The purpose of the analysis was to provide summary data on CT dose derived from a large number of consecutive CT examinations that institutions can use as a starting point for evaluating the CT radiation doses they use in their patients.
Abstract
Purpose
To summarize data on computed tomographic (CT) radiation doses collected from consecutive CT examinations performed at 12 facilities that can contribute to the creation of reference levels.
Materials and Methods
The study was approved by the institutional review boards of the collaborating institutions and was compliant with HIPAA. Radiation dose metrics were prospectively and electronically collected from 199 656 consecutive CT examinations in 83 181 adults and 3871 consecutive CT examinations in 2609 children at the five University of California medical centers during 2013. The median volume CT dose index (CTDIvol), dose-length product (DLP), and effective dose, along with the interquartile range (IQR), were calculated separately for adults and children and stratified according to anatomic region. Distributions for DLP and effective dose are reported for single-phase examinations, multiphase examinations, and all examinations.
Results
For adults, the median CTDIvol was 50 mGy (IQR, 37–62 mGy) for the head, 12 mGy (IQR, 7–17 mGy) for the chest, and 12 mGy (IQR, 8–17 mGy) for the abdomen. The median DLPs for single-phase, multiphase, and all examinations, respectively, were as follows: head, 880 mGy · cm (IQR, 640–1120 mGy · cm), 1550 mGy · cm (IQR, 1150–2130 mGy · cm), and 960 mGy · cm (IQR, 690–1300 mGy · cm); chest, 420 mGy · cm (IQR, 260–610 mGy · cm), 880 mGy · cm (IQR, 570–1430 mGy · cm), and 550 mGy · cm (IQR 320–830 mGy · cm); and abdomen, 580 mGy · cm (IQR, 360–860 mGy · cm), 1220 mGy · cm (IQR, 850–1790 mGy · cm), and 960 mGy · cm (IQR, 600–1460 mGy · cm). Median effective doses for single-phase, multiphase, and all examinations, respectively, were as follows: head, 2 mSv (IQR, 1–3 mSv), 4 mSv (IQR, 3–8 mSv), and 2 mSv (IQR, 2–3 mSv); chest, 9 mSv (IQR, 5–13 mSv), 18 mSv (IQR, 12–29 mSv), and 11 mSv (IQR, 6–18 mSv); and abdomen, 10 mSv (IQR, 6–16 mSv), 22 mSv (IQR, 15–32 mSv), and 17 mSv (IQR, 11–26 mSv). In general, values for children were approximately 50% those for adults in the head and 25% those for adults in the chest and abdomen.
Conclusion
These summary dose data provide a starting point for institutional evaluation of CT radiation doses.
© RSNA, 2015
Introduction
Radiation doses for computed tomography (CT) vary considerably across institutions (1–3), and health oversight and quality organizations in the United States and Europe are urging facilities to measure and standardize CT doses (4–9). Diagnostic reference levels and summaries of CT radiation doses commonly used in practice could guide radiology facilities in quality improvement efforts to optimize CT. However, few CT diagnostic reference levels in the United States are based on large numbers of actual patient examinations; many U.S. guidelines are based on selected data submitted to demonstrate a facility’s best work (10–12), and these may not reflect the routine radiation doses to which patients are exposed. In contrast, guidelines and reference levels have been created in Europe to standardize CT radiation doses (13–18).
The University of California Dose Optimization and Standardization Endeavor is a collaboration across the University of California medical centers (University of California at Davis, Irvine, Los Angeles, San Diego, and San Francisco) to assess and optimize CT doses (19). We performed this study to summarize doses collected from consecutive CT examinations performed at 12 facilities. We provide this snapshot of summary dose data to help institutions evaluate CT doses and contribute to the development of radiation dose benchmarks (20). We believe these data can contribute to the creation of meaningful reference levels in the United States.
Materials and Methods
The University of California at San Francisco Committee on Human Research approved the study and waived the requirement to obtain informed consent. The other hospital institutional review boards relied on this approval. The study complied with requirements of the Health Insurance Portability and Accountability Act.
