Abstract
OBJECTIVE
To evaluate subjects with type 1 diabetes for hepatic glycogen depletion after repeated doses of glucagon, simulating delivery in a bihormonal closed-loop system.
RESEARCH DESIGN AND METHODS
Eleven adult subjects with type 1 diabetes participated. Subjects underwent estimation of hepatic glycogen using 13C MRS. MRS was performed at the following four time points: fasting and after a meal at baseline, and fasting and after a meal after eight doses of subcutaneously administered glucagon at a dose of 2 µg/kg, for a total mean dose of 1,126 µg over 16 h. The primary and secondary end points were, respectively, estimated hepatic glycogen by MRS and incremental area under the glucose curve for a 90-min interval after glucagon administration.
RESULTS
In the eight subjects with complete data sets, estimated glycogen stores were similar at baseline and after repeated glucagon doses. In the fasting state, glycogen averaged 21 ± 3 g/L before glucagon administration and 25 ± 4 g/L after glucagon administration (mean ± SEM) (P = NS). In the fed state, glycogen averaged 40 ± 2 g/L before glucagon administration and 34 ± 4 g/L after glucagon administration (P = NS). With the use of an insulin action model, the rise in glucose after the last dose of glucagon was comparable to the rise after the first dose, as measured by the 90-min incremental area under the glucose curve.
CONCLUSIONS
In adult subjects with well-controlled type 1 diabetes (mean A1C 7.2%), glycogen stores and the hyperglycemic response to glucagon administration are maintained even after receiving multiple doses of glucagon. This finding supports the safety of repeated glucagon delivery in the setting of a bihormonal closed-loop system.
Introduction
Diabetes remains the leading cause of renal failure, nontraumatic lower-limb amputations, and new cases of blindness among adults (1). The risks of these complications can be substantially reduced with reduction of hyperglycemia, but more aggressive therapy increases the risk of hypoglycemia significantly (2). Because of the difficulty of managing diabetes, there are ongoing efforts to develop a closed-loop system (3). Closed-loop systems generally consist of a glucose sensor from which data are collected and input into an algorithm, which calculates a varying insulin rate for delivery via a pump. The difficulty of delivering insulin subcutaneously in such a fashion is related to its relatively slow onset and prolonged effect (4). The slow onset often leads to hyperglycemia after meals, and the prolonged effect increases the risk of hypoglycemia prior to the next meal.
In contrast to insulin, the action of glucagon is rapid and short-lived, even when given subcutaneously (5). In 1963, Kadish (6) published a study introducing the concept of delivering glucagon in the context of a closed-loop system. In such a system, insulin is delivered to treat hyperglycemia and maintain euglycemia, while glucagon is delivered to prevent or treat hypoglycemia. Our group (7,8) and others (9–11) have successfully treated patients with type 1 diabetes using a bihormonal closed-loop system. Glucagon was well tolerated in these studies and significantly reduced the frequency of hypoglycemia compared with a placebo (7). Glucagon administration did not, however, completely eliminate hypoglycemic episodes, and in one study (12) failed to prevent hypoglycemia in one-third of cases, raising the possibility of hepatic glycogen depletion.
Even individuals without diabetes have been shown to have a brisk hyperglycemic response to a single glucagon dose of 0.5 mg, but a poor response to a second dose (13). Lower doses, such as those given via a closed-loop system, have not been studied. Individuals with type 1 diabetes are likely to be at higher risk for glycogen depletion due to lower glycogen stores after an overnight fast (14). In particular, poorly controlled diabetes is associated with reduced glycogen synthesis and glycogen breakdown, which improved with intensive diabetes control and nearly normalized after a year (15). These studies suggest that in individuals with type 1 diabetes, except possibly in those with excellent control, impairments in hepatic glycogen synthesis and breakdown are common. There is an unmet need to better understand whether people with type 1 diabetes are capable of responding normally to repeated small doses of glucagon. If the response to glucagon is preserved and glycogen stores are not depleted, these findings would support the safety of repeated glucagon delivery in a bihormonal closed-loop system.
