Abstract
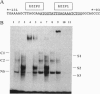
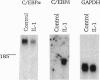
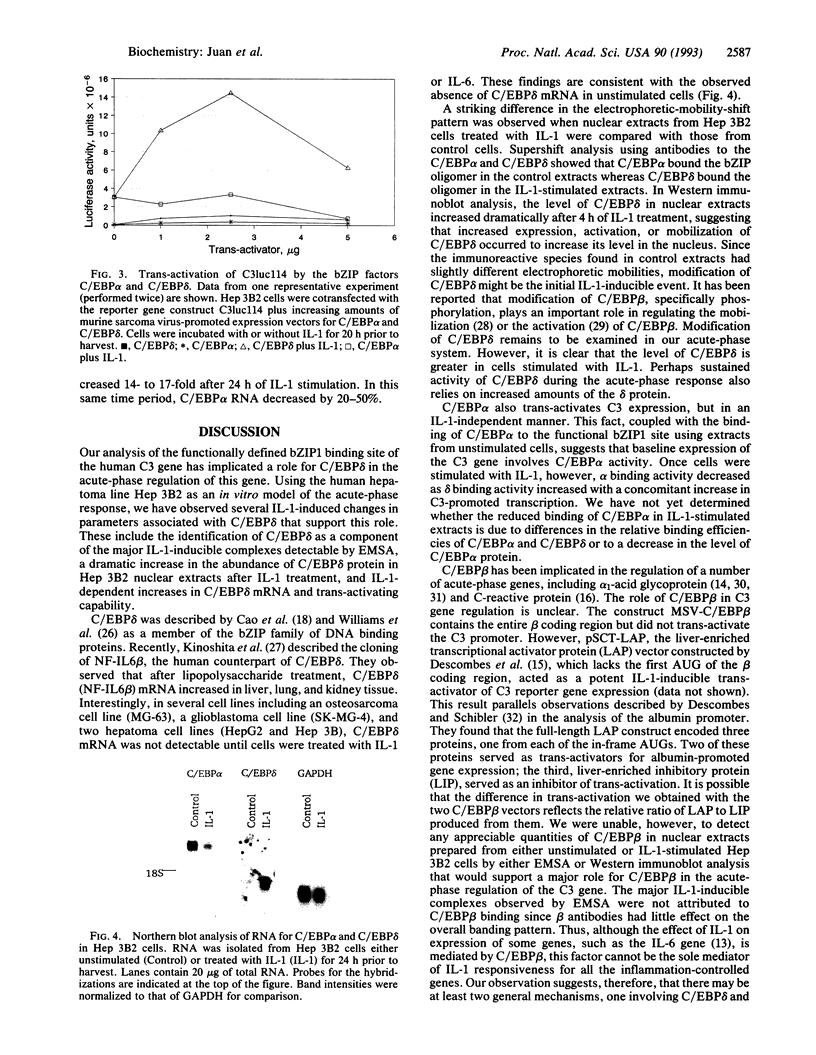
C3, the third component of complement, is critical in the host immune response in that it is involved in both the classical and alternative pathways of complement activation. We have previously shown that a region (bp -127 to -70) within the C3 promoter is indispensable for conferring interleukin 1 (IL-1) responsiveness to this gene. A sequence comparison reveals two CCAAT/enhancer binding protein (C/EBP) consensus sequences, basic DNA binding region and leucine zippers 1 and 2 (bZIP1 and bZIP2), within this region. Site-directed mutagenesis of the more 3' C/EBP site (bZIP1) in the C3 promoter significantly reduced the basal level of expression and the IL-1 responsiveness of the reporter gene, whereas mutation in the second, more 5', C/EBP consensus sequence (bZIP2) had a minimal effect on basal expression and IL-1 inducibility. Electrophoretic-mobility-shift assays, with and without antibodies to the different C/EBP proteins that "supershift" protein-DNA complexes, demonstrated that proteins binding at the 3' C/EBP site formed several complexes. Antibodies to C/EBP alpha supershifted the majority of complexes formed with extracts from control cells. Antibodies directed against C/EBP delta supershifted the major IL-1-inducible complexes. Western immunoblot analyses showed that the level of C/EBP delta protein was increased dramatically in the nuclei of Hep 3B2 cells after 4 h of IL-1 treatment. When Hep 3B2 cells were cotransfected with a C/EBP delta expression vector and a construct with a C3 promoter and a reporter gene, C/EBP delta was able to trans-activate the C3 promoter in an IL-1-responsive manner. The data strongly suggest that C/EBP delta is the major protein responsible for regulating the acute-phase expression of the human C3 gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam T., An M. R., Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992 Mar 15;267(8):5021–5024. [PubMed] [Google Scholar]
- Alam T., Papaconstantinou J. Interaction of acute-phase-inducible and liver-enriched nuclear factors with the promoter region of the mouse alpha 1-acid glycoprotein gene-1. Biochemistry. 1992 Feb 25;31(7):1928–1936. doi: 10.1021/bi00122a005. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baumann H. Hepatic acute phase reaction in vivo and in vitro. In Vitro Cell Dev Biol. 1989 Feb;25(2):115–126. doi: 10.1007/BF02626167. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Chang C. J., Chen T. T., Lei H. Y., Chen D. S., Lee S. C. Molecular cloning of a transcription factor, AGP/EBP, that belongs to members of the C/EBP family. Mol Cell Biol. 1990 Dec;10(12):6642–6653. doi: 10.1128/mcb.10.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P., Chojkier M., Lichtsteiner S., Falvey E., Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990 Sep;4(9):1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991 Nov 1;67(3):569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988 Aug 25;334(6184):721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- Huang J. H., Rienhoff H. Y., Jr, Liao W. S. Regulation of mouse serum amyloid A gene expression in transfected hepatoma cells. Mol Cell Biol. 1990 Jul;10(7):3619–3625. doi: 10.1128/mcb.10.7.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki H., Akira S., Sugita T., Nishio Y., Hashimoto S., Pawlowski T., Suematsu S., Kishimoto T. Reciprocal expression of NF-IL6 and C/EBP in hepatocytes: possible involvement of NF-IL6 in acute phase protein gene expression. New Biol. 1991 Jan;3(1):63–70. [PubMed] [Google Scholar]