We collected data for all diagnostic CT scans obtained in 2013 at 12 facilities associated with the University of California medical centers. The CT examinations were performed with 34 scanners from five manufacturers: (a) Discovery CT750 HD, Lightspeed Ultra, Lightspeed 16, Lightspeed Plus, and Lightspeed VCT (GE Healthcare, Little Chalfont, Buckinghamshire, England); (b) Bodytom (Neurologica, Danvers, Mass); (c) iCT 128 and iCT 256 (Philips, Amsterdam, the Netherlands); (d) Sensation 16, Sensation 64, Somatom Definition, and Somatom Definition AS (Siemens, Erlangen, Germany); and (e) Aquilion and Aquilion One (Toshiba, Tokyo, Japan). Among these were one eight-section scanner and five 16-section scanners. The remaining scanners were 64-section units or higher.
Scan data were deidentified at the facility where they were obtained and uploaded to a single server by using Radimetrics (Bayer HealthCare, Whippany, NJ) (21), a software tool for monitoring and tracking patient radiation exposures from CT. Radimetrics extracts dose metrics from the Digital Imaging and Communications in Medicine tags through direct connections with the scanners or picture archiving and communication systems. Radimetrics calculates patient diameter from the midscan length to calculate the size-specific dose estimate (SSDE). To calculate effective dose, Radimetrics uses the library of Cristy phantoms (22) and matches patients to a particular phantom on the basis of age, weight, or diameter. For each phantom in the library, a set of Monte Carlo simulations are prerun for various scanning protocols with different examination parameters, and organ doses are calculated. The organ doses are used to calculate effective dose according to published International Commission on Radiological Protection publication 103 tissue-weighting factors (23). These data were then anonymized and electronically sent to a single server at the University of California at San Francisco, where the data were downloaded for analysis. CT examinations as part of positron emission tomography/CT and those performed for research, radiation oncology, surgical, or interventional procedures were excluded.
We report four dose metrics. Volume CT dose index (CTDIvol) reflects the average per-section radiation exposure, measured in milligrays referenced to a 16- or 32-cm acrylic cylindric phantom. SSDEs are reported in the chest and abdomen; this calculated measure essentially scales the CTDIvol for patient size by using the midscan region diameter (24). For a given CTDIvol, a smaller patient will have a higher SSDE, reflecting greater radiation exposure per unit of tissue, whereas a larger patient will have a smaller SSDE, reflecting lower radiation exposure per unit of tissue. Although this measure has been described only in the abdomen, we also report it in the chest because institutions are increasingly using it in this way. Dose-length product (DLP) is the product of CTDIvol and scan length, measured in milligray-centimeters, and reflects total radiation output for a CT scan. Effective dose, measured in millisieverts, combines information about radiation received by the patient with scan location to reflect organs irradiated and the potential for deleterious effects of radiation— primarily the risk of developing cancer. Data were collected for each radiating event (“scan”) and then combined and reported according to examination (including at least one radiating event). Although CTDIvol and DLP directly reflect energy output from the scanner, effective dose is a calculated measure that considers both the energy output of the scanner and the potential harm to the patient that is influenced by age and size (25). This measure is most accurate for estimating doses in populations and cannot be considered a precise reflection of risk in individual patients. When an anatomic region was imaged multiple times, as in multiphase studies, we averaged the SSDE and CTDIvol for each radiation event and added the DLPs and effective doses from each radiating event to obtain the total metrics for the examination. All results are presented at the examination level.
We used Radimetrics to extract patient sex, age, date and time of the examination, scan region, study description, protocol name, scanner manufacturer and model, CTDIvol, and DLP. Radimetrics also provided SSDE and effective dose. Examinations were characterized as single phase for single irradiating events or multiphase for more than one irradiating event. Among multiphase examinations, we calculated the mean, median, and mode of the number of phases. When determining the number of phases, we did not include brief scans obtained to determine timing for the injection of iodinated contrast material.
The median dose and interquartile range (IQR) were calculated separately for children and adults (age ≥14 years) and according to anatomic region. We report results for head, chest, or abdomen (all examinations that included the abdomen and/or the pelvis), combined chest and abdomen, neck, and sinus. These regions account for 79% of CT scans in adults and 71% of CT scans in children. We used software (SAS, version 9.3; SAS Institute, Cary, NC) for all analyses.
Results
Overall, 199 656 CT examinations were performed in 83 181 adults and 3871 CT examinations were performed in 2609 children during 2013. The examinations were done on 34 scanners across 12 facilities; all scanners were multidetector units, all had automated tube current modulation capability (which was used for 92% of scans in adults and 88% of scans in children), and 21 were equipped with iterative reconstruction for all or part of the year.