Research Design and Methods
Subjects
Eleven subjects with type 1 diabetes who were receiving insulin pump therapy were each admitted for one 40-h study. All subjects provided written informed consent before entering the study. Eight females and three males were studied, with a mean age of 40.5 ± 5 years, a duration of diabetes of 12.5 ± 2.2 years, an HbA1c level of 7.2 ± 0.1%, and a weight of 70.3 ± 4.2 kg (mean ± SEM). All subjects were required to be between 18 and 65 years of age, and to have a BMI between 18 and 35 kg/m2. Women of childbearing age were required to have a negative urine pregnancy test result prior to participation. Patients with a history of cardiovascular, cerebrovascular, kidney, or liver disease or any other uncontrolled chronic medical conditions were excluded from the study. Other exclusion criteria included adrenal insufficiency, oral or parenteral corticosteroid use, active foot ulceration, bleeding disorder, seizure disorder, history of weight loss of ≥5 pounds over the prior month, active alcohol or substance abuse, or any contraindication to an MRI scan.
Protocol
The research protocol was approved by the Oregon Health & Science University Institutional Review Board, and this study was registered with ClinicalTrials.gov (clinical trial reg. no. NCT01986231). Subjects were given standardized meals that were consumed at home at 10:00 a.m. and 3:00 p.m. on the day of admission to the clinical research center. Subjects were subsequently admitted to the clinical research center in the evening. Each subject’s insulin pump was stopped, and each subject was treated intravenously with regular insulin throughout the remainder of the study to a target glucose concentration of 90–130 mg/dL guided by an insulin infusion protocol previously described by Goldberg et al. (16). Each subject received 8 doses of glucagon (GlucaGen; Novo Nordisk, Copenhagen, Denmark) 2 μg/kg, s.c. Glucagon was reconstituted with sterile water to a concentration of 1 mg/mL just prior to each dose to avoid any degradation or aggregation prior to administration. The study schedule, including glucagon dosing interval and MRS scans, is presented in Table 1.
Table 1.
Study schedule with timing of MRS scans, meals, and glucagon doses
| Study time | Event |
|---|---|
| Day 1, 10:00 a.m. (at home) | Breakfast #1 |
| Day 1, 3:00 p.m. (at home) | Lunch #1 |
| Day 1, 6:00 p.m. | MRI #1 |
| Day 1, 8:00 p.m. | Dinner #1 |
| Day 2, 9:00 a.m. | MRI #2 |
| Day 2, 10:00 a.m. | Breakfast |
| Day 2, Noon | Glucagon #1 |
| Day 2, 3:00 p.m. | Lunch |
| Day 2, 5:00 p.m. | Glucagon #2 |
| Day 2, 6:00 p.m. | MRI #3 |
| Day 2, 7:00 p.m. | Glucagon #3 |
| Day 2, 8:00 p.m. | Dinner #2 |
| Day 2, 10:00 p.m. | Glucagon #4 |
| Day 2, Midnight | Glucagon #5 |
| Day 3, 2:00 a.m. | Glucagon #6 |
| Day 3, 4:00 a.m. | Glucagon #7 |
| Day 3, 6:00 a.m. | Glucagon #8 |
| Day 3, 9:00 a.m. | MRI #4 |
Venous blood glucose levels were measured every 15 min via a HemoCue 201 glucose analyzer (HemoCue AB, Ängelholm, Sweden). The frequency of venous blood glucose measurement was increased to every 10 min for the 3 h after the first and last glucagon doses were administered. The rate of the intravenous administration of regular insulin was fixed over this 3-h time period. If the blood glucose level fell to <90 mg/dL, dextrose was infused to avoid hypoglycemia. All meals during the study were standardized to contain 50% carbohydrates. Calorie content was based on a weight-maintaining diet. The meals for each individual on study day 1 were identical to the meals on study day 2. Insulin aspart (Novolog; Novo Nordisk) was administered subcutaneously just prior to meals at 75% of the subject’s typical dose.