- Kinoshita S., Akira S., Kishimoto T. A member of the C/EBP family, NF-IL6 beta, forms a heterodimer and transcriptionally synergizes with NF-IL6. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1473–1476. doi: 10.1073/pnas.89.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A., Ganapathi M. K., Schultz D., Brabenec A., Weinstein J., Kelley M. F., Kushner I. Transforming growth factor beta 1 regulates production of acute-phase proteins. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1491–1495. doi: 10.1073/pnas.87.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz R., Ziff E. cAMP stimulates the C/EBP-related transcription factor rNFIL-6 to trans-locate to the nucleus and induce c-fos transcription. Genes Dev. 1991 Oct;5(10):1754–1766. doi: 10.1101/gad.5.10.1754. [DOI] [PubMed] [Google Scholar]
- Neta R., Vogel S. N., Sipe J. D., Wong G. G., Nordan R. P. Comparison of in vivo effects of human recombinant IL 1 and human recombinant IL 6 in mice. Lymphokine Res. 1988 Winter;7(4):403–412. [PubMed] [Google Scholar]
- Poli V., Cortese R. Interleukin 6 induces a liver-specific nuclear protein that binds to the promoter of acute-phase genes. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8202–8206. doi: 10.1073/pnas.86.21.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V., Mancini F. P., Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990 Nov 2;63(3):643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- Prowse K. R., Baumann H. Hepatocyte-stimulating factor, beta 2 interferon, and interleukin-1 enhance expression of the rat alpha 1-acid glycoprotein gene via a distal upstream regulatory region. Mol Cell Biol. 1988 Jan;8(1):42–51. doi: 10.1128/mcb.8.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom S. A., Komm B. S., Ponce-de-Leon H., Yi Z., Teuscher C., Lyttle C. R. Estrogen regulation of tissue-specific expression of complement C3. J Biol Chem. 1989 Oct 5;264(28):16941–16947. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M., Cao Z., Rosenfeld M. G. Calcium-regulated phosphorylation within the leucine zipper of C/EBP beta. Science. 1992 Apr 17;256(5055):370–373. doi: 10.1126/science.256.5055.370. [DOI] [PubMed] [Google Scholar]
- Williams S. C., Cantwell C. A., Johnson P. F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991 Sep;5(9):1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Juan T. S., Wilde M. D., Fey G. H., Darlington G. J. A 58-base-pair region of the human C3 gene confers synergistic inducibility by interleukin-1 and interleukin-6. Mol Cell Biol. 1990 Dec;10(12):6181–6191. doi: 10.1128/mcb.10.12.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won K. A., Baumann H. NF-AB, a liver-specific and cytokine-inducible nuclear factor that interacts with the interleukin-1 response element of the rat alpha 1-acid glycoprotein gene. Mol Cell Biol. 1991 Jun;11(6):3001–3008. doi: 10.1128/mcb.11.6.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P., Sipe J., Dinarello C. A., Colten H. R. Structure of a human serum amyloid A gene and modulation of its expression in transfected L cells. J Biol Chem. 1987 Nov 15;262(32):15790–15795. [PubMed] [Google Scholar]