For adults, the most common areas imaged were the abdomen (63 167 of 199 656 examinations [32%]), head (32 663 of 199 656 examinations [16%]), chest (26 857 of 199 656 examinations [13%]), combined chest and abdomen (26 998 of 199 656 examinations [14%]), sinus (3950 of 199 656 examinations [2%]), and neck (3472 of 199 656 examinations [2%]) (Table 1). Overall, 21% of examinations (n = 42 549) were of other anatomic areas, and the doses from these examinations are not summarized herein. In children, the most frequently imaged areas were the head (1282 of 3871 examinations [33%]), abdomen (708 of 3871 examinations [18%]), chest (355 of 3871 examinations [9%]), combined chest and abdomen (84 of 3871 examinations [2%]), sinus (185 of 3871 examinations [5%]), and neck (119 of 3871 examinations [3%]) (Table 1). Overall, 29% of examinations (n = 1138) were of other anatomic areas; the doses from these scans are not summarized herein (Table 2). Multiphase scanning was used in 42% of examinations performed in adults and 14% of examinations performed in children (Tables 1 and 2). Among children and adults who underwent multiphase examinations, the median number of phases was two and the mode was two (ie, most multiphase studies used two phases). The mean number of phases was 2.5 for children and 2.9 for adults.
Table 1.
Radiation Dose Metrics in Adults
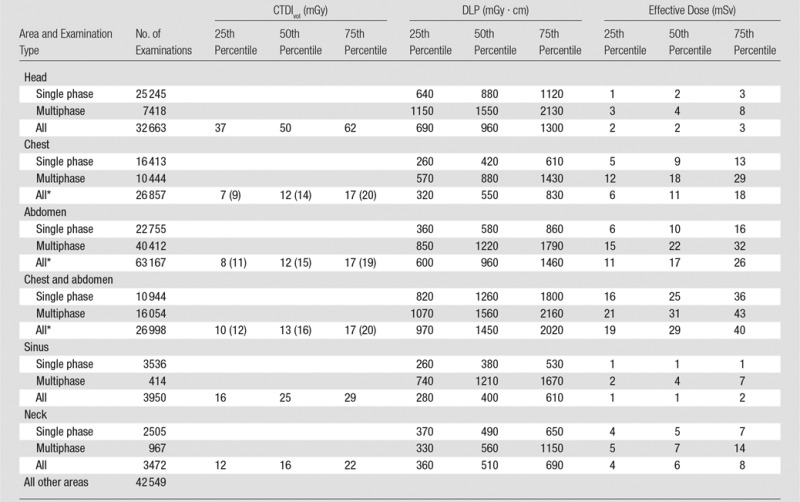
*Numbers in parentheses are SSDEs, which reflect an adjusted CTDIvol measurement.
Table 2.
Radiation Dose Metrics in Children
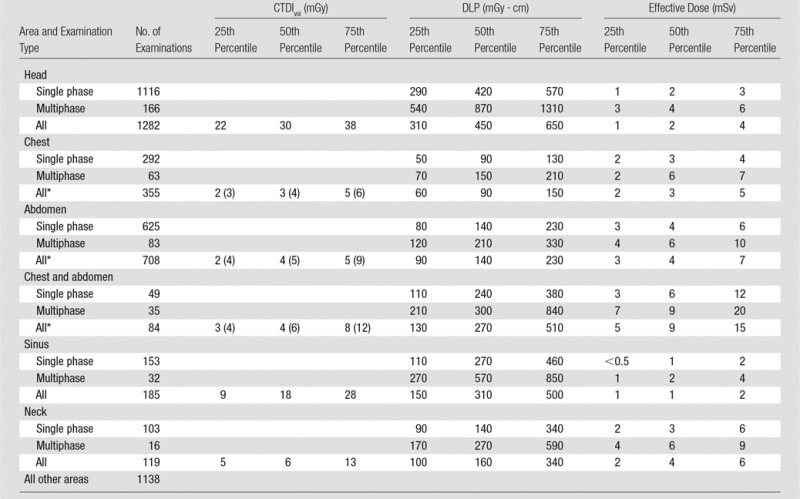
Note.—Examinations were performed in children younger than 1 year (n = 483 [12.5%]), 1–4 years (n = 949 [24.5%]), 5–9 years (n = 991 [25.6%]), and 10–14 years (n = 1448 [37.4%]).
*Numbers in parentheses are SSDEs, which reflect an adjusted CTDIvol measurement.