Semiquantitative measurements of in vivo hepatic glycogen were performed by 13C MRS at 7 T. Hepatic glycogen levels were determined immediately after entry into the study at 6:00 p.m., after fasting overnight at 9:00 a.m., and again at 6:00 p.m. and 9:00 a.m. following an identical feeding pattern but with added administration of glucagon. The method used followed an adaptation of the methodology previously reported by Shulman et al. (17) and Jue et al. (18). This methodology is akin to MRI, with the same safety profile. However, there is no spatial resolution of signal and therefore no imaging; only the total signal in the interrogation volume is collected, and, rather than water 1H nuclei, the 13C nucleus is probed. The spectroscopic nature of this technique allows 13C nuclei to be differentiated on the basis of their chemical environments. The C1 carbon of glycogen is shifted far from all other in vivo carbon resonances (∼100.5 parts per million), and it therefore readily allows for determining changes in hepatic glycogen concentrations (19).
A complete description of the 13C MRS data acquisition methods and analytical methods are provided in the Supplementary Data. In brief, all spectra were acquired on a Siemens Magnetom 7 T MRI system. Data acquisition was performed using a home-built 1H/13C surface dual-tune coil. The 1H coil was used for shimming and coil localization, and to acquire 1H water proton images of the liver. These images were used to perform volume corrections during data analysis. 13C MRS spectra were acquired, on resonance, for the C1 glycogen carbon (74.7370 MHz) using 11,000 transients and a repetition time of 160 ms; the total acquisition time was ∼25 min. Each spectrum was acquired in the presence of a 13C-enriched acetonitrile (Sigma-Aldrich, St. Louis, MO) external standard. 13C signal intensities were determined by a line-fitting routine. The intensity of the C1 glycogen signal was then related to the in vivo glycogen concentration by comparing it to a standardization curve generated on phantoms of oyster glycogen (Sigma-Aldrich). Axial and sagittal 1H images were used to correct for differences in the coil volume occupied by the liver and phantom. In this way, it was possible to obtain a semiquantitative measure of hepatic glycogen concentration (in grams per liter).
Statistical Analysis
The primary end point was the comparison of estimated hepatic glycogen as determined by 13C MRS after fasting overnight on study day 1 versus study day 2 (i.e., before and after repeated doses of glucagon) and after lunch on study day 1 versus study day 2. This outcome was modeled as a continuous variable using generalized estimating equations to account for correlations between repeated measures on the same individuals. In this glycogen model, we included indicator variables for fasting and the administration of glucagon, as well as for their interaction. MRS data from three subjects were excluded. The scans were not obtained in the first subject because of an error in equipment setup. In a second subject, minimal hepatic glycogen was detected in all scans because of excessive separation of the coil from the liver by a thick subcutaneous adipose layer. A third subject did not complete the final MRS procedure because of illness.
The secondary end point was the rise in glucose after the first versus the last glucagon dose, as measured by the incremental area under the curve (AUC) for glucose for the 90-min interval after glucagon administration (20). Data from all 11 subjects were included. A three-compartment glucoregulatory model was used to account for the effect of meals, intravenous dextrose administration, and insulin administration, therefore isolating the effect of glucagon. We used an insulin pharmacokinetics model reported by Hovorka et al. (21) and an insulin pharmacodynamics model reported by Wilinska et al. (22). All 19 model parameters were fit for the first overnight period. Based on these estimated parameters, glucose values for the entire time course of the study were estimated. Any response due to glucagon was not modeled, thus differentiating the change in glucose due to glucagon administration. The modeled glucose values were subtracted from the true values to produce the response due to glucagon for each glucagon dose. Subjects were evaluated within a hospital setting, during which time they were sedentary, which could have led to reduced insulin sensitivity. To evaluate the possibility that reduced insulin sensitivity affected the outcome of the study, a separate simulation was completed whereby insulin sensitivity was modeled to decline linearly from 100% down to 50% over the course of the study.
