficking during epithelial polarization. Development 2014; 141:2796-802; PMID:25005475; http://dx.doi.org/ 10.1242/dev.105403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lohia M, Qin Y, Macara IG. The Scribble polarity protein stabilizes E-cadherin/p120-catenin binding and blocks retrieval of E-cadherin to the Golgi. PLoS One 2012; 7:e51130; PMID:23226478; http://dx.doi.org/ 10.1371/journal.pone.0051130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Richardson EC, Pichaud F. Crumbs is required to achieve proper organ size control during Drosophila head development. Development 2010; 137:641-50; PMID:20110329; http://dx.doi.org/ 10.1242/dev.041913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farnie G, Clarke RB. Mammary stem cells and breast cancer–role of Notch signalling. Stem Cell Rev 2007; 3:169-75; PMID:17873349; http://dx.doi.org/ 10.1007/s12015-007-0023-5 [DOI] [PubMed] [Google Scholar]
- 85.Hernandez A, Serrano-Bueno G, Perez-Castineira JR, Serrano A. Intracellular proton pumps as targets in chemotherapy: V-ATPases and cancer. Curr Pharm Des 2012; 18:1383-94; PMID:22360554; http://dx.doi.org/ 10.2174/138161212799504821 [DOI] [PubMed] [Google Scholar]
- 86.Otero-Rey EM, Somoza-Martin M, Barros-Angueira F, Garcia-Garcia A. Intracellular pH regulation in oral squamous cell carcinoma is mediated by increased V-ATPase activity via over-expression of the ATP6V1C1 gene. Oral Oncol 2008; 44:193-9; PMID:17467328; http://dx.doi.org/ 10.1016/j.oraloncology.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 87.Lu X, Qin W, Li J, Tan N, Pan D, Zhang H, Xie L, Yao G, Shu H, Yao M, et al.. The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res 2005; 65:6843-9; PMID:16061667; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-3822 [DOI] [PubMed] [Google Scholar]
- 88.Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y, Yasui N, Yoneda T. The a3 isoform vacuolar type H(+)-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res 2011; 9:845-55; PMID:21669964; http://dx.doi.org/ 10.1158/1541-7786.MCR-10-0449 [DOI] [PubMed] [Google Scholar]
- 89.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, et al.. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 2009; 69:2260-8; PMID:19276390; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 2011; 11:671-7; PMID:21833026; http://dx.doi.org/ 10.1038/nrc3110 [DOI] [PubMed] [Google Scholar]
- 91.Maycotte P, Gearheart CM, Barnard R, Aryal S, Mulcahy Levy JM, Fosmire SP, Hansen RJ, Morgan MJ, Porter CC, Gustafson DL, et al.. STAT3-mediated autophagy dependence identifies subtypes of breast cancer where autophagy inhibition can be efficacious. Cancer Res 2014; 74:2579-90; PMID:24590058; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maycotte P, Thorburn A. Targeting autophagy in breast cancer. World J Clin Oncol 2014; 5:224-40; PMID:25114840; http://dx.doi.org/ 10.5306/wjco.v5.i3.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morgan MJ, Gamez G, Menke C, Hernandez A, Thorburn J, Gidan F, Staskiewicz L, Morgan S, Cummings C, Maycotte P, et al.. Regulation of autophagy and chloroquine sensitivity by oncogenic RAS in vitro is context-dependent. Autophagy 2014; 10:1814-26; PMID:25136801; http://dx.doi.org/ 10.4161/auto.32135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.White E. Exploiting the bad eating habits of Ras-driven cancers. Genes Dev 2013; 27:2065-71; PMID:24115766; http://dx.doi.org/ 10.1101/gad.228122.113 [DOI] [PMC free article] [PubMed] [Google Scholar]