The median radiation doses and IQRs in adults are reported in Table 1. Median CTDIvol values were as follows: head, 50 mGy (IQR, 37–62 mGy); chest, 12 mGy (IQR, 7–17 mGy); and abdomen, 12 mGy (IQR, 8–17 mGy). Median SSDE values were 14 mGy (IQR, 9–20 mGy) in the chest and 15 mGy (IQR, 11–19 mGy) in the abdomen. Median DLPs for single-phase, multiphase, and all examinations, respectively, were as follows: head, 880 mGy · cm (IQR, 640–1120 mGy · cm), 1550 mGy · cm (IQR, 1150–2130 mGy · cm), and 960 mGy · cm (IQR, 690–1300 mGy · cm); chest, 420 mGy · cm (IQR, 260–610 mGy · cm), 880 mGy · cm (IQR, 570–1430 mGy · cm), and 550 mGy · cm (IQR, 320–830 mGy · cm); and abdomen, 580 mGy · cm (IQR 360–860 mGy · cm), 1220 mGy · cm (IQR, 850–1790 mGy · cm), and 960 mGy · cm (IQR, 600–1460 mGy · cm). Median effective doses for single-phase, multiphase, and all examinations, respectively, were as follows: head, 2 mSv (IQR, 1–3 mSv), 4 mSv (IQR, 3–8 mSv), and 2 mSv (IQR, 2–3 mSv); chest, 9 mSv (IQR, 5–13 mSv), 18 mSv (IQR, 12–29 mSv), and 11 mSv (IQR, 6–18 mSv); and abdomen, 10 mSv (IQR 6–16 mSv), 22 mSv (IQR, 15–32 mSv), and 17 mSv (IQR, 11–26 mSv). Effective doses and DLPs for multiphase examinations were approximately twice those for single-phase examinations.
In children, median and IQRs were substantially lower than those in adults (Table 2). Median CTDIvol values were as follows: head, 30 mGy (IQR, 22–38 mGy); chest, 3 mGy (IQR, 2–5 mGy); and abdomen, 4 mGy (IQR, 2–5 mGy). The doses for CT of the head were approximately 50% of those used in adults, and doses for CT of the chest and abdomen were approximately 25% of those used in adults. The median abdominal SSDE was 5 mGy (IQR, 4–9 mGy), and the median chest SSDE was 4 mGy (IQR, 3–6 mGy); these findings indicate that values in children were also considerably lower than those in adults. Most examinations in children were single phase, with the following median DLPs: head, 450 mGy (IQR, 310-650 mGy); chest, 90 mGy (IQR, 60-150 mGy); and abdomen, 140 mGy (IQR, 90-230 mGy). The median effective dose was 2 mSv (IQR, 1-4 mSv) for the head, 3 mSv (IQR, 2–5 mSv) for the chest, and 4 mSv (IQR, 3–7 mSv) for the abdomen.
Table 3 summarizes the 75th percentiles of the doses described above and can be used as a simple point of reference with which imaging centers can compare their own doses.
Table 3.
Summary of 75th Percentile Data
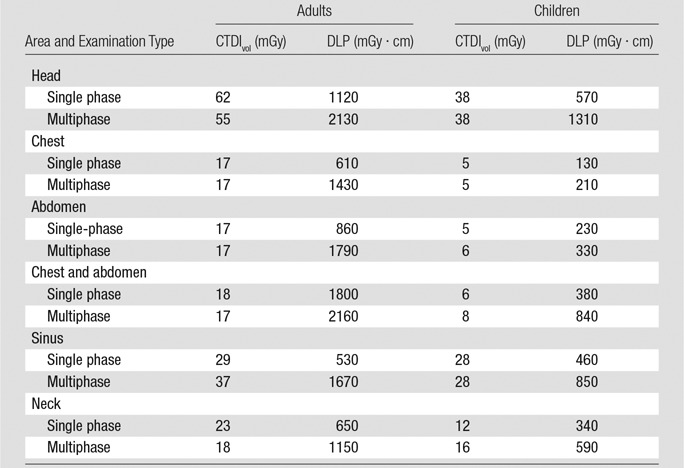
Note.—Data can be used as a point of reference.
Discussion
Herein we provide summary radiation dose data for CT dose metrics in adults and children based on a large number of consecutive scans obtained at the University of California medical centers. The measures, organized according to anatomic region and number of phases, offer practical data that CT imaging facilities can use as a starting point for assessing their own doses when more detailed and protocol-specific targets are not available. Our approach parallels the CT radiation dose measure adopte d by the National Quality Forum in 2011 (20). Furthermore, because the United States lacks summary data on radiation doses for CT based on large numbers of patients, our data can contribute to the creation of radiation dose benchmarks by oversight and health care quality organizations.