To test for changes in the mean rise in glucose due to glucagon after each dose, we used a generalized estimating equation model with robust variance estimators and dose number as a categorical independent variable. Wald tests were used to examine the significance of the overall model and of coefficients representing changes from the first dose at each subsequent dose. SEs and 95% CIs were calculated using the standard delta method for such models.
All analyses were performed with Stata version 13.1 (StataCorp LP, College Station, TX) or MATLAB version R2014a (MathWorks, Natick, MA). Statistical significance was accepted at P < 0.05. Data are represented as the mean ± SEM, unless otherwise noted.
Results
Glucagon Doses
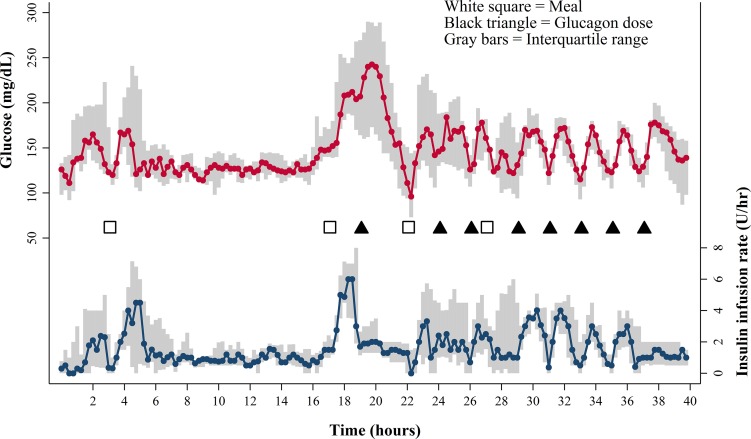
All subjects received eight doses of glucagon, each 2 µg/kg, with a mean dose of 140.7 ± 8.2 µg and a total dose of 1,125.8 ± 65.5 µg over 16 h. Median glucose values and insulin infusion rates for the entire study are shown in Fig. 1. Dextrose was infused infrequently and only for the treatment of hypoglycemia with a mean dose of 253 ± 164 mL of 10% dextrose, equivalent to 25 g of carbohydrate, over 44 h.
Figure 1.
Median venous blood glucose values in milligrams per deciliter (top graph) and median insulin infusion rates in units per hour (bottom graph). The interquartile range is shown in gray. Meals (white squares) and glucagon doses (black triangles) are also shown. Note that glucose levels consistently rose after each glucagon dose. The insulin infusion rate was fixed after the first and last glucagon dose, but was otherwise adjusted to bring the glucose concentration into the target range.
Glycogen Stores After Repeated Doses of Glucagon in Subjects With Type 1 Diabetes
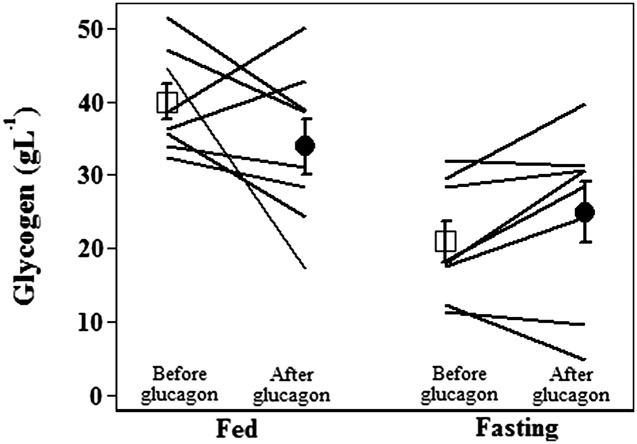
At baseline, prior to the administration of glucagon, hepatic glycogen declined significantly after an overnight fast as expected (−19 g/L, P < 0.001). Repeated administration of glucagon did not significantly lower glycogen stores. In the fasting state, glycogen averaged 21 ± 3 g/L before glucagon administration and 25 ± 4 g/L after glucagon administration (P = NS; see Fig. 2). In the fed state, the difference before and after glucagon was larger, though still not statistically significant: glycogen averaged 40 ± 2 g/L before glucagon administration and 34 ± 4 g/L after glucagon administration (P = NS).