Facilities can use these summary data in two ways. First, they can compare their dose distributions to our reported values to determine whether their doses are within this attainable range. If distributions are considerably higher (eg, if medians are higher than our 75th percentiles), the institutions could review protocols and scanner settings. Institutions can also take a more aggressive approach to optimization as suggested by several authors, wherein institutions try to get doses below the 25th percentile (16). Indeed, institutions may already be able to obtain optimization of images below the 25th percentile we report. Second, in the absence of broadly accepted U.S. diagnostic reference levels, these summary data could be applied in a manner similar to that with which reference levels are used in the United Kingdom (26): A technologist setting scanner parameters for a patient could compare prescan CTDIvol and DLP values reported by the scanner with our summary data. If the planned scan would lead to doses higher than our 75th percentile—or even higher than the 50th percentile, depending on how aggressively an institution wants to optimize doses—with no clear clinical or patient-specific indications to exceed this level, a radiologist or physicist could be consulted to determine whether altering scanning parameters might be possible. The 75th percentiles for CTDIvol and DLP in adults and children are summarized in the one-page reference table for printing and posting at scanners.
Our reference doses differ from Dose Check—an alert function available on CT scanners indicating high preprogrammed CTDIvol values—in several important ways. First, if Dose Check values are set at levels based on the American Association of Physicists in Medicine recommendations (27), technologists will be notified for the top 5% of examinations. The ostensible purpose of this is to reduce the highest outlier doses by notifying technologists when they are likely to occur, without burdening technologists with too many alerts. In contrast, the reference values summarized in Table 3 are set at the highest 25% of examinations and are meant to increase dose awareness among technologists and radiologists regarding doses that can be routinely attained. The goal is to encourage dose optimization activities in order to reach these values on most patients while avoiding alerts. Reflecting these different thresholds, the value recommended by the American Association of Physicists in Medicine for head CT in adults is 80 mGy, whereas we report 62 mGy.
A second difference is that while Dose Check is triggered on the basis of expected CTDIvol values, we report dose data for four different metrics that institutions can use to understand where they may have higher than typical doses. For example, if an institution tends to use scanning lengths that are longer than average, or uses multiphase examinations more often than average, the DLP and effective dose metrics will reflect this, whereas CTDIvol values may not.
Institution-level radiation doses are often assessed by medical physicists, who compare doses within specific, narrowly defined protocols developed for particular clinical indications, where decisions are made to balance dose with image quality concerns. Although these efforts are a necessary part of dose optimization, assessing overall institutional performance according to protocol is difficult without widely adopted standards for naming or using protocols. The United States has thousands of protocol names, and use is not consistent across institutions—even for common indications (1). Furthermore, even if the protocols were named consistently, comparisons within protocols would not provide all of the information because they would not reflect how frequently the protocols were used. For example, comparing quad-phase liver examinations from one institution to another would indicate only how well the institution optimized dose for that examination, not whether the institution is overusing that examination type.
Our data, organized according to anatomic region, offer a practical way to compare CT dose levels across institutions and patients, and this approach has been used in other countries to compare practice (16–18). U.S. national efforts on creating CT dose-level benchmarks have primarily used survey data in which institutions report typical scanning parameters. Although the results are important and provide a starting point for optimizing radiation dose, they may not reflect the actual doses to which patients are exposed. For example, the International Atomic Energy Agency conducted a survey of radiation dose for CT in children across 40 underdeveloped countries. Although more than 90% of facilities said they had protocols specific for children, these protocols were not available or were used at no more than half of these facilities (5).
The reported metrics offer a variety of information that institutions can use for quality improvement activities. CTDIvol reflects specific scanning parameter choices and possible radiologist preferences related to image quality, and SSDE scales these parameter choices to the approximate size of the patient. DLP and effective dose reflect specific scanning parameter choices as well as the use of multiphase scanning. Factors that can be optimized to reduce patient dose without compromising diagnostic accuracy include reducing the number and scan length of multiphase scans; using dose reduction software for iterative reconstruction; standardizing scan protocols; and educating physicians, radiologists, and technologists on optimal diagnostic protocol selection. All dose-optimization mechanisms should be considered when CT doses are higher than dose targets.
We calculated effective dose by using Radimetrics, but other commercial and free shareware tools are available for calculating effective dose. Furthermore, simple methods and conversion factors exist for converting DLP, which is reported by more than 95% of U.S. CT scanners, to effective dose (28,29). Effective dose has the advantage of wide use and easy comparison with radiation from other imaging tests and environmental exposures.
Few U.S. data have assessed doses by anatomic area for a large number of consecutive scans to compare with the results we report. The American College of Radiology created reference levels as part of their CT certification program, but these levels are based on submissions of a small number of self-identified “best” cases rather than on typical cases or random cases from a facility. For example, facilities applying for American College of Radiology CT accreditation submit a small sample of images, usually three or four for each CT unit; the American College of Radiology assumes that the images submitted are examples of the facility’s best, not average, work (11). Although not directly comparable, the summary data reported herein are 20%–65% lower than the American College of Radiology reference levels (12). The American College of Radiology has also collected dose data as part of their Dose Index Registry, but these data have not been reported except for a single renal stone protocol (3) and the data from the registry are not publicly available. The National Council on Radiation Protection & Measurements report number 172 published benchmarks in the United States, but these do not reflect typical practice or large numbers of patients (12).
In contrast, there are considerable data and benchmarks from European countries with which to compare our data (13–18). As reference levels are typically set at the 75% level, we use the data presented in Table 3 to compare with these European publications. Pantos et al (18) summarized doses from 42 published studies, reporting similar doses in the head (mean effective dose, 1.9 vs 2.0 mSv) but lower doses in the chest (mean effective dose, 7.5 vs 12 mSv) and abdomen (mean effective dose, 15 vs 17 mSv). Tack et al (17) reported audit results in Luxembourg following a nationwide dose optimization effort and also found similar doses in the head (CTDIvol, 52 vs 50 mGy) and lower doses in the chest (CTDIvol, 7 vs 12 mGy) and abdomen (CTDIvol, 10 vs 12 mGy). Stamm (16) conducted a broad review of diagnostic reference levels (set at the 75th percentile) that were generally lower than our 75th percentile in distribution. For example, he reported reference levels for the DLP in the brain of 783–1149 mGy · cm (vs 75th percentile of 1300 mGy · cm in our cohort), in the chest of 400–627 mGy · cm (vs 830 mGy · cm in our cohort), and in the abdomen of 534–1629 mGy · cm (vs 1470 mGy · cm in our population). Thus, our 75th percentiles fall above or at the high end of the reference-level distributions reported (16). European radiologists may accept more noise in their images than do their U.S. colleagues, perhaps because of greater awareness and concern regarding the potential risks of radiation. As a result, their view of how to define optimized protocols may be different, which could account for these dose differences. There are no data with which to compare clinical outcomes as they may vary by dose.
The strengths of our study are the large sample size and automated data collection. The study also has several limitations. We do not describe dose for some CT examinations that crossed multiple regions (eg, head and chest) because estimating doses for these examinations is inaccurate. However, our data cover most CT scans obtained. We report on the number of machines equipped with iterative reconstruction software, but we do not know when the software was used. We report the median doses and the 75th percentiles because these thresholds are common in radiology (30,31). However, these measures do not necessarily reflect best practices, and many European countries are already using lower doses. For institutions in the United States that already optimize their doses (eg, by tailoring each CT examination to the clinical question being asked and to the size of the patient), they may be able to use doses that are considerably lower than those we report. The reference values for children are based on a relatively small number of examinations, and we did not have a sufficient sample size to report the metrics within age strata. Furthermore, we anticipate that future technical improvements might permit the use of doses considerably lower than these values. Our summary data might not be appropriate for facilities with a specific case mix—for example, a urology practice that evaluates only patients with renal stones. Finally, optimization must also focus on obtaining clinically adequate images at any dose level.
The purpose of the analysis was to provide summary data on CT dose based on a large number of consecutive CT examinations that institutions can use as a starting point for evaluation of the CT radiation doses they use in their patients. Quality organizations may use these data to contribute to nationally representative reference levels.
We provide data on common CT scans as a starting point for developing standards for routine CT. We expect that we and others will continue to update and add to these data for the creation of benchmarks. Institutions can use our metrics as a starting point to assess their CT doses and, when appropriate, apply a variety of approaches to achieve CT doses that optimize effective patient diagnosis and safety.