Figure 2.
Estimated individual and group mean glycogen stores with SEM before (white squares) and after (black circles) glucagon administration in subjects with type 1 diabetes, under fed and fasting conditions (n = 8). Liver glycogen stores after eight doses of glucagon were similar to baseline levels.
Glucose Response to Glucagon Administration After Repeated Doses of Glucagon
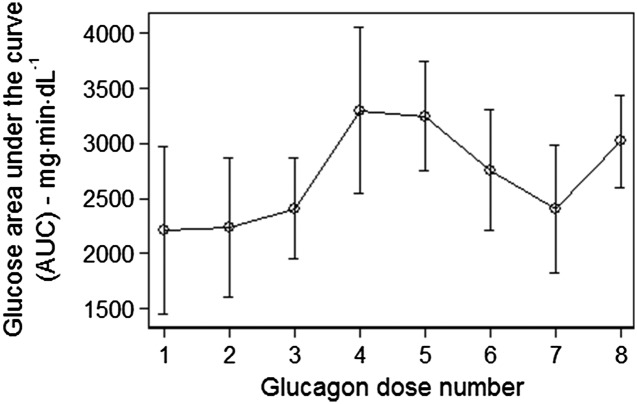
The rise in glucose level after the last dose of glucagon was comparable to the rise after the first dose as measured by the 90-min incremental AUC for glucose (2,209 ± 757 vs. 3,018 ± 415 mg ⋅ min ⋅ dL−1, P = NS; see Fig. 3). Similarly, there was no significant difference in AUC when the model included a 50% decline in insulin sensitivity that may have occurred due to subject inactivity during the hospital stay (2,209 ± 757 vs. 2,812 ± 410 mg ⋅ min ⋅ dL−1, P = NS). The average AUC after any dose of glucagon was 2,698 ± 248 mg ⋅ min ⋅ dL−1, and there were no significant differences across all eight doses. See Supplementary Table 1 for unadjusted and adjusted values.
Figure 3.
Mean incremental glucose AUC with SEM after each of eight doses of glucagon. The modeled AUC accounting for the effects of meals, dextrose, and insulin was subtracted from the observed AUC to isolate the effect of each glucagon dose.
Conclusions
Glucagon raises circulating glucose levels primarily via glycogenolysis, and to a lesser degree gluconeogenesis (23). In this study, we assessed the effect of repeated doses of glucagon on glycogen stores and on circulating glucose levels. To rigorously assess for glycogen depletion in this study, we gave glucagon doses that were higher than the typical doses used in our previous closed-loop studies (1,126 µg/day in this study vs. 282 µg/day in the previous studies) (7,8,24). The doses given in this study are comparable to those given in a study by Russell et al. (9), but were significantly higher than those given by Haidar et al. (10), and higher than those given by our group (7,8,24). Despite these glucagon doses, we found that glycogen stores were not significantly diminished after repeated glucagon doses relative to baseline in both the fasting and fed states. There was, however, a trend toward a modest decline in glycogen stores in the fed state, with six of eight subjects showing a decline. This study was not powered to detect modest differences, and a much larger study would likely demonstrate a modest difference in the fed state. The data indicate that there is not a marked (>50%) reduction in glycogen stores, and subjects retained their hyperglycemic response to glucagon even in the fasting state after receiving in excess of 1 mg glucagon. These findings suggest that glycogen stores can be replenished even following multiple doses of glucagon. These findings also provide reassurance that repeated glucagon dosing in the setting of a bihormonal closed-loop system is unlikely to cause marked glycogen depletion over the short term. Glycogen depletion must be avoided, as it would leave a person with diabetes at higher risk for severe hypoglycemia.
In light of these findings, it is improbable that glycogen depletion was responsible for glucagon failing to prevent hypoglycemia in one-third of cases in our past study (12). It is more likely that the failure of glucagon to prevent hypoglycemia was related to a high prevailing insulin effect, which has been shown to dominate over the effect of glucagon at high doses (25). The delay in the detection of impending hypoglycemia by the glucose sensor also was a contributor (12). Currently available sensors have significantly better accuracy in the hypoglycemic range (26), allowing for earlier glucagon delivery. Improved sensor technology and adjusting glucagon dosing based on insulin-on-board have led to significantly fewer glucagon failures (24).
It is important to note that the subjects in this study were eating on a regular basis, had well-controlled diabetes, and were studied over a short period of time. Fasting for a prolonged period would induce glycogen depletion, and automated glucagon delivery would not be appropriate in such circumstances, such as during an episode of gastroenteritis. It is also unknown whether individuals with poorly controlled diabetes would respond to repeated glucagon doses in the same manner as those with well-controlled diabetes.
There remain hurdles to the commercialization of a bihormonal closed-loop system. Glucagon is an unstable protein in aqueous solution, and currently available emergency kits are unsuitable for use in a portable pump. For this reason, glucagon was reconstituted immediately prior to each dose in this study. Multiple efforts are underway to create a stable liquid glucagon formulation (27–30). The logistics of delivering multiple hormones are also complicated, and are being addressed by companies such as Tandem Diabetes Care, where the development of a dual-chamber pump is underway. Prior to Food and Drug Administration approval of such systems, longer and larger outpatient clinical trials will certainly be required.
Supplementary Material
Article Information
Acknowledgments. The authors thank Tom Barbara, PhD (Oregon Health & Science University [OHSU]), for building the custom 1H/13C coil used in this study and Manoj Sammi, PhD (OHSU), and William Woodward, BS (OHSU), for assistance with data acquisition. The authors also thank Kevin Yuen, MD (OHSU), and Michael Chu, MD (OHSU), for their assistance in conducting the studies.
Funding. This work was supported by grants from JDRF, M.J. Murdock Charitable Trust, the HEDCO Foundation, the Good Samaritan Foundation (Portland, OR), and the National Institutes of Health (grant T32-DK-007674). This publication was also supported by the Oregon Clinical and Translational Research Institute and the National Center for Advancing Translational Sciences at the National Institutes of Health (grant UL1-TR-000128).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. J.R.C., P.J., and W.K.W. have a financial interest in Pacific Diabetes Technologies, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by Oregon Health & Science University. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.R.C. and D.B. conducted the study and the data analysis and wrote the article. J.E.Y. and W.K.W. conducted the study and the data analysis and edited the article. P.A.B. conducted the study and edited the article. Y.C. conducted MRS scans. K.R. conducted the data analysis and wrote the article. J.M.S., P.J., and R.R. conducted the data analysis. M.W. conducted MRS scans and wrote the article. J.R.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, PA, 8–12 June 2012.
Footnotes
Clinical trial reg. no. NCT01986231, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-0754/-/DC1.
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA, U.S. Department of Health and Human Services, 2014 [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 3.Peyser T, Dassau E, Breton M, Skyler JS. The artificial pancreas: current status and future prospects in the management of diabetes. Ann N Y Acad Sci 2014;1311:102–123 [DOI] [PubMed] [Google Scholar]
- 4.Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet 2001;40:641–659 [DOI] [PubMed] [Google Scholar]
- 5.Graf CJ, Woodworth JR, Seger ME, Holcombe JH, Bowsher RR, Lynch R. Pharmacokinetic and glucodynamic comparisons of recombinant and animal-source glucagon after IV, IM, and SC injection in healthy volunteers. J Pharm Sci 1999;88:991–995 [DOI] [PubMed] [Google Scholar]
- 6.Kadish AH. Automation control of blood sugar a servomechanism for glucose monitoring and control. Trans Am Soc Artif Intern Organs 1963;9:363–367 [PubMed] [Google Scholar]
- 7.Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs PG, El Youssef J, Castle J, et al. Automated control of an adaptive bihormonal, dual-sensor artificial pancreas and evaluation during inpatient studies. IEEE Trans Biomed Eng 2014;61:2569–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014;371:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haidar A, Legault L, Dallaire M, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ 2013;185:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol 2015;3:17–26 [DOI] [PubMed] [Google Scholar]