Advance in Knowledge
■ This article provides radiation dose data for a large number of consecutive CT scans from five large academic medical centers for several anatomic areas; because the data reflect all CT scans obtained in the anatomic areas reported and are not classified according to the clinical indication for scanning, sites that want to understand how their overall dose data compare with those from other institutions can use these data as a starting point for the evaluation of their own CT radiation dose.
Implication for Patient Care
■ The summary dose data herein provide a sample of CT doses that can be readily attained by institutions and that institutions can use to assess their own doses and to guide their optimization processes; these efforts can reduce patient exposure to ionizing radiation.
Received November 25, 2014; revision requested January 21, 2015; revision received February 13; final version accepted February 22.
Funding: This research was supported by the National Institutes of Health (grant R01CA181191 and award no. A1209013).
Supported by the Center for Health Quality and Innovation, University of California Office of the President, Centers for Disease Control and Prevention (Cooperative Agreement No. 5U48DP001908), and Patient-Centered Outcomes Research Institute (Program Award 7043).
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
See also the article by Bring and Miller in this issue.
Disclosures of Conflicts of Interest: R.S. disclosed no relevant relationships. M.M. disclosed no relevant relationships. N.W. disclosed no relevant relationships. T.R.N. disclosed no relevant relationships. J.M.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: received a one-time royalty from a patent issued and licensed to Samsung. C.H.C. disclosed no relevant relationships. R.G. disclosed no relevant relationships. D.J.H. disclosed no relevant relationships. M.K. disclosed no relevant relationships. R.L. disclosed no relevant relationships. M.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received a grant from Siemens Healthcare; is a paid speaker for Toshiba America Medical Systems. Other relationships: disclosed no relevant relationships. A.S. disclosed no relevant relationships. D.L.M. disclosed no relevant relationships.
Abbreviations:
- CTDIvol
- volume CT dose index
- DLP
- dose-length product
- IQR
- interquartile range
- SSDE
- size-specific dose estimate
References
- 1.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009;169(22):2078–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA 2012;307(22):2400–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukasiewicz A, Bhargavan-Chatfield M, Coombs L, et al. Radiation dose index of renal colic protocol CT studies in the United States: a report from the American College of Radiology National Radiology Data Registry. Radiology 2014;271(2):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sentinel Event Alert System . The Joint Commission. http://www.jointcommission.org/assets/1/18/SEA_471.PDF. Published August 24, 2011. Accessed DATE.
- 5.Vassileva J, Rehani MM, Applegate K, et al. IAEA survey of paediatric computed tomography practice in 40 countries in Asia, Europe, Latin America and Africa: procedures and protocols. Eur Radiol 2013;23(3):623–631. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration . Initiative to reduce unnecessary radiation exposure from medical imaging [white paper]. http://www.fda.gov/Radiation-EmittingProducts/RadiationSafety/RadiationDoseReduction/ucm2007191.htm. Accessed April 27, 2015.
- 7.Rehani MM. ICRP and IAEA actions on radiation protection in computed tomography. Ann ICRP 2012;41(3-4):154–160. [DOI] [PubMed] [Google Scholar]
- 8.Brisse HJ, Aubert B. CT exposure from pediatric MDCT: results from the 2007–2008 SFIPP/ISRN survey [in French]. J Radiol 2009;90(2):207–215. [DOI] [PubMed] [Google Scholar]
- 9.Muhogora WE, Ahmed NA, Almosabihi A, et al. Patient doses in radiographic examinations in 12 countries in Asia, Africa, and Eastern Europe: initial results from IAEA projects. AJR Am J Roentgenol 2008;190(6):1453–1461. [DOI] [PubMed] [Google Scholar]
- 10.McCollough C, Branham T, Herlihy V, et al. Diagnostic reference levels from the ACR CT accreditation program. J Am Coll Radiol 2011;8(11):795–803. [DOI] [PubMed] [Google Scholar]
- 11.American College of Radiology . CT accreditation program requirements. Reston, Va: American College of Radiology, 2013. [Google Scholar]