- 12.Castle JR, Engle JM, El Youssef J, Massoud RG, Ward WK. Factors influencing the effectiveness of glucagon for preventing hypoglycemia. J Diabetes Sci Technol 2010;4:1305–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockton JA, Poucher SM. Single dose glucagon (0.5 mg IV bolus) administration in healthy human volunteers is a robust model for assessment of glycogenolysis: characterisation of the glucose excursion after glucagon challenge. J Pharmacol Toxicol Methods 2007;55:86–90 [DOI] [PubMed] [Google Scholar]
- 14.Kishore P, Gabriely I, Cui MH, et al. Role of hepatic glycogen breakdown in defective counterregulation of hypoglycemia in intensively treated type 1 diabetes. Diabetes 2006;55:659–666 [DOI] [PubMed] [Google Scholar]
- 15.Bischof MG, Krssak M, Krebs M, et al. Effects of short-term improvement of insulin treatment and glycemia on hepatic glycogen metabolism in type 1 diabetes. Diabetes 2001;50:392–398 [DOI] [PubMed] [Google Scholar]
- 16.Goldberg PA, Siegel MD, Sherwin RS, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care 2004;27:461–467 [DOI] [PubMed] [Google Scholar]
- 17.Shulman GI, Rothman DL, Chung Y, et al. 13C NMR studies of glycogen turnover in the perfused rat liver. J Biol Chem 1988;263:5027–5029 [PubMed] [Google Scholar]
- 18.Jue T, Rothman DL, Shulman GI, Tavitian BA, DeFronzo RA, Shulman RG. Direct observation of glycogen synthesis in human muscle with 13C NMR. Proc Natl Acad Sci U S A 1989;86:4489–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruetter R, Magnusson I, Rothman DL, Avison MJ, Shulman RG, Shulman GI. Validation of 13C NMR measurements of liver glycogen in vivo. Magn Reson Med 1994;31:583–588 [DOI] [PubMed] [Google Scholar]
- 20.Wolever TM. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br J Nutr 2004;91:295–301 [DOI] [PubMed] [Google Scholar]
- 21.Hovorka R, Canonico V, Chassin LJ, et al. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas 2004;25:905–920 [DOI] [PubMed] [Google Scholar]
- 22.Wilinska ME, Chassin LJ, Schaller HC, Schaupp L, Pieber TR, Hovorka R. Insulin kinetics in type-I diabetes: continuous and bolus delivery of rapid acting insulin. IEEE Trans Biomed Eng 2005;52:3–12 [DOI] [PubMed] [Google Scholar]
- 23.Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 1996;98:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakhtiani PA, El Youssef J, Duell AK, et al. Factors affecting the success of glucagon delivered during an automated closed-loop system in type 1 diabetes. J Diabetes Complications 2015;29:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Youssef J, Castle JR, Bakhtiani PA, et al. Quantification of the glycemic response to microdoses of subcutaneous glucagon at varying insulin levels. Diabetes Care 2014;37:3054–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kropff J, Bruttomesso D, Doll W, et al. Accuracy of two continuous glucose monitoring systems: a head-to-head comparison under clinical research centre and daily life conditions. Diabetes Obes Metab 2015;17:343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson MA, Caputo N, Castle JR, David LL, Roberts CT Jr, Ward WK. Stable liquid glucagon formulations for rescue treatment and bi-hormonal closed-loop pancreas. Curr Diab Rep 2012;12:705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakhtiani PA, Caputo N, Castle JR, et al. A novel, stable, aqueous glucagon formulation using ferulic acid as an excipient. J Diabetes Sci Technol 2015;9:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newswanger B, Ammons S, Phadnis N, et al. Development of a highly stable, nonaqueous glucagon formulation for delivery via infusion pump systems. J Diabetes Sci Technol 2015;9:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pohl R, Li M, Krasner A, De Souza E. Development of stable liquid glucagon formulations for use in artificial pancreas. J Diabetes Sci Technol 2015;9:8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.