- 12.National Council on Radiation Protection and Measurements . Reference levels and achievable doses in medical and dental imaging: recommendations for the United States. Bethesda, Md: National Council on Radiation Protection and Measurements, 2012. [Google Scholar]
- 13.Verdun FR, Gutierrez D, Vader JP, et al. CT radiation dose in children: a survey to establish age-based diagnostic reference levels in Switzerland. Eur Radiol 2008;18(9):1980–1986. [DOI] [PubMed] [Google Scholar]
- 14.Galanski M, Nagel HD, Stamm G. Paediatric CT exposure practice in the federal republic of Germany: results of nationwide survey in 2005–2006. Hannover, Germany: Medizinische Hochschule, 2007. [Google Scholar]
- 15.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. Doses from computed tomography (CT) examinations in the UK: 2003 review. Publication no. NRPB-W67. Chilton, England: National Radiation Protection Board, 2005. [Google Scholar]
- 16.Stamm G. Collective radiation dose from MDCT: critical review of survey studies. In: Tack D, Kalra MK, Gevenois PA, eds. Radiation dose from multidetector CT. 2nd ed. Berlin, Germany: Springer, 2012; 209–229. [Google Scholar]
- 17.Tack D, Jahnen A, Kohler S, et al. Multidetector CT radiation dose optimisation in adults: short- and long-term effects of a clinical audit. Eur Radiol 2014;24(1):169–175. [DOI] [PubMed] [Google Scholar]
- 18.Pantos I, Thalassinou S, Argentos S, Kelekis NL, Panayiotakis G, Efstathopoulos EP. Adult patient radiation doses from non-cardiac CT examinations: a review of published results. Br J Radiol 2011;84(1000):293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UC DOSE Collaboration: The University of California Dose Optimization and Standardization Endeavor . http://radiology.ucsf.edu/research/labs/radiology-outcomes-research/UCDOSE. Published 2014. Accessed April 25, 2014.
- 20.Keegan J, Miglioretti DL, Gould R, Donnelly LF, Wilson ND, Smith-Bindman R. Radiation dose metrics in CT: assessing dose using the National Quality Forum CT patient safety measure. J Am Coll Radiol 2014;11(3):309–315. [DOI] [PubMed] [Google Scholar]
- 21.Radimetrics Enterprise Platform: Dose Management Solution . Bayer HealthCare. http://www.radiologysolutions.bayer.com/products/ct/dosemanagement/rep/. Accessed February 5, 2015.
- 22.Cristy M. Mathematical phantoms representing children of various ages for use in estimates of internal dose. U.S. Nuclear Regulatory Commission Rep. NUREG/CR-1159 (also Oak Ridge National Laboratory Rep. ORNL/NUREG/TM-367). Oak Ridge, Tenn: Oak Ridge National Laboratory, 1980. [Google Scholar]
- 23.The 2007 Recommendations of the International Commission on Radiological Protection . ICRP publication 103. Ann ICRP 2007:37(2–4). [DOI] [PubMed] [Google Scholar]
- 24.Boone J, Strauss K, Cody D, et al. Size-specific dose estimates (SSDE) in pediatric and adult body CT examinations. Report of AAPM Task Group 204. College Park, Md: American Association of Physicists in Medicine, 2011. [Google Scholar]
- 25.Radiological protection and safety in medicine: a report of the International Commission on Radiological Protection. Ann ICRP 1996;26(2):1–47. [Published correction appears in Ann ICRP 1997;27(2):61.] [PubMed] [Google Scholar]
- 26.Wall BF. Implementation of DRLs in the UK. Radiat Prot Dosimetry 2005;114(1-3):183–187. [DOI] [PubMed] [Google Scholar]
- 27.Dose Notifications CT. and Alerts: What are they, how do they work, and important information for successful implementation. American Association of Physicists in Medicine (AAPM) Working Group on Standardization of CT Nomenclature and Protocols. http://www.aapm.org/pubs/CTProtocols/documents/NotificationsAlertsSlides.pdf. Published April 16, 2014. Accessed January 27, 2015.
- 28.Huda W, Magill D, He W. CT effective dose per dose length product using ICRP 103 weighting factors. Med Phys 2011;38(3):1261–1265. [DOI] [PubMed] [Google Scholar]
- 29.Huda W. Computing effective doses from dose-length product in CT. Radiology 2008;248(1):321; author reply 321–322. [DOI] [PubMed] [Google Scholar]
- 30.American College of Radiology . BIRADS atlas. 5th ed. Reston, Va: American College of Radiology, 2013. [Google Scholar]
- 31.Sickles EA, Miglioretti DL, Ballard-Barbash R, et al. Performance benchmarks for diagnostic mammography. Radiology 2005;235(3):775–790. [DOI] [PubMed] [Google Scholar]


